
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Valentino Natoli | + 1982 word(s) | 1982 | 2021-07-05 05:09:51 | | | |
| 2 | Peter Tang | Meta information modification | 1982 | 2021-07-06 03:31:20 | | | | |
| 3 | Peter Tang | + 7 word(s) | 1989 | 2021-07-07 10:48:26 | | |
Video Upload Options
The tongue is able to quickly reflect the state of health or disease of the human body. Tongue inspection is an important diagnostic approach. It is a unique method that allows to explore the pathogenesis of diseases based on the guiding principles of the holistic concept that involves the observation of changes in the lining of the tongue in order to understand the physiological functions and pathological changes of the body. It is a potential method of screening and early detection of cancer.
1. Introduction
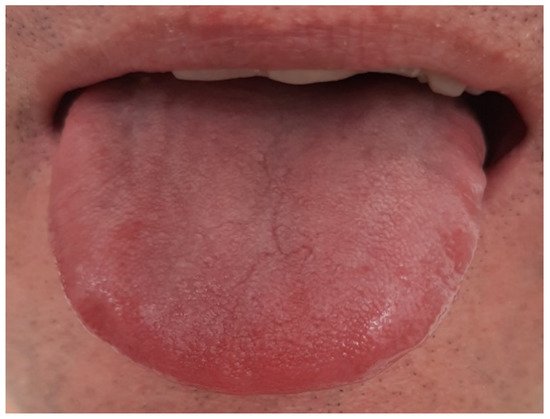
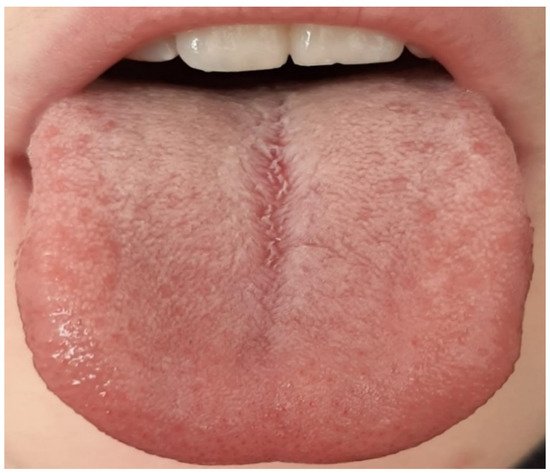
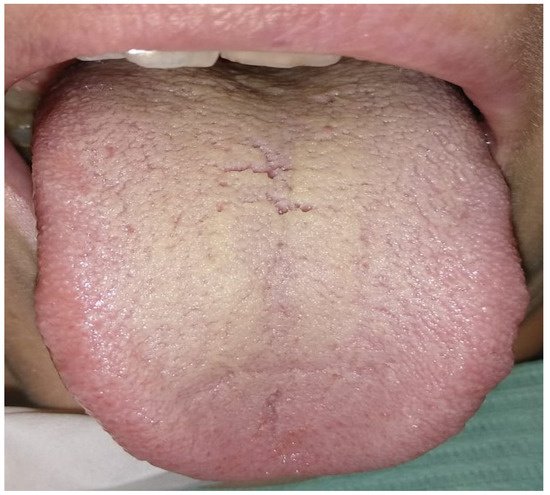
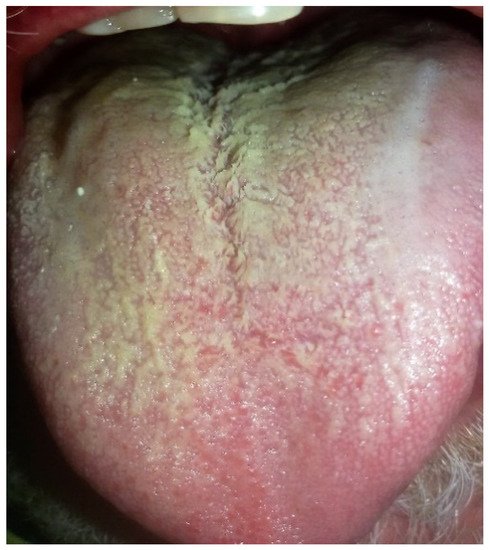
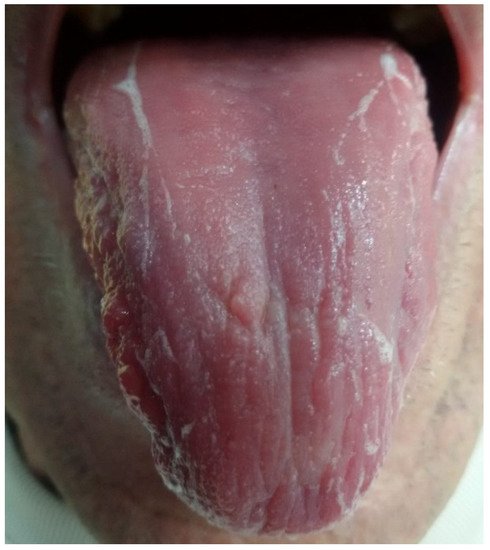
|
Description |
|---|
|
Thin white, thick white, and fat white tongue coating |
|
Tongue coating thin yellow, thick yellow, and fat yellow |
|
Thin black and gray tongue coating and thick gray black |
|
Value |
Description |
|---|---|
|
0 |
No visible patina on the back of the tongue |
|
1 |
Patina present only on the posterior III of the tongue |
|
2 |
Patina that completely covers the dorsal surface of the tongue but does not mask the underlying mucosa |
|
3 |
Very thick patina covering the entire dorsal surface of the tongue |
2. Current Insights on Tongue and Systemic Connections Microbiota
3. Conclusions
References
- Sun, B.; Zhou, D.; Tu, J.; Lu, Z. Evaluation of the Bacterial Diversity in the Human Tongue Coating Based on Genus-Specific Primers for 16S rRNA Sequencing. BioMed Res. Int. 2017, 2017, 1–12.
- Anastasi, J.K.; Currie, L.M.; Kim, G.H. Understanding diagnostic reasoning in TCM practice: Tongue diagnosis. Altern. Ther. Heal. Med. 2009, 15, 18–28.
- Ye, J.; Cai, X.; Yang, J.; Sun, X.; Hu, C.; Xia, J.; Shen, J.; Su, K.; Yan, H.; Xu, Y.; et al. Bacillus as a potential diagnostic marker for yellow tongue coating. Sci. Rep. 2016, 6, 32496.
- Asakawa, M.; Takeshita, T.; Furuta, M.; Kageyama, S.; Takeuchi, K.; Hata, J.; Ninomiya, T.; Yamashita, Y. Tongue Microbiota and Oral Health Status in Community-Dwelling Elderly Adults. mSphere 2018, 3, e00332-18.
- Welch, J.L.M.; Utter, D.; Rossetti, B.J.; Welch, D.B.M.; Eren, A.M.; Borisy, G.G. Dynamics of tongue microbial communities with single-nucleotide resolution using oligotyping. Front. Microbiol. 2014, 5, 568.
- Cui, J.; Cui, H.; Yang, M.; Du, S.; Li, J.; Li, Y.; Liu, L.; Zhang, X.; Li, S. Tongue coating microbiome as a potential biomarker for gastritis including precancerous cascade. Protein Cell 2019, 10, 496–509.
- Kageyama, S.; Asakawa, M.; Takeshita, T.; Ihara, Y.; Kanno, S.; Hara, T.; Takahashi, I.; Yamashita, Y. Transition of Bacterial Diversity and Composition in Tongue Microbiota during the First Two Years of Life. mSphere 2019, 4, e00187-19.
- Jiang, B.; Liang, X.; Chen, Y.; Ma, T.; Liu, L.; Li, J.; Jiang, R.; Chen, T.; Zhang, X.; Li, S. Integrating next-generation sequencing and traditional tongue diagnosis to determine tongue coating microbiome. Sci. Rep. 2012, 2, srep00936.
- Hsu, C.-H.; Yu, M.-C.; Lee, C.-H.; Lee, T.-C.; Yang, S.-Y. High Eosinophil Cationic Protein Level in Asthmatic Patients with “Heat” Zheng. Am. J. Chin. Med. 2003, 31, 277–283.
- Zhao, Y.; Mao, Y.-F.; Tang, Y.-S.; Ni, M.-Z.; Liu, Q.-H.; Wang, Y.; Feng, Q.; Peng, J.-H.; Hu, Y.-Y. Altered oral microbiota in chronic hepatitis B patients with different tongue coatings. World J. Gastroenterol. 2018, 24, 3448–3461.
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2011, 61, 582–588.
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association ofFusobacteriumspecies in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220.
- Ballini, A.; Di Palma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131.
- Ramírez, J.H.; Parra, B.; Gutiérrez, S.; Arce, R.M.; Jaramillo, A.; Ariza, Y.; Contreras, A. Biomarkers of cardiovascular disease are increased in untreated chronic periodontitis: A case control study. Aust. Dent. J. 2014, 59, 29–36.
- Fava, F.; Danese, S. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J. Gastroenterol. 2011, 17, 557–566.
- Ahn, J.; Chen, C.Y.; Hayes, R.B. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012, 23, 399–404.
- Besnard, P.; Christensen, J.E.; Brignot, H.; Bernard, A.; Passilly-Degrace, P.; Nicklaus, S.; De Barros, J.-P.P.; Collet, X.; Lelouvier, B.; Servant, F.; et al. Author Correction: Obese Subjects With Specific Gustatory Papillae Microbiota and Salivary Cues Display an Impairment to Sense Lipids. Sci. Rep. 2018, 8, 9773.
- Chen, L.Y.; Lu, G.Z. Analysis of Tongue Images in 114 Patients with Gastric Cancer. Zhong Yi Za Zhi 2011, 52, 1935–1938.
- Miyazaki, H.; Sakao, S.; Katoh, Y.; Takehara, T. Correlation Between Volatile Sulphur Compounds and Certain Oral Health Measurements in the General Population. J. Periodontol. 1995, 66, 679–684.
- Xu, J.; Xiang, C.; Zhang, C.; Xu, B.; Wu, J.; Wang, R.; Yang, Y.; Shi, L.; Zhang, J.; Zhan, Z. Microbial biomarkers of common tongue coatings in patients with gastric cancer. Microb. Pathog. 2019, 127, 97–105.
- Huang, S.; Yang, F.; Zeng, X.; Chen, J.; Li, R.; Wen, T.; Li, C.; Wei, W.; Liu, J.; Chen, L.; et al. Preliminary characterization of the oral microbiota of Chinese adults with and without gingivitis. BMC Oral Health 2011, 11, 33.
- Kistler, J.O.; Booth, V.; Bradshaw, D.J.; Wade, W.G. Bacterial Community Development in Experimental Gingivitis. PLoS ONE 2013, 8, e71227.
- He, Y.; Gong, D.; Shi, C.; Shao, F.; Shi, J.; Fei, J. Dysbiosis of oral buccal mucosa microbiota in patients with oral lichen planus. Oral Dis. 2017, 23, 674–682.
- Cao, Y.; Qiao, M.; Tian, Z.; Yu, Y.; Xu, B.; Lao, W.; Ma, X.; Li, W. Comparative Analyses of Subgingival Microbiome in Chronic Periodontitis Patients with and Without IgA Nephropathy by High Throughput 16S rRNA Sequencing. Cell. Physiol. Biochem. 2018, 47, 774–783.
- Healy, C.; Moran, G.P. The microbiome and oral cancer: More questions than answers. Oral Oncol. 2019, 89, 30–33.
- Mager, D.L.; Haffajee, A.D.; Devlin, P.M.; Norris, C.M.; Posner, M.R.; Goodson, J.M. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J. Transl. Med. 2005, 3, 27.
- Allali, I.; Boukhatem, N.; Bouguenouch, L.; Hardi, H.; Boudouaya, H.A.; Cadenas, M.B.; Ouldim, K.; Amzazi, S.; Azcarate-Peril, M.A.; Ghazal, H. Gut microbiome of Moroccan colorectal cancer patients. Med Microbiol. Immunol. 2018, 207, 211–225.
- Downes, J.; Sutcliffe, I.C.; Booth, V.; Wade, W.G. Prevotella maculosa sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microbiol. 2007, 57, 2936–2939.
- Tsigarida, A.; Dabdoub, S.; Nagaraja, H.; Kumar, P. The Influence of Smoking on the Peri-Implant Microbiome. J. Dent. Res. 2015, 94, 1202–1217.
- Ehrenfest, D.M.D.; Del Corso, M.; Inchingolo, F.; Charrier, J.-B. Selecting a relevant in vitro cell model for testing and comparing the effects of a Choukroun’s platelet-rich fibrin (PRF) membrane and a platelet-rich plasma (PRP) gel: Tricks and traps. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 409–411.
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J. Clin. Microbiol. 2008, 46, 1407–1417.
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process: Ecological perspectives. J. Dent. Res. 2010, 90, 294–303.
- Goodson, J.; Groppo, D.; Halem, S.; Carpino, E. Is Obesity an Oral Bacterial Disease? J. Dent. Res. 2009, 88, 519–523.
- Takeshita, T.; Kageyama, S.; Furuta, M.; Tsuboi, H.; Takeuchi, K.; Shibata, Y.; Shimazaki, Y.; Akifusa, S.; Ninomiya, T.; Kiyohara, Y.; et al. Bacterial diversity in saliva and oral health-related conditions: The Hisayama Study. Sci. Rep. 2016, 6, 22164.
- Carlsson, J.; Grahnén, H.; Jonsson, G.; Wikner, S. Early Establishment of Streptococcus salivarius in the Mouths of Infants. J. Dent. Res. 1970, 49, 415–418.
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42.
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326.
- Mason, M.R.; Chambers, S.; Dabdoub, S.; Thikkurissy, S.; Kumar, P.S. Characterizing oral microbial communities across dentition states and colonization niches. Microbiome 2018, 6, 1–10.
- Seerangaiyan, K.; Jüch, F.; Winkel, E.G. Tongue coating: Its characteristics and role in intra-oral halitosis and general health—A review. J. Breath Res. 2017, 12, 034001.
- Loesche, W.J. Microbiology and treatment of halitosis. Curr. Infect. Dis. Rep. 2003, 5, 220–226.
- NISC Comparative Sequencing Program; Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64.
- Ballini, A.; Gnoni, A.; De Vito, D.; DiPalma, G.; Cantore, S.; Isacco, C.G.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of probiotics on the occurrence of nutrition absorption capacities in healthy children: A randomized double-blinded placebo-controlled pilot study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657.
- Inchingolo, F.; Martelli, F.S.; Isacco, C.G.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115.
- Cantore, S.; Ballini, A.; De Vito, D.; Abbinante, A.; Altini, V.; DiPalma, G.; Inchingolo, F.; Saini, R. Clinical results of improvement in periodontal condition by administration of oral probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1329–1334.
- Inchingolo, F.; Santacroce, L.; Ballini, A.; Topi, S.; DiPalma, G.; Haxhirexha, K.; Bottalico, L.; Charitos, I.A. Oral Cancer: A Historical Review. Int. J. Environ. Res. Public Health 2020, 17, 3168.
- Topi, S.; Santacroce, L.; Bottalico, L.; Ballini, A.; Inchingolo, A.D.; Dipalma, G.; Charitos, I.A.; Inchingolo, F. Gastric Cancer in History: A Perspective Interdisciplinary Study. Cancers 2020, 12, 264.
- Boccellino, M.; Di Stasio, D.; Di Palma, G.; Cantore, S.; Ambrosio, P.; Coppola, M.; Quagliuolo, L.; Scarano, A.; Malcangi, G.; Borsani, E.; et al. Steroids and growth factors in oral squamous cell carcinoma: Useful source of dental-derived stem cells to develop a steroidogenic model in new clinical strategies. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8730–8740.
- Yang, I.; Nell, S.; Suerbaum, S. Survival in hostile territory: The microbiota of the stomach. FEMS Microbiol. Rev. 2013, 37, 736–761.
- Gibbons, R.J.; Houte, J.V. Bacterial Adherence in Oral Microbial Ecology. Annu. Rev. Microbiol. 1975, 29, 19–42.
- Cabre, M.; Serra-Prat, M.; Palomera, E.; Almirall, J.; Pallares, R.; Clavé, P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing 2010, 39, 39–45.
- Abe, S.; Ishihara, K.; Adachi, M.; Okuda, K. Tongue-coating as risk indicator for aspiration pneumonia in edentate elderly. Arch. Gerontol. Geriatr. 2008, 47, 267–275.
- Shimazaki, Y.; Tomioka, M.; Saito, T.; Nabeshima, F.; Ikematsu, H.; Koyano, K.; Yamashita, Y. Influence of oral health on febrile status in long-term hospitalized elderly patients. Arch. Gerontol. Geriatr. 2009, 48, 411–414.
- Marik, P.E.; Kaplan, D. Aspiration Pneumonia and Dysphagia in the Elderly. Chest 2003, 124, 328–336.
- Sjögren, P.; Nilsson, E.; Forsell, M.; Johansson, O.; Hoogstraate, J.; Sjögren, P. A Systematic Review of the Preventive Effect of Oral Hygiene on Pneumonia and Respiratory Tract Infection in Elderly People in Hospitals and Nursing Homes: Effect Estimates and Methodological Quality of Randomized Controlled Trials: Oral hygiene and pneumonia in elderly. J. Am. Geriatr. Soc. 2008, 56, 2124–2130.
- Zhou, Y.; Gao, H.; Mihindukulasuriya, A.K.; La Rosa, P.S.; Wylie, K.M.; Vishnivetskaya, T.; Podar, M.; Warner, B.; Tarr, I.P.; Nelson, E.D.; et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013, 14, R1.
- Humphrey, L.L.; Fu, R.; Buckley, D.I.; Freeman, M.; Helfand, M. Periodontal Disease and Coronary Heart Disease Incidence: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2008, 23, 2079–2086.
- Ide, R.; Hoshuyama, T.; Wilson, D.; Takahashi, K.; Higashi, T. Periodontal Disease and Incident Diabetes: A Seven-Year Study. J. Dent. Res. 2011, 90, 41–46.
- Yoneda, M.; Naka, S.; Nakano, K.; Wada, K.; Endo, H.; Mawatari, H.; Imajo, K.; Nomura, R.; Hokamura, K.; Ono, M.; et al. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012, 12, 16.
- Iwauchi, M.; Horigome, A.; Ishikawa, K.; Mikuni, A.; Nakano, M.; Xiao, J.; Odamaki, T.; Hironaka, S. Relationship between oral and gut microbiota in elderly people. Immun. Inflamm. Dis. 2019, 7, 229–236.
- Dawes, C. Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dent. Assoc. 2008, 139, 18S–24S.
- Isacco, C.G.; Ballini, A.; De Vito, D.; Nguyen, K.C.D.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L. Rebalance the Oral Microbiota as Efficacy Tool in Endocrine, Metabolic, and Immune Disorders. Endocr. Metab. Immune Dis. Drug Targets 2021, 21, 777–784.
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725.
- Ceccarelli, F.; Orrù, G.; Pilloni, A.; Bartosiewicz, I.; Perricone, C.; Martino, E.; Lucchetti, R.; Fais, S.; Vomero, M.; Olivieri, M.; et al. Porphyromonas gingivalisin the tongue biofilm is associated with clinical outcome in rheumatoid arthritis patients. Clin. Exp. Immunol. 2018, 194, 244–252.




