3. Secondary Metabolites
To date, the structures and the average content of primary metabolites occurring in apples, are well known, for their nutritional value
[26][42]. Therefore, in recent years, research has been most focused on the extraction of secondary metabolites to be used in pharmacological studies for their beneficial properties
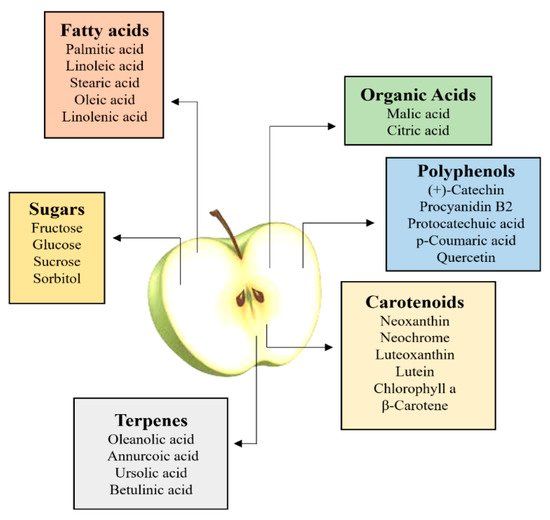
[32][21]. Food and pharmaceutical studies have addressed their attention on fruits and vegetables’ bioactive components considered healthy for the treatment and prevention of human diseases. Among the different classes of apples, secondary metabolites, polyphenols, carotenoids, organic acids and terpenes are the main phytochemicals
[6].
Although several classes of bioactive compounds occurring in apples, their well-known antioxidant properties are mainly attributed to phenolic compounds. These compounds exhibit several of double bonds and hydroxyl groups in their structures, which are responsible of their antioxidant activity
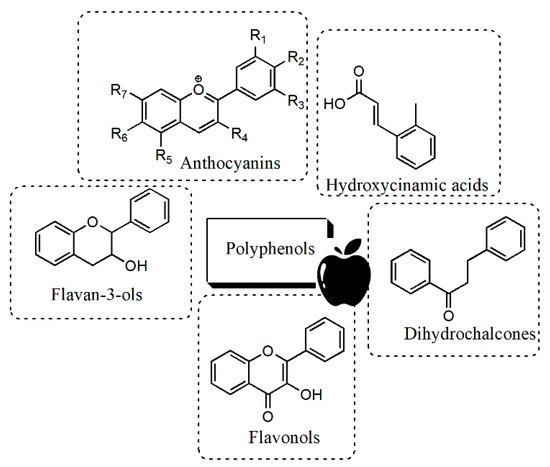
[33][48]. There are five major groups of polyphenolic compounds found in apples: hydroxycinnamic acids (primarily chlorogenic acid), flavan-3-ols, i.e., (+)-catechin, (−)-epicatechin and anthocyanidins, flavonols (mainly different quercetin glycosides), dihydrochalcones (such as phloridzin) and anthocyanins
[34][35][36][49,50,51] (
Figure 2). A high percentage (60%) of the total phenolic concentration in apple peel is represented by the monomeric and polymeric flavan-3-ols, while flavonols), hydroxycinnamic acids, dihydrochalcones and anthocyanins account, respectively, for the 18%, 9%, 8% and 5% of the total phenol content
[37][52].
Figure 2. Main classes of polyphenols occurring in apples.
Carotenoid pigments in the skin of apples contribute to fruit coloration, and therefore to their attractiveness, but in the flesh, their concentrations are low. Indeed, fruits of commercial apple cultivars show relatively low concentrations of carotenoids (<2.5 µg/g of fresh weight), in comparison with non-commercial apples, such as the rootstock cultivar “Aotea”, that show relatively high carotenoid concentrations
[38][53].
Alongside with sugars, aromatic volatile compounds and organic acids are responsible for the taste and flavor of apples (
Figure 3). In addition, organic acids are the main soluble constituents that influence the shelf life of fresh fruits and ripeness; consequently, they can be used as an index of consumer acceptability
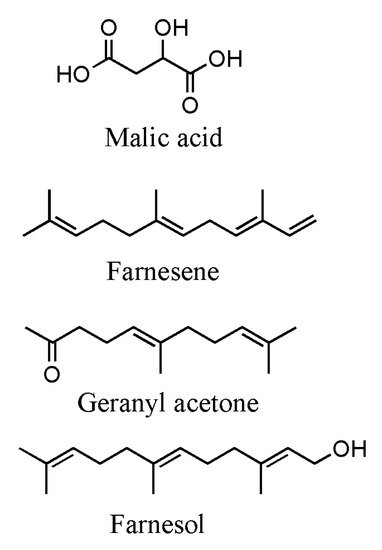
[39][54]. In cultivated apple, malic acid is the predominantly detectable organic acid, while malic acid and citric acid are the predominant organic acids in wild apple species
[40][55]. In regard to apple aroma profiles, many volatile organic compounds (VOCs) contribute to the overall sensory quality. These compounds include carboxylic esters, alcohols, aldehydes and ketones. Various terpenes have also been identified; however, they only contribute a relatively minor component of total VOCs produced
[41][56]. Some of the terpene occurring in apples are α-farnesene, geranyl acetone and farnesol
[42][43][57,58]. α-farnesene, which is an acyclic branched sesquiterpene, is highly occurring in ripe fruits; moreover, others monoterpenes, cyclic sesquiterpenes and terpene derivatives have also been identified in floral and vegetative tissues. Many of these compounds are constitutively produced in relatively low amounts also as response to insect infestation and they could directly affect apple pest behavior
[41][56]. More polar and less volatile terpenes, i.e., triterpenoid compounds, have been also identified in apples, namely pomaceic, annurcoic, euscaphic, pomolic, corosolic, maslinic, oleanolic, betulinic and ursolic acid
[44][59].
Figure 3. Main compounds responsible for the taste and flavor of apples.
3.1. Extraction
Phytochemical compounds from apples were extracted by using various extraction methods based on the application of different solvents and by heating and/or mixing. The most used techniques were Soxhlet extraction, maceration and hydro-distillation
[45][46][47][60,61,62]. Conventional Soxhlet extraction still remains one of the most relevant approach to extract volatile compounds from apples
[47][62]. The sample is placed in a thimble-holder, where it’s slowly filled with condensed solvent from a distillation flask. Once the liquid has reached an overflow level, the whole contents of the thimble-holder is aspirated by a siphon, which unloads it back into the distillation flask, leaving the extracted analytes in the bulk liquid
[29][45]. On the contrary, during maceration, the sample is immersed for a variable time in a solvent inside an airtight container in order to allow the analyte transferring
[48][63]. Vacuum hydro-distillation, instead, uses water vapor to recover volatile and apolar components from fruit tissues, and, as Soxhlet, it has been mainly used for the extraction of apple aroma, because it gave the extracts closest to the fresh fruit
[45][60]. The duration of the extraction process, and the large number of organic solvents used are the major drawbacks of these techniques. In fact, alternative approaches have emerged in an attempt to mitigate limitations of the conventional ones. The innovation is largely focused on finding technological solutions to diminish or even prevent the use of organic solvents in extraction processes, in order to obtain more highly purified products containing fewer additional toxins
[49][64]. The new methods include solid–liquid extractions (namely microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE), accelerated solvent extraction (ASE), supercritical fluid extraction (SFE)) and solid-phase microextraction (SPME). Organic solvents, such as hexane, acetone, methanol, ethanol or water, have been generally used under atmospheric pressure. The choice of the solvent largely depended on the polarity of the analytes.
Extraction of polyphenols from apples has been carried out by using methods that differ for many variables, such as solvent, time, temperature and number of extractions; therefore, the data reported in the literature are not always easily comparable. According to Bai et al.
[50][65], the extraction of polyphenols from apples can easily be achieved via MAE, by using ethanol as extracting solvent, in a ratio to raw material of 22.9/1. Microwave-assisted extraction is based on the use of microwave energy to heat solvents in contact with a sample to allow the analytes partitioning from the sample matrix into the solvent
[51][66]. Compared to Soxhlet and maceration, MAE results in a greater efficiency at a shorter time for the extraction of phenolic compounds from apple pomace. Moreover, accelerated operation can be regarded as a major advantage of MAE, which is useful especially at the industrial scale
[46][61]. Bekele et al.
[52][24] used a methanol extraction by adding pre-chilled MeOH (−20 °C) to apple pulp and extracting at 70 °C in a homogenizer. The extract was then centrifuged and dried under nitrogen flow. This extraction method was not selective for polyphenols; in fact, it allowed the extraction of many hydrophilic metabolites. Furthermore, this protocol of extraction was found to be not very efficient for complete extraction of all apple polyphenols, because less polar molecules could remain inside the matrix, which is discarded. In order to avoid this problem, a multi-step extraction can be used
[53][54][67,68]. A first step involves the treatment of the dried apples cortex with hexane, to remove lipids, carotenoids and chlorophyll. Then, an extraction with methanol dissolves sugars, organic acids and phenolic compounds of low molecular weight. Lastly, the resulting residue is treated with aqueous acetone (4:6), in order to extract polymerized polyphenols. Sanoner et al.
[53][67] applied this protocol by blending the apple powder with each solvent for 5 min, using an Ultra-Turrax blender, and the mixture was filtered through a G3 sintered glass filter. Moreover, UAE, ASE and SFE play an important role as real potential sustainable technique for industrial applications for polyphenols extraction. In recent works, ASE conditions have been optimized for the extraction of polyphenols from apple peel and pulp, achieving good recovery and repeatability. ASE allows to reduce the volume of solvent required for the extraction, to shorten the analysis time and the handling necessary to obtain precise results
[55][69]. As regard to SFE, several works aimed to recover phenolic compounds applying CO
2 in supercritical conditions without and with ethanol (5%) as co-solvent. As CO
2 is non-polar, it is not a favorable solvent for polar polyphenols. However, the addition of organic co-solvents which could improve the solvating power and the yield of the extraction, such as ethanol, methanol and acetone, could be a suitable strategy
[56][70]. Instead, Stefova et al.
[57][71] used UAE with a methanol: water mixture for the extraction of polyphenols. Ultrasound can reduce the operating temperature of extraction for thermolabile compounds; moreover, the cavitation process that occurs during sonication causes the rupture of cell walls, thus enhancing solvent contact with available extractable cell material
[58][72]. UAE has also been used for the extraction of organic acids; water or alcoholic mixtures were used as extraction solvents
[26][40][59][60][42,55,73,74].
In regard to carotenoids and vitamins, their extraction from apple skin and pomace is usually carried out with acetone or a solution of acetone/petroleum ether as solvent
[3][38][3,53]. Since carotenoids are strongly susceptible to oxidative degradation, due to the high number of conjugated double bonds, extraction must be performed in dark conditions or by adding antioxidant compounds, such as butylated hydroxytoluene (BHT), to the extraction solvent, in order to prevent photo-isomerization phenomena
[61][75].
Unlike apple polar triterpenoids, such as betulinic and ursolic acid, whose extraction is carried out by using polar solvents (i.e., methanol and acetone)
[62][76], a separate discussion is needed for the extraction of terpenes from apple tissues. As they are non-polar and highly volatile compounds, the better method for their extraction is the solid-phase microextraction (SPME), followed by their analysis by gas chromatographic (GC) methods. With SPME, the analytes are absorbed from the liquid or gaseous sample onto an absorbent coated fused silica fiber, which is part of the syringe needle, for a fixed time. The fiber is then inserted directly into a GC injection port for thermal desorption
[63][77]. Other advantages of this technique are the absence of solvents and the possibility to separate and pre-concentrate the analytes in a single step
[64][78]. Therefore, it is really helpful for the extraction of volatile compounds from apples, due to their low concentration and the complexity of the matrix
[65][79]. As fiber coatings, DVB/CAR/PDMS (Divinylbenzene/Carboxen/Polydimethylsiloxane) and PDMS/DVB have been mainly used because they offer higher extraction efficiency and a clear pattern of volatile compounds
[42][66][57,80]. Despite its several advantages, the limited amount of stationary phase on the fused silica fiber used for the SPME often does not ensure enough sensitivity and reproducibility. To overcome this problem, Madrera et al.
[67][81] successfully applied stir bar sorption extraction (SBSE), a variation of SPME consisting on the use of magnetized bars covered with an absorbent polymer, for the extraction of apple pomace aroma. SBSE has the same advantages than SPME, but its sensibility increases around 100-fold because it uses a greater amount of stationary phase.
3.2. Chromatographic Methods
The determination of secondary metabolites in plant-based foods remains an analytical challenge, due to their low concentration and the complexity and diversity of their structures. The structural complexity of secondary metabolites often hinders most attempts to quantify these compounds by analytical methods not including preliminary separation steps
[12][68][12,91]. In effect, to conduct a reliable detection and quantification of the main phytochemicals occurring in fruits, chromatographic methods (both LC and GC) either coupled with UV–Vis, fluorescence, or mass spectrometry (MS) detection, represent the gold standards methods
[69][70][71][72][73]. The following paragraphs deepen on the chromatographic methods that have been used for the determination of the main apple secondary metabolites[92,
i.e.93,
phenolics94,
organic acids95,
carotenoids and terpenes96].
The most common method for the separation and analysis of polyphenols and organic acids occurring in apple fruits is the high-performance liquid chromatography (HPLC) due to its high-resolution, efficiency, reproducibility and relatively short analysis time, without derivatization and no restriction on sample volatility [74][75][76][77][78][79]. Recent advances in apple phytochemicals analysis show a tendency for the application of environmentally friendly and faster techniques. This is evidenced, for example by the recently developed separative techniques, such as Ultrahigh-Performance Liquid Chromatography (UHPLC) and Ultra-Fast Liquid Chromatography that came from the evolution of packing materials used to improve resolution, and also contributed to such advances. Recently, UHPLC has been proposed for the analysis of polyphenols [80][81][82][83][84][85]. In general, peak efficiency and chromatographic resolution provided in UHPLC are higher than conventional HPLC. In addition, UHPLC methods can be considered more cost-effective because they typically need 80% less organic solvents than conventional HPLC methods [68]. Both HPLC and UHPLC can be easily coupled to a variety of detectors for polyphenols detection, including UV–Vis and MS.The most common method for the separation and analysis of polyphenols and organic acids occurring in apple fruits is the high-performance liquid chromatography (HPLC) due to its high-resolution, efficiency, reproducibility and relatively short analysis time, without derivatization and no restriction on sample volatility [97,98,99,100,101,102]. Recent advances in apple phytochemicals analysis show a tendency for the application of environmentally friendly and faster techniques. This is evidenced, for example by the recently developed separative techniques, such as Ultrahigh-Performance Liquid Chromatography (UHPLC) and Ultra-Fast Liquid Chromatography that came from the evolution of packing materials used to improve resolution, and also contributed to such advances. Recently, UHPLC has been proposed for the analysis of polyphenols [103,104,105,106,107,108]. In general, peak efficiency and chromatographic resolution provided in UHPLC are higher than conventional HPLC. In addition, UHPLC methods can be considered more cost-effective because they typically need 80% less organic solvents than conventional HPLC methods [91]. Both HPLC and UHPLC can be easily coupled to a variety of detectors for polyphenols detection, including UV–Vis and MS.
To date, no single chromatographic methods capable of separating the different types of phenolic compounds, occurring in apples, are available. It is necessary to optimize the stationary phase, mobile phase, gradient elution, temperature and flow rate for each group of compounds
[86][109]. Moreover, polyphenols stereochemistry, molecular weight, polarity and degree of polymerization could influence compounds retention. However, the reported methods for the separation of phenolics, as well as their glycosides, have been carried out mainly by reverse phase liquid chromatography (RPLC), on silica-based C18-bonded phase columns
[57][83][87][88][71,106,110,111]. The average particle diameter of HPLC packings is typically 3–10 μm. With columns of smaller particle size, a larger number of plates per unit time is provided, with respect to columns with larger particle size
[89][112]. As mobile phase, binary mixtures of aqueous formic acid or acetic acid and acetonitrile (ACN) or methanol (MeOH) as organic modifiers have been employed
[57][83][87][88][71,106,110,111]. Typically, gradient elutions have been preferred, since multiple-step gradients are more suitable for complex mixtures, such as apple extracts. Although RPLC has been mostly chosen for apple polyphenols separation, Hollands et al.
[90][113] used hydrophilic interaction chromatography (HILIC) to develop a robust and reliable analytical method for the extraction, separation and identification of monomeric and oligomeric procyanidins in apple extracts. HILIC separation mechanism is opposite to that of RP systems: polar stationary phase retains polar analytes, which are eluted by mixture of organic solvent (usually acetonitrile) and water
[91][114]. Due to the complexity of procyanidins oligomers structures in the apple extract, normal phase silica columns were found to be not suitable for quantification purposes, particularly at a higher degree of polymerization. Instead, HILIC column ensured a better resolution of the chromatographic peaks
[90][113].
As for phenolic compounds, reverse phase liquid chromatography is used also for the detection of organic acids by C18-bonded phase column. However, a method based on the use of an Aminex HPX cation-exchange column and an elution solvent consisting of sulfuric acid in bi-distilled water has been also reported for the quantification of malic acid
[92][115]. The Aminex HPX-series of strong cation-exchange resins are prepared from a sulfonated polystyrene-divinylbenzene copolymer and are available in prepacked columns. One of the major problems experienced with the use of these columns are related to the co-elution of non-acid components and the poor resolution of the chromatographic peaks, thus limiting their use. Moreover, the ion exchange’s separation mechanism implies that organic acids should be in their ionic form, so that a severe control of pH is required
[93][116].
As regard detection, the most commonly used detector for HPLC is a variable-wavelength UV or UV–Vis detector, because both phenolics and organic acids absorb very well in the UV region
[32][94][19][88][95][96][97][98][99][21,28,35,111,117,118,119,120,121]. The use of low UV detection wavelength, which ranged between 185 nm and 254 nm, allows to achieve high sensitivities in the determination of organic acids
[100][122]. Moreover, for phenol compounds, HPLC–DAD provides extensive information; however, no single wavelength is ideal for monitoring all classes of phenolics, because they display absorbency maxima at different wavelengths (s belonging to flavanols, phenolics acids, dihydrochalcones and flavonols. Among them, quercetin glycosides were found to be the main polyphenols in the peel (203 ± 108 mg/100 g) and phenolic acids (10 ± 5 mg/100 g) in the flesh. Instead, by Liaudanskas et al.
[95] [117] flavonols were quantitated at 360 nm. The method implemented by these authors allowed the identification of 11 analytes: procyanidin B1, (+)-catechin, chlorogenic acid, procyanidin B2, (−)-epicatechin, rutin, hyperoside, isoquercitrin, avicularin, quercitrin and phloridzin. The elution order of quercetin-3-
O-glycosides was as follows: quercetin-3-
O-rutinoside (rutin), quercetin-3-
O-galactoside (hyperoside), quercetin-3-
O-glucoside (iso-quercitrin), quercetin-3-
O-arabinoside (avicularin) and quercetin-3-
O-rhamnoside (quercitrin), mainly according to other studies aimed at the evaluation of the quercetin derivatives profile in food extracts
[69][92].
HPLC–UV for the quantification of polyphenols on
Malus extracts allowed to reach limits of detection (LOD) and limits of quantification (LOQ) in the range 0.2–5.8 µg/mL and 0.1–7.1 µg/mL, respectively. Fluoresce detector (FL) in some cases offers higher selectivity and sensitivity compared to UV–Vis detection methods, so that it could be offered as a robust and reliable alternative or to be complementary to UV–Vis detection systems. For example, Teleszko et al.
[83] [106] determined the polyphenolic profile in leaves and fruits of 2 cultivars of
Malus domestica by UPLC–PDA–FL. In this case, the identification was also achieved, by using LC–MS/MS. Although LC–UV–Vis or LC–UV–FL are cheap and robust techniques for the quantification of polyphenols, their identification could be uniquely achieved through the comparison of retention times and UV–Vis spectra with those of authentic standards. Moreover, phenolic compounds are often linked to saccharidic moieties that are not UV-active, thus preventing the correct polyphenols identification.
Considering these difficulties, in many cases, it is necessary to use a more sensitive and selective detector such as a mass spectrometer to an LC system (LC–MS), as it allows unequivocal identification of the analytes thanks to the possibility to conduct MS/MS experiments
[72][86][101][102][103][104][95,109,123,124,125,126]. Liquid chromatography coupled to mass spectrometry (LC–MS) or tandem mass spectrometry (LC–MS/MS) are among the most widely used techniques for the analysis of polyphenols occurring in apple fruits
[105][106][107][108][109][127,128,129,130,131]. The employment of these methods is particularly helpful not only for their quantitative determination but also for their characterization and structural elucidation, especially when MS
n fragmentation can be achieved
[68][91]. For the ionization of apple polyphenols in LC–MS, electrospray ionization (ESI) in negative mode has been, by far, the most generalized interface employed
[106][108][110][128,130,132]. The negative ionization mode provides the highest sensitivity and results in limited fragmentation of flavonoids. Instead, for the identification of anthocyanins, positive ionization mode is mainly chosen, as it gives the best results
[111][133]. As an extra-certainty to the molecular mass determination, the combination of both ionization modes (positive and negative) in MS
n scan could be implemented
[13]. Other less common techniques used in the analysis of polyphenols areatmospheric pressure ionization techniques, such as atmospheric pressure chemical ionization (APCI). For instance, LC–APCI–MS in positive ionization mode was proposed for the characterization of apple polyphenols by Alonso-Salces et al.
[112][134], who reported for the first time five isorhamnetin glycosides, two hydroxyphloretin glycosides and quercetin in apple peel.
As mass analyzers, multiple types are available and have been proposed for phenols detection, among them triple quadrupole (QqQ)
[113][135], linear ion-trap
[80][103], time-of-flight
[80][103], Orbitrap
[82][105] and QTrap
[114][136], among others. QTrap mass analyzers are hybrid instruments combining a quadrupole and a liner ion-trap in a similar configuration to a QqQ instrument and they gaining popularity for the analysis of food products. LC–MS methods offer a better selectivity compared to LC–UV methods. In this regard, Verdu et al.
[106][128] developed an UHPLC–UV and UHPLC–MS/MS for the quantification of phenolic compounds in apple juices. The developed methods were validated for 15 major compounds based on linearity, limits of detection and quantification, recovery and precision tests. A comparison of the quantifications showed that both UHPLC–UV and UHPLC–MS/MS had an excellent correlation for major compounds, quantified in 120 different samples. However, the slope value showed an overestimation of the UV detector for chlorogenic acid, explained by the co-elution of unknown UV-absorbing minor compounds, highlighting the advantage of using MS as detector and the selected reaction monitoring (SRM) mode to quantify highly concentrated samples. LC coupled to high-resolution mass spectrometry (HRMS), which provides accurate mass measurements, has recently obtained popularity due to its ability to give more comprehensive information concerning the exact molecular mass, elemental composition and detailed molecular structure of a given compound. LC–HRMS provides data of exceptional quality regarding apple metabolites. Indeed, it is currently used to aid in the identification of a broader range of phenolic compounds. High-resolution MS/MS has several advantages; indeed, they greatly improve the sensitivity and the accuracy of the mass measurements, thus allowing a simplified identification of the analytes and a differentiation between molecular formulas having the same nominal masses
[108][130].
UV and MS are, often, both used for the identification and quantification of apple polyphenols
[115][116][137,138]. Bizjak et al.
[115][137] studied the changes of the concentrations of sugars, organic acids and a wide range of polyphenols as well as total phenolic compounds in the “Braeburn” apple peel during the advanced maturation of apples in two growing, by coupling HPLC to both detectors. A total of 21 phenolics, belonging to five groups, namely hydroxycinnamic acids, dihydrochalcones, flavonols, flavanols and anthocyanins, were identified and quantified. Identification was performed by comparing the retention times and their UV−Vis spectra from 200 to 600 nm and confirmed by MS and MS
2 data that were acquired in positive and negative ions mode by using full-scan-data-dependent MS scanning from
m/
z 115 to 2000. The results obtained could be useful to understand the evolution and highest concentration of primary and secondary metabolites in the last stages of apple ripening and their relation as well. Instead, Ramirez-Ambrosi et al.
[13] used ultrahigh performance liquid chromatography with diode array detection coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry (UHPLC–DAD–ESI–Q-ToF-MS), in order to obtain polyphenolic profile of apples, apple pomace and apple juice from Asturian cider apples, in a single run of 22 min. This method allowed the automatic and simultaneous acquisition of accurate mass to charge values, overcoming chromatographic co-elution problems. With this technique, a large number of phenolic acids, organic acids and flavonoids were identified
[13] [13].