
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paulo Dani | + 1193 word(s) | 1193 | 2021-06-25 05:16:03 | | | |
| 2 | Enzi Gong | Meta information modification | 1193 | 2021-06-25 10:06:28 | | |
Video Upload Options
One of the most frequently and widely used pyrethroids is deltamethrin, often employed for the control of household insect pests. The presence of three chiral centers translates into eight possible different stereoisomers, with only one of them having insecticidal activity. Active deltamethrin (a-DLM) has an S configuration at the α−benzyl carbon and a 1-R-cis configuration at the cyclopropane ring.
1. Overview
Pyrethroids are among the insecticidal compounds indicated by the World Health Organization for mitigation of vector-borne diseases. Active deltamethrin (with chiral configuration α-S,1-R-cis) is one of the most effective pyrethroids characterized by low toxicity to humans, and it is currently tested as active ingredient for insecticidal paints. Nevertheless, several degradation processes can occur and affect the insecticidal efficacy in the complex paint matrix. In the present study, a detailed NMR analysis of deltamethrin stability has been carried out under stress conditions, mimicking a water-based insecticidal paint environment. Two novel by-products, having a diastereomeric relationship, were identified and their structure was elucidated by combining NMR, HPLC, GC-MS, and ESI-MS analyses. These compounds are the result from a nucleophilic addition involving deltamethrin and one of its major degradation products, 3-phenoxybenzaldehyde. Given the known toxicity of the aldehyde, this reaction could represent a way to reduce its concentration into the matrix. On the other hand, the toxicology of these compounds towards humans should be addressed, as their presence may adversely affect the performance of deltamethrin-containing products.
2. Pyrethroids
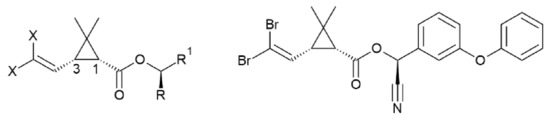
3. Conclusions
References
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. Morb. Mortal. Wkly. Rep. 2018, 67, 496.
- Sherrard-Smith, E.; Griffin, J.T.; Winskill, P.; Corbel, V.; Pennetier, C.; Djenontin, A.; Moore, S.; Richardson, J.H.; Muller, P.; Edi, C.; et al. Systematic review of indoor residual spray efficacy and effectiveness against Plasmodium falciparum in Africa. Nat. Commun. 2018, 9, 4982.
- Beier, J.C.; Keating, J.; Githure, J.I.; Macdonald, M.B.; Impoinvil, D.E.; Novak, R.J. Integrated vector management for malaria control. Malar. J. 2008, 7, S4.
- Messenger, L.A.; Rowland, M. Insecticide-treated durable wall lining (ITWL): Future prospects for control of malaria and other vector-borne diseases. Malar. J. 2017, 16, 213.
- Maloney, K.M.; Ancca-Juarez, J.; Salazar, R.; Borrini-Mayori, K.; Niemierko, M.; Yukich, J.O.; Naquira, C.; Keating, J.A.; Levy, M.Z. Comparison of insecticidal paint and deltamethrin against Triatoma infestans (Hemiptera: Reduviidae) feeding and mortality in simulated natural conditions. J. Vector Ecol. 2013, 38, 6–11.
- Hazra, D.K. Pesticidal Paints: An Integral Approach to Colour your Imagination. Biodivers. Int. J. 2018, 2, 95–96.
- Schiøler, K.L.; Alifrangis, M.; Kitron, U.; Konradsen, F. Insecticidal Paints: A Realistic Approach to Vector Control? PLOS Negl. Trop. Dis. 2016, 10, e0004518.
- Ho, J.; Mudraboyina, B.; Spence-Elder, C.; Resendes, R.; Cunningham, M.F.; Jessop, P.G. Water-borne coatings that share the mechanism of action of oil-based coatings. Green Chem. 2018, 20, 1899–1905.
- Del Amo, B.; Romagnoli, R.; Deyá, C.; González, J.A. High performance water-based paints with non-toxic anticorrosive pigments. Prog. Org. Coat. 2002, 45, 389–397.
- Kang, K.; Lu, J.; McLoughlin, D.; Olsen, J.H. Insecticidal Paints. U.S. Patents 2014/0288026 A1, 25 September 2014.
- U.S. President’s Malaria Initiative FY 2020 Guidance; USAID: Washington, DC, USA, 2019.
- Sibanda, M.M.; Focke, W.W.; Labuschagne, F.J.W.J.; Moyo, L.; Nhlapo, N.S.; Maity, A.; Muiambo, H.; Massinga, P.; Crowther, N.A.S.; Coetzee, M.; et al. Degradation of insecticides used for indoor spraying in malaria control and possible solutions. Malar. J. 2011, 10, 307.
- Amelotti, I.; Catalá, S.S.; Gorla, D.E. Experimental evaluation of insecticidal paints against Triatoma infestans (Hemiptera: Reduviidae), under natural climatic conditions. Parasites Vectors 2009, 2, 30.
- Elliott, M. Properties and applications of pyrethroids. Environ. Health Perspect. 1976, 14, 1–2.
- Berg, H. Pesticide use in rice and rice–fish farms in the Mekong Delta, Vietnam. Crop. Prot. 2001, 20, 897–905.
- Hirano, M. Characteristics of pyrethroids for insect pest control in agriculture. Pestic. Sci. 1989, 27, 353–360.
- Wouters, W.; van den Bercken, J. Action of pyrethroids. Gen. Pharmacol. Vasc. Syst. 1978, 9, 387–398.
- Pawa, A.S.; Mali, G.V.; Deshmukh, H.V. Biodegradation of Deltamethrin by using Indigenous Bacteria Isolated from Contaminated Soil. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 258–265.
- Cycoń, M.; Żmijowska, A.; Piotrowska-Seget, Z. Enhancement of deltamethrin degradation by soil bioaugmentation with two different strains of Serratia marcescens. Int. J. Environ. Sci. Technol. 2014, 11, 1305–1316.
- Rehman, H.; Aziz, A.T.; Saggu, S.; Abbas, Z.; Mohan, A.; Ansari, A. Systematic review on pyrethroid toxicity with special reference to deltamethrin. J. Entomol. Zool. Stud. 2014, 2, 60–70.
- Mestres, R.; Mestres, G. Deltamethrin: Uses and Environmental Safety. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 1992; pp. 1–18.
- Maguire, R.J.; Carey, J.H.; Hart, J.H.; Tkacz, R.J.; Lee, H.B. Persistence and fate of deltamethrin sprayed on a pond. J. Agric. Food Chem. 1989, 37, 1153–1159.
- Perschke, H.; Hussain, M. Chemical isomerization of deltamethrin in alcohols. J. Agric. Food Chem. 1992, 40, 686–690.
- Tariq, S.R.; Ahmed, D.; Farooq, A.; Rasheed, S.; Mansoor, M. Photodegradation of bifenthrin and deltamethrin-effect of copper amendment and solvent system. Env. Monit Assess. 2017, 189, 71.
- Nahri-Niknafs, B.; Ahmadi, A. Photodegradation of Deltamethrin and Fenvalerate under Simulated Solar Light Irradiation and Identification of Photoproducts. Rev. Chim. 2013, 64, 828–831.
- Elliott, M. The pyrethroids: Early discovery, recent advances and the future. Pestic. Sci. 1989, 27, 337–351.
- Kaneko, H. Pyrethroid Chemistry and Metabolism; Elsevier Academic Press Inc.: San Diego, CA, USA, 2010; pp. 1635–1663.
- Maguire, R.J. Aquatic Environmental Fate of Deltamethrin. Water Sci. Technol. 1992, 25, 99–102.
- Ruzo, L.O.; Casida, J.E. Degradation of decamethrin on cotton plants. J. Agric. Food Chem. 1979, 27, 572–575.
- Acharya, B.N.; Nivsarkar, M.; Saxena, C.; Kaushik, M.P. Effects of the process of the incorporation of deltamethrin on slow release property of insecticidal paint. Pigment. Resin Technol. 2004, 33, 21–25.
- Lee, H.L.; Ahmad, N.W.; Ghani, A.A. Paint Composition. U.S. Patent 6,881,248 B2, 19 April 2005.




