
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesco Ciani | + 682 word(s) | 682 | 2021-06-17 10:02:52 | | | |
| 2 | Enzi Gong | + 118 word(s) | 800 | 2021-06-29 10:40:39 | | | | |
| 3 | Francesco Ciani | Meta information modification | 800 | 2021-06-29 12:54:00 | | | | |
| 4 | Enzi Gong | + 26 word(s) | 826 | 2021-06-30 08:46:25 | | | | |
| 5 | Enzi Gong | + 3 word(s) | 829 | 2021-07-01 04:39:50 | | |
Video Upload Options
Particulate Bound Hg (PBM) consists of all airborne particulate containing Hg, including both stable condensed and gaseous forms adsorbed on atmospheric particulate matter (PM); it is operationally sampled and quantified by pulling air through a glass fiber or a quartz filter. PBM usually includes all those particles with a diameter <2.5 μm, even if its characterization depends on the pore size of the filter used for its collection. The accurate dimensional characterization is then essential to estimate the dry deposition of PBM, as well as any other particulate pollutant; the particles diameters directly influence gravitational sedimentation and the PBM residence time in the atmosphere. In addition, PBM chemical speciation, as well as for the other Hg forms, is fundamental to understand PBM bioavailability and therefore the effects on human .
1. Overview
Mercury (Hg) is ubiquitously distributed in the environment, naturally occurring in the Earth’s crust at average concentrations of about 0.05 mg kg−1[1]. Hg in the atmosphere is present both as gaseous forms, i.e., GEM and reactive gaseous Hg (RGM, Hg2+), and as particulate bound Hg (PBM). GEM represents the most abundant (>95%) form of Hg in the atmosphere, with high stability and long lifetime (from 0.5 to 2 years) thanks to chemical inertness[2]. Due to this long persistence, Hg is considered one of the major global environmental pollutants[3]. PBM consists of all airborne particulate containing Hg, including both stable condensed and gaseous forms adsorbed on atmospheric particulate matter (PM); it is operationally sampled and quantified by pulling air through a glass fiber or a quartz filter[4]. PBM usually includes all those particles with a diameter <2.5 μm, even if its characterization depends on the pore size of the filter used for its collection[5]. The accurate dimensional characterization is then essential to estimate the dry deposition of PBM, as well as any other particulate pollutant; the particles diameters directly influence gravitational sedimentation and the PBM residence time in the atmosphere[6]. In addition, PBM chemical speciation, as well as for the other Hg forms, is fundamental to understand PBM bioavailability and therefore the effects on human .
2. Analysis Method for PBM
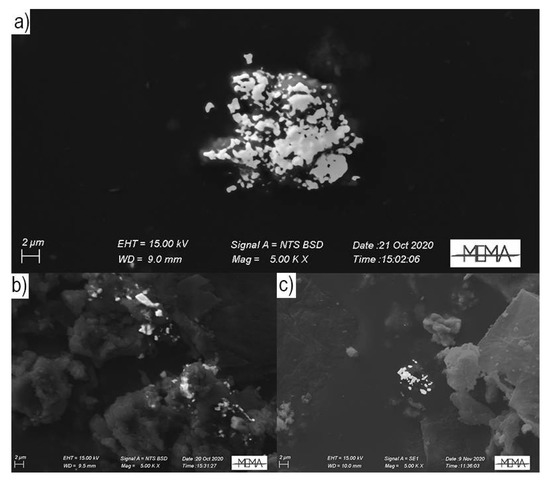
3. Conclusions
References
- Rudnick, R.L.; Gao, S.; Holland, H.D.; Turekian, K.K.. Composition of the continental crust. In The Crust.; Holland, H.D., Turekian, K.K, Eds.; University of Maryland: Maryland, MD, USA, 2003; pp. 1–64.
- William H. Schroeder; John Munthe; Atmospheric mercury—An overview. Atmospheric Environment 1998, 32, 809-822, 10.1016/s1352-2310(97)00293-8.
- Jung-Duck Park; Wei Zheng; Human Exposure and Health Effects of Inorganic and Elemental Mercury. Journal of Preventive Medicine and Public Health 2012, 45, 344-352, 10.3961/jpmph.2012.45.6.344.
- Mary M. Lynam; Gerald J. Keeler; Comparison of methods for particulate phase mercury analysis: sampling and analysis. Analytical and Bioanalytical Chemistry 2002, 374, 1009-1014, 10.1007/s00216-002-1584-4.
- Selin, E.S.. Atmospheric Chemistry, Modeling, and Biogeochemistry of Mercury. In Mercury in the Environment: Pattern and Process; Bank, M.S., Eds.; University of California Press: Berkeley, CA, USA, 2012; pp. 73-79.
- Pyung-Rae Kim; Young-Ji Han; Thomas M. Holsen; Seung-Muk Yi; Atmospheric particulate mercury: Concentrations and size distributions. Atmospheric Environment 2012, 61, 94-102, 10.1016/j.atmosenv.2012.07.014.
- Goldstein, J.; Newbury, D.E.; Joy, D.C.; Lyman, C.E.; Echlin, P.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-Ray Microanalysis; Springer:: New York, NY, USA, 2003; pp. 61-98.
- Francesco Ciani; Valentina Rimondi; Pilario Costagliola; Atmospheric mercury pollution: the current methodological framework outlined by environmental legislation. Air Quality, Atmosphere & Health 2021, 15, 1-13, 10.1007/s11869-021-01044-4.




