
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuliana Bianco | + 5265 word(s) | 5265 | 2021-06-28 08:38:27 | | | |
| 2 | Vivi Li | + 30 word(s) | 5295 | 2021-07-07 04:09:59 | | |
Video Upload Options
Apples represent a greater proportion of the worldwide fruit supply, due to their availability on the market and to the high number of existing cultivar varieties and apple-based products (fresh fruit, fruit juice, cider and crushed apples). Several studies on apple fruit metabolites are available, with most of them focusing on their healthy properties’ evaluation. In general, the metabolic profile of apple fruits strongly correlates with most of their peculiar characteristics, such as taste, flavor and color. At the same time, many bioactive molecules could be identified as markers of a specific apple variety. Therefore, a complete description of the analytical protocols commonly used for apple metabolites’ characterization and quantification could be useful for researchers involved in the identification of new phytochemical compounds from different apple varieties.
1. Introduction
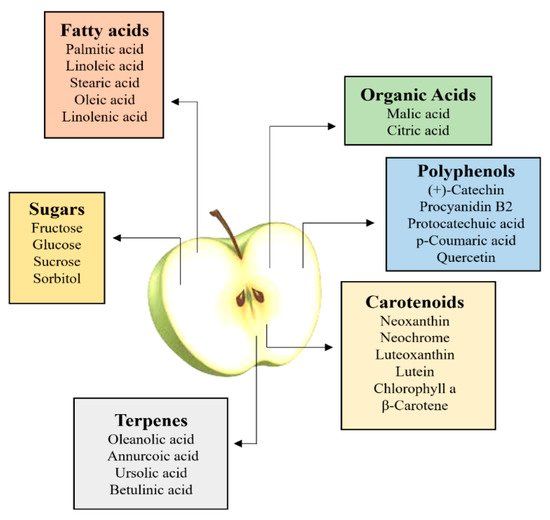
2. Primary Metabolites: Extraction and Analysis
2.1. Sugars
2.2. Fatty Acids
3. Secondary Metabolites
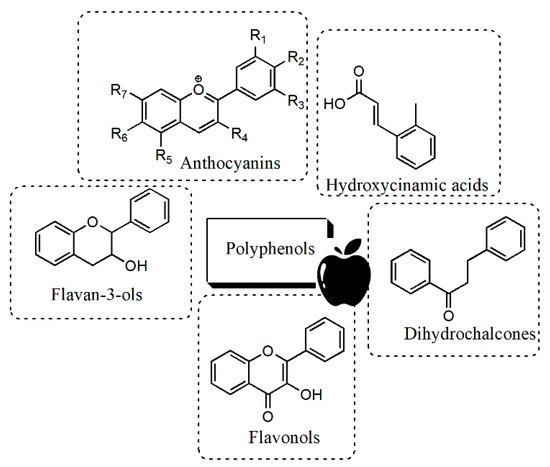
3.1. Extraction
3.2. Chromatographic Methods
References
- Bevilacqua, M.; Bro, R.; Marini, F.; Rinnan, Å.; Rasmussen, M.A.; Skov, T. Recent chemometrics advances for foodomics. TrAC Trends Anal. Chem. 2017, 96, 42–51.
- Herrero, M.; Simò, C.; Garcia-Canasa, V.; Ibanez, E.; Cifuentes, A. Foodomics: MS-based strategies in modern food science and nutrition. Mass Spectrom. Rev. 2012, 31, 49–69.
- Giordani, E.; Doumett, S.; Nin, S.; Del Bubba, M. Selected primary and secondary metabolites in fresh persimmon (Diospyros kaki Thunb.): A review of analytical methods and current knowledge of fruit composition and health benefits. Food Res. Int. 2011, 44, 1752–1767.
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products-A review. Sci. Hortic. 2015, 184, 179–192.
- Huber, G.M.; Rupasinghe, H.P.V. Phenolic profiles and antioxidant properties of apple skin extracts. J. Food Sci. 2009, 74, 693–700.
- Feliciano, R.P.; Antunes, C.; Ramos, A.; Serra, A.T.; Figueira, M.E.; Duarte, C.M.M.; de Carvalho, A.; Bronze, M.R. Characterization of traditional and exotic apple varieties from Portugal. Part 1—Nutritional, phytochemical and sensory evaluation. J. Funct. Foods 2010, 2, 35–45.
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401.
- Mohanta, S.; Singh, S.K.; Kumar, B.; Gulati, M.; Jyoti, J.; Som, S.; Panchal, S.; Melkani, I.; Banerjee, M.; Sinha, S.K.; et al. Solidification of liquid Modified Apple Polysaccharide by its adsorption on solid porous carriers through spray drying and evaluation of its potential as binding agent for tablets. Int. J. Biol. Macromol. 2018, 120, 1975–1998.
- Ajanaku, C.; Echeme, J.; Mordi, R.; Bolade, O.; Okoye, S.; Jonathan, H.; Ejilude, O. In-vitro antibacterial, phytochemical, antimycobacterial activities and GC-MS analyses of Bidens pilosa leaf extract. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 721–725.
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Amboni, R.D.D.M.C.; Denardi, F.; Fett, R. Physico-chemical and antioxidant properties of six apple cultivars (Malus domestica Borkh) grown in southern Brazil. Sci. Hortic. 2009, 122, 421–425.
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15.
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835.
- Ramirez-Ambrosi, M.; Abad-Garcia, B.; Viloria-Bernal, M.; Garmon-Lobato, S.; Berrueta, L.A.; Gallo, B. A new ultrahigh performance liquid chromatography with diode array detection coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry analytical strategy for fast analysis and improved characterization of phenolic compounds in ap. J. Chromatogr. A 2013, 1316, 78–91.
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10.
- Guan, Y.; Peace, C.; Rudell, D.; Verma, S.; Evans, K. QTLs detected for individual sugars and soluble solids content in apple. Mol. Breed. 2015, 35, 1–13.
- Karkacier, M.; Erbas, M.; Uslu, M.K.; Aksu, M. Comparison of different extraction and detection methods for sugars using amino-bonded phase HPLC. J. Chromatogr. Sci. 2003, 41, 331–333.
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC-ELSD. Food Chem. 2014, 145, 784–788.
- Filip, M.; Vlassa, M.; Coman, V.; Halmagyi, A. Simultaneous determination of glucose, fructose, sucrose and sorbitol in the leaf and fruit peel of different apple cultivars by the HPLC-RI optimized method. Food Chem. 2016, 199, 653–659.
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in “Honeycrisp” apple flesh. Food Chem. 2010, 123, 1013–1018.
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of Physiological Characteristics, Soluble Sugars, Organic Acids and Volatile Compounds in “Orin” Apples (Malus domestica) at Different Ripening Stages. Molecules 2021, 26, 807.
- Liu, Y.; Ying, Y.; Yu, H.; Fu, X. Comparison of the HPLC method and FT-NIR analysis for quantification of glucose, fructose, and sucrose in intact apple fruits. J. Agric. Food Chem. 2006, 54, 2810–2815.
- Liu, Y.-d.; Ying, Y. Bin Measurement of sugar content in Fuji apples by FT-NIR spectroscopy. J. Zhejiang Univ. Sci. 2004, 5, 651–655.
- Arain, S.; Sherazi, S.T.H.; Bhanger, M.I.; Memon, N.; Mahesar, S.A.; Rajput, M.T. Prospects of fatty acid profile and bioactive composition from lipid seeds for the discrimination of apple varieties with the application of chemometrics. Grasas y Aceites 2012, 63, 175–183.
- Baeza-Jiménez, R.; López-Martínez, L.X.; García-Varela, R.; García, H.S. Lipids in fruits and vegetables: Chemistry and biological activities. Fruit Veg. Phytochem. Chem. Hum. Health Second Ed. 2017, 1, 423–449.
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121.
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93.
- Chiu, H.H.; Kuo, C.H. Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples. J. Food Drug Anal. 2020, 28, 60–73.
- Duroňová, K.; Márová, I.; Čertík, M.; Obruča, S. Changes in lipid composition of apple surface layer during long-term storage in controlled atmosphere. Chem. Pap. 2012, 66, 940–948.
- Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty acid composition, physicochemical properties, antioxidant and cytotoxic activity of apple seed oil obtained from apple pomace. J. Sci. Food Agric. 2014, 94, 929–934.
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of wild apple (Malus spp.) by-products as a source of essential fatty acids, tocopherols and phytosterols with antimicrobial activity. Plants 2018, 7, 90.
- Seppanen-Laakso, T.; Laakso, I.; Hiltunen, R. Analysis of fatty acids by gas chromatography, and its relevance to research on health and nutrition. Anal. Chim. Acta 2002, 465, 39–62.
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants 2018, 7, 20.
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530.
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353.
- Alonso-Salces, R.M.; Barranco, A.; Abad, B.; Berrueta, L.A.; Gallo, B.; Vicente, F. Polyphenolic Profiles of Basque Cider Apple Cultivars and Their Technological Properties. J. Agric. Food Chem. 2004, 52, 2938–2952.
- Vanzani, P.; Rossetto, M.; Rigo, A.; Vrhovsek, U.; Mattivi, F.; D’Amato, E.; Scarpa, A.M. Major phytochemicals in apple cultivars: Contribution to peroxyl radical trapping efficiency. J. Agric. Food Chem. 2005, 53, 3377–3382.
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181.
- Ampomah-Dwamena, C.; Deinoprat, S.; Lewis, D.; Sutherland, P.; Volz, R.; Allan, A.C. Metabolic and gene expression analysis of apple (Malus × domestica) carotenogenesis. J. Exp. Bot. 2012, 63, 4497–4511.
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, 1064–1070.
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of predominant organic acid components in malus species: Correlation with apple domestication. Metabolites 2018, 8, 74.
- Nieuwenhuizen, N.J.; Green, S.A.; Chen, X.; Bailleul, E.J.D.; Matich, A.J.; Wang, M.Y.; Atkinson, R.G. Functional Genomics Reveals That a Compact Terpene Synthase Gene Family Can Account for Terpene Volatile Production in Apple. Plant Physiol. 2020, 161, 787–804.
- Ferreira, L.; Perestrelo, R.; Caldeira, M.; Câmara, J.S. Characterization of volatile substances in apples from Rosaceae family by headspace solid-phase microextraction followed by GC-qMS. J. Sep. Sci. 2009, 32, 1875–1888.
- Mehinagic, E.; Prost, C.; Demaimay, M. Optimization of extraction of apple aroma by dynamic headspace and influence of saliva on extraction of volatiles. J. Agric. Food Chem. 2004, 52, 5175–5182.
- Sut, S.; Poloniato, G.; Malagoli, M.; Dall’acqua, S. Fragmentation of the main triterpene acids of apple by LC- APCI-MSn. J. Mass Spectrom. 2018, 53, 882–892.
- Mehinagic, E.; Prost, C.; Demaimay, M. Representativeness of Apple Aroma Extract Obtained by Vacuum Hydrodistillation: Comparison of Two Concentration Techniques. J. Food Sci. 2003, 68, 2411–2415.
- Rezaei, S.; Rezaei, K.; Haghighi, M.; Labbafi, M. Solvent and Solvent to Sample Ratio as Main Parameters in the Microwave-assisted Extraction of Polyphenolic Compounds from Apple Pomace. Food Sci. Biotechnol. 2013, 22, 1269–1274.
- Rowan, D.D. Volatile metabolites. Metabolites 2011, 1, 41–63.
- Müller, E.; Berger, R.; Blass, E.; Sluyts, D.; Pfennig, A. Liquid-liquid Extraction. Encicl. Ind. Chem. 2000, 21, 250–303.
- Naboulsi, I.; Aboulmouhajir, A. Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review. Acad. J. Med. Plants 2018, 6, 223–240.
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758.
- Eskilsson, C.S.; Bjorklund, E. Analytcal-scale microwave-assisted extrcation. J. Chromatogr. A 2000, 902, 227–250.
- Bekele, E.A.; Annaratone, C.E.P.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Geeraerd, A.H. Multi-response optimization of the extraction and derivatization protocol of selected polar metabolites from apple fruit tissue for GC-MS analysis. Anal. Chim. Acta 2014, 824, 42–56.
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.F. Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853.
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.F. Reversed-Phase HPLC following Thiolysis for Quantitative Estimation and Characterization of the Four Main Classes of Phenolic Compounds in Different Tissue Zones of a French Cider Apple Variety (Malus domestica Var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705.
- Alonso-Salces, R.M.; Korta, E.; Barranco, A.; Berrueta, L.A.; Gallo, B.; Vicente, F. Determination of polyphenolic profiles of Basque cider apple varieties using accelerated solvent extraction. J. Agric. Food Chem. 2001, 49, 3761–3767.
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261.
- Stefova, M.; Petkovska, A.; Ugarkovic, S.; Stanoeva, J.P. Strategy for optimized use of LC-MSn for determination of the polyphenolic profiles of apple peel, flesh and leaves. Arab. J. Chem. 2019, 12, 5180–5186.
- Pingret, D.; Fabiano-Tixier, A.S.; Le Bourvellec, C.; Renard, C.M.G.C.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81.
- Bizjak, J.; Weber, N.; Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Alam, Z.; Stich, K.; Halbwirth, H.; Veberic, R. Influence of phostrade Ca on color development and anthocyanin content of “Braeburn” apple (Malus domestica Borkh.). HortScience 2013, 48, 193–199.
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Effects of location within the tree canopy on carbohydrates, organic acids, amino acids and phenolic compounds in the fruit peel and flesh from three apple (Malus 3 domestica) cultivars. Hortic. Res. 2014, 1, 1–7.
- Rodríguez-Bernaldo de Quirós, A.; Costa, H.S. Analysis of carotenoids in vegetable and plasma samples: A review. J. Food Compos. Anal. 2006, 19, 97–111.
- Fan, J.; Liao, D.; Zhang, X. Ultrasonic assisted extraction of ursolic acid from apple pomace: A novel and facile technique. Sep. Sci. Technol. 2016, 6395, 1344–1350.
- Prosen, H.; Zupančič-Kralj, L. Solid-phase microextraction. TrAC Trends Anal. Chem. 1999, 18, 272–282.
- Bianco, G.; Novario, G.; Zianni, R.; Cataldi, T.R.I. Comparison of two SPME fibers for the extraction of some off-flavor cork-taint compounds in bottled wines investigated by GC-HRMS. Anal. Bioanal. Chem. 2009, 393, 2019–2027.
- Schmutzer, G.R.; Magdas, A.D.; David, L.I.; Moldovan, Z. Determination of the Volatile Components of Apple Juice Using Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry. Anal. Lett. 2014, 47, 1683–1696.
- Risticevic, S.; Souza-Silva, E.A.; Gionfriddo, E.; DeEll, J.R.; Cochran, J.; Hopkins, W.S.; Pawliszyn, J. Application of in vivo solid phase microextraction (SPME) in capturing metabolome of apple (Malus ×domestica Borkh.) fruit. Sci. Rep. 2020, 10, 1–11.
- Sbse-gc-ms, S.; Rodr, R. Determination of Volatile Compounds in Apple Pomace by Stir Bar Sorptive Extraction and Gas Chromatography-Mass. J. Food Sci. 2011, 76, C1326–C1334.
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. TrAC Trends Anal. Chem. 2017, 88, 1–24.
- Pascale, R.; Acquavia, M.A.; Cataldi, T.R.I.; Onzo, A.; Coviello, D.; Bufo, S.A.; Scrano, L.; Ciriello, R.; Guerrieri, A.; Bianco, G. Profiling of quercetin glycosides and acyl glycosides in sun-dried peperoni di Senise peppers (Capsicum annuum L.) by a combination of LC-ESI (—)-MS/MS and polarity prediction in reversed-phase separations. Anal. Bioanal. Chem. 2020, 412, 3005–3015.
- Pascale, R.; Bianco, G.; Cataldi, T.R.I.; Buchicchio, A.; Losito, I.; Altieri, G.; Genovese, F.; Tauriello, A.; Di Renzo, G.C.; Lafiosca, M.C. Investigation of the Effects of Virgin Olive Oil Cleaning Systems on the Secoiridoid Aglycone Content Using High Performance Liquid Chromatography–Mass Spectrometry. J. Am. Oil Chem. Soc. 2018, 95, 665–671.
- Pascale, R.; Bianco, G.; Cataldi, T.R.I.; Kopplin, P.S.; Bosco, F.; Vignola, L.; Uhl, J.; Lucio, M.; Milella, L. Mass spectrometry-based phytochemical screening for hypoglycemic activity of Fagioli di Sarconi beans (Phaseolus vulgaris L.). Food Chem. 2018, 242, 497–504.
- Bianco, G.; Pascale, R.; Carbone, C.F.; Acquavia, M.A.; Cataldi, T.R.I.; Schmitt-Kopplin, P.; Buchicchio, A.; Russo, D.; Milella, L. Determination of soyasaponins in Fagioli di Sarconi beans (Phaseolus vulgaris L.) by LC-ESI-FTICR-MS and evaluation of their hypoglycemic activity. Anal. Bioanal. Chem. 2018, 410, 1561–1569.
- Bianco, G.; Pascale, R.; Lelario, F.; Bufo, S.A.; Cataldi, T.R.I. Investigation of Glucosinolates by Mass Spectrometry. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 431–461. ISBN 978-3-319-25462-3.
- Ventura, G.; Calvano, C.D.; Losito, I.; Bianco, G.; Pascale, R.; Palmisano, F.; Cataldi, T.R.I. Effect of pH and mobile phase additives on the chromatographic behaviour of an amide-embedded stationary phase: Cyanocobalamin and its diaminemonochloro-platinum(II) conjugate as a case study. J. Sep. Sci. 2019, 42, 1155–1162.
- Pascale, R.; Bianco, G.; Coviello, D.; Cristina Lafiosca, M.; Masi, S.; Mancini, I.M.; Bufo, S.A.; Scrano, L.; Caniani, D. Validation of a liquid chromatography coupled with tandem mass spectrometry method for the determination of drugs in wastewater using a three-phase solvent system. J. Sep. Sci. 2020, 43, 886–895.
- Natale, A.; Nardiello, D.; Palermo, C.; Muscarella, M.; Quinto, M.; Centonze, D. Development of an analytical method for the determination of polyphenolic compounds in vegetable origin samples by liquid chromatography and pulsed amperometric detection at a glassy carbon electrode. J. Chromatogr. A 2015, 1420, 66–73.
- Dordevic, B.; Durovic, D.; Zec, G.; Radovic, A.; Vulic, T. Bio-chemical properties and susceptibility to fire blight (Erwinia amylovora Burrill) of scab-resistant apple cultivars (Malus domestica Borkh.). Folia Hortic. 2019, 31, 253–261.
- Petkovska, A.; Gjamovski, V.; Stanoeva, J.P.; Stefova, M. Characterization of the polyphenolic profiles of peel, flesh and leaves of Malus domestica cultivars using UHPLC-DAD-HESI-MSn. Nat. Prod. Commun. 2017, 12, 35–42.
- Marks, S.C.; Mullen, W.; Crozier, A. Flavonoid and chlorogenic acid profiles of English cider apples. J. Sci. Food Agric. 2007, 87, 719–728.
- Jakobek, L.; García-Villalba, R.; Tomás-Barberán, F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Compos. Anal. 2013, 31, 199–211.
- De Paepe, D.; Servaes, K.; Noten, B.; Diels, L.; De Loose, M.; Van Droogenbroeck, B.; Voorspoels, S. An improved mass spectrometric method for identification and quantification of phenolic compounds in apple fruits. Food Chem. 2013, 136, 368–375.
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic characterization and antioxidant activity of malus domestica and prunus domestica cultivars from Costa Rica. Foods 2018, 7, 15.
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746.
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Viskelis, J.; Pukalskas, A.; Janulis, V. Phenolic Profiles and Contribution of Individual Compounds to Antioxidant Activity of Apple Powders. J. Food Sci. 2016, 81, C1055–C1061.
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of pectinase treatment on extraction of antioxidant phenols from pomace, for the production of puree-enriched cloudy apple juices. Food Chem. 2011, 127, 623–631.
- López-fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A review. Antioxidants 2020, 9, 479.
- Serni, E.; Venir, E.; Romano, G.; Guerra, W.; Robatscher, P. Determination of Major Phenolics Content in Dried Apples from Three New Cultivars (Malus domestica Borkh.) Using HPLC-UV-FL with Pentafluorophenyl Stationary Phase. Food Anal. Methods 2020, 13, 863–871.
- Wojdyło, A.; Oszmiański, J. Antioxidant activity modulated by polyphenol contents in apple and leaves during fruit development and ripening. Antioxidants 2020, 9, 567.
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447.
- Hollands, W.J.; Voorspoels, S.; Jacobs, G.; Aaby, K.; Meisland, A.; Garcia-Villalba, R.; Tomas-Barberan, F.; Piskula, M.K.; Mawson, D.; Vovk, I.; et al. Development, validation and evaluation of an analytical method for the determination of monomeric and oligomeric procyanidins in apple extracts. J. Chromatogr. A 2017, 1495, 46–56.
- Bernal, J.; Ares, A.M.; Pól, J.; Wiedmer, S.K. Hydrophilic interaction liquid chromatography in food analysis. J. Chromatogr. A 2011, 1218, 7438–7452.
- Veberic, R.; Schmitzer, V.; Petkovsek, M.M.; Stampar, F. Impact of Shelf Life on Content of Primary and Secondary Metabolites in Apple (Malus domestica Borkh.). J. Food Sci. 2010, 75, 461–468.
- Blake, J.D.; Clarke, M.L.; Richards, G.N. Determination of organic acids in sugar cane process juice by high-performance liquid chromatography: Improved resolution using dual aminex HPX-87H cation-exchange columns equilibrated to different temperatures. J. Chromatogr. A 1987, 398, 265–277.
- Arnous, A.; Meyer, A.S. Comparison of methods for compositional characterization of grape (Vitis vinifera L.) and apple (Malus domestica) skins. Food Bioprod. Process. 2008, 86, 79–86.
- Liaudanskas, M.; Viškelis, P.; Jakštas, V.; Raudonis, R.; Kviklys, D.; Milašius, A.; Janulis, V. Application of an optimized HPLC method for the detection of various phenolic compounds in apples from Lithuanian cultivars. J. Chem. 2014, 2014.
- Sowa, A.; Zgórka, G.; Szykuba, A.; Franiczek, R.; Gbikowska, B.; Gamian, A.; Sroka, Z. Analysis of polyphenolic compounds in extracts from leaves of some malus domestica cultivars: Antiradical and antimicrobial analysis of these extracts. BioMed Res. Int. 2016, 2016.
- Le Bourvellec, C.; Bouzerzour, K.; Ginies, C.; Regis, S.; Plé, Y.; Renard, C.M.G.C. Phenolic and polysaccharidic composition of applesauce is close to that of apple flesh. J. Food Compos. Anal. 2011, 24, 537–547.
- Le Bourvellec, C.; Bureau, S.; Renard, C.M.G.C.; Plenet, D.; Gautier, H.; Touloumet, L.; Girard, T.; Simon, S. Cultivar and year rather than agricultural practices affect primary and secondary metabolites in apple fruit. PLoS ONE 2015, 10, e0141916.
- Zhang, L.; Xu, Q.; You, Y.; Chen, W.; Xiao, Z.; Li, P.; Ma, F. Characterization of quercetin and its glycoside derivatives in Malus germplasm. Hortic. Environ. Biotechnol. 2018, 59, 909–917.
- Mato, I.; Suárez-Luque, S.; Huidobro, J.F. A review of the analytical methods to determine organic acids in grape juices and wines. Food Res. Int. 2005, 38, 1175–1188.
- Pascale, R.; Bianco, G.; Calace, S.; Masi, S.; Mancini, I.M.; Mazzone, G.; Caniani, D. Method development and optimization for the determination of benzene, toluene, ethylbenzene and xylenes in water at trace levels by static headspace extraction coupled to gas chromatography–barrier ionization discharge detection. J. Chromatogr. A 2018, 1548, 10–18.
- Onzo, A.; Acquavia, M.A.; Cataldi, T.R.I.; Ligonzo, M.; Coviello, D.; Pascale, R.; Martelli, G.; Bondoni, M.; Scrano, L.; Bianco, G. Coceth sulfate characterization by electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, 1–10.
- Lelario, F.; De Maria, S.; Rivelli, A.R.; Russo, D.; Milella, L.; Bufo, S.A.; Scrano, L. LC-FTICR-MS and IRMPD in a Commercial Variety and and their Anticholinesterase and Antioxidant Activities. Toxins 2019, 11, 230.
- Lelario, F.; Labella, C.; Napolitano, G.; Scrano, L.; Bufo, S.A. Fragmentation study of major spirosolane-type glycoalkaloids by collision-induced dissociation linear ion trap and infrared multiphoton dissociation Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 2395–2406.
- Šavikin, K.; Živković, J.; Zdunić, G.; Godevac, D.; Dordević, B.; Dojčinović, B.; Dordević, N. Phenolic and mineral profiles of four Balkan indigenous apple cultivars monitored at two different maturity stages. J. Food Compos. Anal. 2014, 35, 101–111.
- Verdu, C.F.; Gatto, J.; Freuze, I.; Richomme, P.; Laurens, F.; Guilet, D. Comparison of two methods, UHPLC-UV and UHPLC-MS/MS, for the quantification of polyphenols in cider apple juices. Molecules 2013, 18, 10213–10227.
- Kolniak-Ostek, J.; Oszmiański, J.; Wojdyło, A. Effect of l-ascorbic acid addition on quality, polyphenolic compounds and antioxidant capacity of cloudy apple juices. Eur. Food Res. Technol. 2013, 236, 777–798.
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/quantification of free and bound phenolic acids in peel and pulp of apples (Malus domestica) using high resolution mass spectrometry (HRMS). Food Chem. 2017, 215, 301–310.
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. 2012, 135, 1991–1998.
- Malec, M.; Le Quéré, J.M.; Sotin, H.; Kolodziejczyk, K.; Bauduin, R.; Guyot, S. Polyphenol profiling of a red-fleshed apple cultivar and evaluation of the color extractability and stability in the juice. J. Agric. Food Chem. 2014, 62, 6944–6954.
- Giomaro, G.; Karioti, A.; Bilia, A.R.; Bucchini, A.; Giamperi, L.; Ricci, D.; Fraternale, D. Polyphenols profile and antioxidant activity of skin and pulp of a rare apple from Marche region (Italy). Chem. Cent. J. 2014, 8, 1–10.
- Alonso-Salces, R.M.; Ndjoko, K.; Queiroz, E.F.; Ioset, J.R.; Hostettmann, K.; Berrueta, L.A.; Gallo, B.; Vicente, F. On-line characterisation of apple polyphenols by liquid chromatography coupled with mass spectrometry and ultraviolet absorbance detection. J. Chromatogr. A 2004, 1046, 89–100.
- Denis, M.C.; Furtos, A.; Dudonné, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple Peel Polyphenols and Their Beneficial Actions on Oxidative Stress and Inflammation. PLoS ONE 2013, 8, e53725.
- Todea, D.; Cadar, O.; Simedru, D.; Roman, C.; Tanaselia, C.; Suatean, I.; Naghiu, A. Determination of major-to-trace minerals and polyphenols in different apple cultivars. Not. Bot. Horti Agrobot. Cluj Napoca 2014, 42, 523–529.
- Bizjak, J.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Changes in primary metabolites and polyphenols in the peel of “braeburn” Apples (Malus domestica Borkh.) during advanced maturation. J. Agric. Food Chem. 2013, 61, 10283–10292.
- De Bernonville, T.D.; Gaucher, M.; Guyot, S.; Durel, C.E.; Dat, J.F.; Brisset, M.N. The constitutive phenolic composition of two Malus×domestica genotypes is not responsible for their contrasted susceptibilities to fire blight. Environ. Exp. Bot. 2011, 74, 65–73.




