Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SHIH-CHIEH LEE | + 2494 word(s) | 2494 | 2021-06-23 04:59:55 | | | |
| 2 | Vivi Li | + 411 word(s) | 2905 | 2021-06-24 04:01:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lee, S. Cinnamomum osmophloeum and Oral Mucositis. Encyclopedia. Available online: https://encyclopedia.pub/entry/11194 (accessed on 07 February 2026).
Lee S. Cinnamomum osmophloeum and Oral Mucositis. Encyclopedia. Available at: https://encyclopedia.pub/entry/11194. Accessed February 07, 2026.
Lee, Shih-Chieh. "Cinnamomum osmophloeum and Oral Mucositis" Encyclopedia, https://encyclopedia.pub/entry/11194 (accessed February 07, 2026).
Lee, S. (2021, June 23). Cinnamomum osmophloeum and Oral Mucositis. In Encyclopedia. https://encyclopedia.pub/entry/11194
Lee, Shih-Chieh. "Cinnamomum osmophloeum and Oral Mucositis." Encyclopedia. Web. 23 June, 2021.
Copy Citation
Cinnamon plants (Cinnamomum spp.) are of the genus Lauraceae, native to South and Southeast Asia, and are generally used as food flavors and traditional medicinal plants. Cinnamomum osmophloeum, commonly known as indigenous cinnamon or pseudocinnamon, is endemic to Taiwan’s natural hardwood forests.
C. osmophloeum
biological activities
oral mucositis
cinnamaldehyde
linalool
1. Introduction
Cinnamon plants (Cinnamomum spp.) are of the genus Lauraceae, native to South and Southeast Asia, and are generally used as food flavors and traditional medicinal plants. Cinnamomum osmophloeum, commonly known as indigenous cinnamon or pseudocinnamon, is endemic to Taiwan’s natural hardwood forests [1]. Major components of the essential oils extracted from C. osmophloeum leaves explored by high-performance liquid chromatography (HPLC) are as follows: α-pinene, camphene, benzaldehyde, β-pinene, 3-pheayl pionaldehyde, cis-cinnamaldehyde, trans-cinnamaldehyde, isobornylacetate, eugenol, and cinnamil acetate [2]. The essential oils extracted from C. osmophloeum leaves comprise 101 volatile compounds, as identified by GC/MS analysis, including monoterpenoids, sesquiterpenoids, alcohols, phenols, aldehydes, ketones, esters, acids, and other miscellaneous compounds. It was demonstrated that the linalool chemotypes present in C. osmophloeum were as follows: linalool, trans-cinnamyl acetate, camphor, cinnamaldehyde, 3-phenyl-2-propenal, caryophyllene, coumarin, bornyl acetate, limonene, α-(+)-pinene, estragole, and caryophyllene oxide [3]. In several studies (both in vitro and in vivo), C. osmophloeum has been applied as an alternative natural therapy to treat certain compromised and uncompromised diseases [3][4][5][6][7].
Oral mucositis (OM) is known as the inflammation of oral mucosa, usually occurring as an adverse side-effect of chemotherapy and/or radiation therapy (radiotherapy), and is manifested as atrophy, swelling, erythema, and ulceration [8]. OM occurrence in the hospital might increase costs and deteriorate oral health quality of life [9][10][11]. Hence, oral care treatments, including nutritional care, pain control, oral cleansing, palliation of a dry mouth, bleeding handling, and medicinal interventions have been introduced to decrease the severity of OM after cancer therapy [12]. Patients receiving radiotherapy to head and neck areas are at a significant risk of developing oral mucositis. The risk is lower (less than 50% or little risk) in patients with prolonged chemotherapy, patients receiving surgery, and patients with radiotherapy to non-head and neck areas [10][13]. The underlying pathophysiology of OM is divided into five phases: (1) initiation, (2) primary damage response, (3) signaling and amplification, (4) ulceration (symptomatic phase), and (5) healing [14][15][16]. The first phase (initiation stage) happens after exposure to radiotherapy or chemotherapy. It consists of two events: DNA breakdown and the generation of reactive oxygen species (ROS). DNA strand breakdowns lead to direct injury and death of the cells, and reactive oxygen species play a role as key initiators and mediators of downstream biological events. During the second phase (primary damage response), activator transduction pathways are stimulated by the DNA breaks strand, which can lead to the activation of several transcription factors, including p53 and nuclear factor kappa-B (NF-κB). NF-κB works as a controller for the expression of a broad range of genes, and produces a series of mediators, including pro-inflammatory cytokines and both pro- and anti-apoptotic cellular changes. During phase III (signal amplification stage), pro-inflammatory cytokines deliver a positive reaction to enhance and accelerate the process of wound healing. During phase IV (ulceration phase, also called symptomatic phase), it is common for the mucosal surface to become re-infected with bacteria. Bacterial invasion stimulates macrophage accumulation to conceal additional amounts of pro-inflammatory cytokines. During phase V (healing stage), signals from the connective tissue to the bordering epithelium can activate the migration, propagation, and differentiation of cells, resulting in healed mucosa. A number of biomaterials explored for OM therapy perform their principal mechanisms linked to pathophysiology by depressing pro-inflammatory cytokines. In addition to anti-inflammation, antioxidant, antifungal, antibacterial, and immunomodulator mechanisms of action have been reported [17]. As one of the encouraging biomaterials, C. osmophloeum might be a potential alleviator of OM.
2. Medical-Biological Activities of C. osmophloeum
Table 1. Beneficial biological activities of C. osmophloeum.
| Bioactivity | Chemical Identification | C. osmophloeum Parts | Constituent (s) | Study | Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Anti-inflammatory effect | LC-MS/MS | Leaves | Kaempferitrin | In vitro | Down-regulate the extracellular LDL-R (chronic inflammation-related diabetes mellitus) | Ku et al., (2017) [18] |
| GC-MS | Twigs | Trans-cinnamaldehyde, caryophyllene oxide, L-borneol, L-bornyl acetate, eugenol, β-caryophyllene, E-nerolidol, and cinnamyl | In vitro | Suppressing nitric oxide synthesis by LPS-stimulated macrophages | Tung et al. (2008) [19] | |
| GC-MS | Leaves | Trans-cinnamaldehyde,(-)-aromadendrene, caryophyllene oxide, T-cadinol, and α-cadinol |
In vitro | Suppressing nitric oxide production by LPS-stimulated macrophages | Tung et al. (2010) [20] | |
| GC-MS and HPLC | Leaves | Cinnamaldehyde | In vitro | Cinnamaldehyde inhibits LPS-mediated pro-inflammatory cytokine production | Chao et al. (2008) [21] | |
| TLC | Leaves | NA | In vitro | Inhibition of the production of NO and cytokines (TNF-a and IL-12), from LPS/IFNc-activated macrophages | Fang, Rao & Tzeng (2005) [22] | |
| CC, HPLC, TLC, ESIMS, and GC-MS | Twigs | Kaempferol glycosides | In vitro | Nitric oxide production inhibitory activities | Lin & Chang(2012) [23] | |
| GC-MS | Leaves | Linalool and cinnamaldehyde | In vivo | Inhibition of the expression of molecules in both TLR4 and NLRP3 signaling pathways | Lee et al. (2018) [24] | |
| GC-MS | Leaves | 21 compounds were identified | In vitro | Inhibition of IL-1â and IL-6 production | Chao et al. (2005) [25] | |
| Antibacterial activity | GC | Leaves | Cinnamaldehyde | In vitro | Bactericidal | Chang, Chen & Chang (2001) [26] |
| GC-MS | Leaves | Cinnamaldehyde, cinnamic acid, cinnamyl alcohol, and cinnamyl acetate | In vitro | Bacterial inhibition | Chang et al. (2008) [27] | |
| Antifungal activity | GC-MS | Leaves | Cinnamaldehyde | In vitro | NA | Cheng et al. (2006) [28] |
| GC-MS | Leaves | Cinnamaldehyde | In vitro | NA | Wang, Chen & Chang (2005) [29] | |
| Antioxidant activities | ESIMS | Twigs | Kaempferol-7-O-rhamnoside | In vitro | NA | Chua, Tung, & Chang (2008) [30] |
| GC-MS and GC−FID |
Leaves | Alloaromadendrene | In vitro | NA | Yu et al. (2014) [31] | |
| GC-MS and GC-FID | Leaves | Trans-cinnamaldehyde | In vitro | NA | Yeh et al. (2013) [32] | |
| GC-MS and GC-FID | Leaves | Trans cinnamaldehyde | In vivo | Expression of antioxidative-related genes was pointedly affected by essential oils from C. osmophloeum. | Hsu et al. (2012) [33] | |
| Antidyslipidemic activity | HPLC | Leaves | Kaempferol and kaempferitrin | In vivo | Cholesterol-lowering activity | Lin et al. (2011) [34] |
| Anti-hyperglycemic and antioxidant activities | A modified vanillin-H2SO4 assay A modified acid-butanol assay The AlCl3 method |
Twigs | Proanthocyanidin and tannin contents | In vitro | CoTE has PTP1B inhibitory activity to improve insulin or leptin resistance | Lin et al. (2016) [35] |
| Hepatoprotective effects | NA | Leaves | trans-cinnamaldehyde, ()-aromadendrene, T-cadinol, or R-cadinol | In vivo | The modulation of anti-inflammatory activities (decreased the aspartate aminotransferase (AST), alanine aminotransferase (ALT), tumor necrosis factor-R (TNF-R), and interleukin 6 (IL-6) levels in serum) | Tung et al. (2011) [6] |
| Pancreas Protective Effect and Hypoglycemic activity | GC/MS | Leaves | Linalool | In vivo | 1. Decreased pancreatic values of thiobarbituric acid reactive substances and activities of superoxide dismutase and glutathione reductase 2. Decreased pancreatic levels of interleukin-1β and nitric oxide |
Lee et al. (2013) [3] |
| Prevent Cardiac Hypertrophy | HPLC | Leaves | Cinnamaldehyde | In vivo | The protective role of cinnamaldehyde related to the ERK1/2 signaling pathway. | Yang et al. (2015) [7] |
| Treatment of renal interstitial fibroblasts | NA | Leaves | Cinnamaldehyde | In vitro | Inhibit high glucose-induced hypertrophy (decreased cell size; cellular hypertrophy index; and protein levels of collagen IV, fibronectin, and α-smooth muscle actin). | Chao et al. (2010) [4] |
| Anticancer (liver and oral cancer) | TLC, CC and HPLC | Heart wood and roots | Lignan Esters | In vitro | Tumor cell growth inhibition | Chen et al. (2010) [36] |
| Anti-diabetes | TLC | Twigs | Kaempferol glycosides CO-1 and CO-2 | In vitro | Enhanced adiponectin secretion, and activation of the insulin signaling pathway | Lee et al. (2009) [37] |
| Anti-hyperuricemia effect | GC-MS | Leaves | Cinnamaldehyde | In vivo | Acts as a xanthine oxidase inhibitor and reduces the serum uric acid levels | Wang et al. (2008) [2] |
| Anxiolytic properties | HPLC | Leaves | Linalool | In vivo | Reduced the amount of 5-HT, DA and NE and increased the level of dopamine in striatum | Cheng et al. (2014) [38] |
| Wound Repair Promoter and Antioxidant | NA | Leaves | NA | In vitro and in vivo | Inhibited tyrosinase activity and reduced melanin content | Lee et al. (2015) [39] |
| Anti-inflammatory and anti-cancer properties | NA | Barks | NA | In vivo | The growth inhibition of NO, TNF-, and IL-12, and tumor cell proliferation | Rao et al. (2007) [40] |
| Hypolipidemic effects | NA | Leaves | S-(þ)-linalool | In vivo | Inhibited lipid accumulation through downregulation of 3T3-L1 adipocyte differentiation | Cheng et al. (2018) [41] |
| Effect on the human immune system | HS-GC/MS and HPLC | Leaves | Cinnamaldehyde | In vivo | Cytokines modulatory effect | Lin et al. (2011) [42] |
| Potential skin-whitening and protective agent | NA | Leaves | Cinnamaldehyde and cinnamylacetate | In vitro | Neutralized the IBMX-induced increase in melanin content in B16-F10 cells by inhibiting tyrosinase gene expression at the level of transcription | Lee et al. (2015) [39] |
| Anti-inflammatory effect in intestine | GC/MS | Leaves | Linalool | In vivo | The suppression of the TLR4 pathway by CO and partly by the inhibitory effect of CO on the activity of xanthine oxidase | Lee et al. (2015) [43] |
| Anti-tumor | NA | Leaves | Trans-cinnamaldehyde | In vitro | Trans-cinnamaldehyde triggers suicidal death oferythrocytes, i.e., cells devoid of mitochondria and gene expression. | Theurer et al. (2013) [44] |
| Dietary supplements and treatment of hyperuricemia and gout | GC-MS and GC-FID | Leaves | Cinnamaldehyde | In vitro | The xanthine oxidase inhibitory activity | Huang et al. (2018) [45] |
| Anti-hyperglycemic and antioxidant activities | (MALDI/MS) (RP-HPLC) /MS/MS | Twigs | Proanthocyanidin | In vitro | The proanthocyanidins in CoTE mainly consisted of (epi)catechin and contained at least one A-type linkage. The inhibitory activity of α-glucosidase and α-amylase | Lin et al. (2016) [46] |
NA: Not available; LC: liquid chromatography; MS: mass spectrometry; GC: gas chromatography; HPLC: high-performance liquid chromatography; TLC: thin layer chromatography; CC: column chromatography; ESIMS: electrospray ionization mass spectrometry; GC-FID: gas chromatography−flame ionization detection; MALDI: matrix-assisted laser desorption/ionization; RP: reverse phase.
3. Potential Use of C. osmophloeum for the Treatment of Oral Mucositis (OM)
The current protocols of medicine for chemotherapy are associated with oral mucositis. Cytarabine, high-dose 5-fluororacil, alkylating agents, and platinum-based compounds are highly associated with the incidence of oral mucositis. Actinomycin D, amsacrin, bleomycin, busulfan, capecitabine, carboplatin, chlorambucil, cisplatin, cytarabine, docetaxel, doxorubicin, etoposide, floxuridine, ifofsamide, irinotecan, leucovorin, methotrexate, mitoxantron, oxaliplatin, paclitaxel, plicamycin, tioguanin, vinblastine, vincristine, vindecine, and vinorelbine (the protocol can be combined) are the medicines used for chemotherapy and are reported to lead to the development of oral mucositis [47][48]. In order to prevent oral mucositis, the Multinational Association of Supportive Care in Cancer (MASCC) and International Society of Oral Oncology (ISOO) help clinics by comprising Clinical Practice Guidelines for Oral Mucositis [49]. The recommendations are comprised of basic oral care, growth factors and cytokines, anti-inflammatory agents, laser and other light therapy, cryotherapy, and natural and miscellaneous agents (Table 2). Köstler et al. provided several experimental approaches to treat oral mucositis; they included locally applied non-pharmacological methods, anti-inflammatory and mucosa protectant agents, cytokines, granulocyte colony-stimulating factor (G-CSF, filgrastim) and granulocyte-macrophage colony-stimulating factor (GM-CSF, molgramostim), antiseptic agents, corticosteroids, mouth-coating agents, and dexpanthenol [48].
Table 2. Interventions to prevent oral mucositis (clinical practice guidelines) proposed by the Multinational Association of Supportive Care in Cancer (MASCC) and International Society of Oral Oncology (ISOO).
| Intervention | Protocol | Population | Evidence of Effectiveness |
|---|---|---|---|
| Basic oral care | Tooth brushing, flossing, and one mouth rinse | All age groups and across all cancer treatment modalities | Not strong evidence |
| Growth factors and cytokines | Palifermin (keratinocyte growth factor-1) |
Patients receiving high-dose chemotherapy and total body irradiation, followed by autologous stem cell transplantation for hematological malignancies | Strong evidence |
| Anti-inflammatory agents | Benzydamine mouthwash | Patients with head and neck cancer receiving moderate-dose radiation therapy (up to 50 Grays), without concomitant chemotherapy | Strong evidence |
| Laser and other light therapy | Low-level laser therapy (LLLT) | Patients receiving high-dose chemotherapy for HSCT with or without total body irradiation |
Strong evidence |
| Cryotherapy | The placement of ice chips in the mouth |
Patients receiving bolus dosing of 5-fluorouracil |
Strong evidence |
| Natural and miscellaneous agents | Systemic zinc supplements administered orally (antioxidant effect) | Patients with oral cancer undergoing radiotherapy or chemoradiation | Not strong evidence |
The study of C. osmophloeum and/or its constituents in OM are limited. In one study, the effect of cinnamaldehyde on oral mucositis and an evaluation of the salivary total antioxidant capacity of gamma-irradiated rats were carried out. The saliva samples were taken from the rats in triplicate [50]. In order to evaluate the consequences and severity of mucositis, the conditions of the oral cavity were assessed by using Parkin’s clinical scale, where 0 represents normal mucosa, 0.5 indicates normally pink mucosa, 1 stands for minor red mucosa, 2 is severe red mucosa, 3 is local desquamation, 4 describes exudation and crust around less than half of the lip area, and 5 characterizes exudation and crust for more than half of the lip area [51]. The authors concluded that the clinical effects in the intervention group seemed to be due to the antioxidant, antibacterial, and anti-inflammatory effects of cinnamaldehyde. It is noteworthy to mention that through anti-inflammation and antioxidant mechanisms, cinnamaldehyde would delay the onset of oral mucositis. Moreover, alteration in the oral microflora of existing bacteria in the fourth phase (ulceration phase) could exacerbate the severity of mucositis, whereas cinnamaldehyde alleviated oral mucositis via its antibacterial properties [50]. A recent investigation reported the effect of cinnamon bark fractions (an essential oil and an aqueous extract) on Candida albicans growth inhibition (growth, biofilm formation, and adherence properties) and oral epithelial cells (barrier integrity and inflammatory response) [52]. The anti-adherence and anti-inflammatory properties of proanthocyanidins, a family of polyphenols containing flavan-3-ol oligomers and polymers, are used to treat oral infections [52][53]. The two pro-inflammatory cytokines, IL-6 and IL-8, which serve as important cytokines in the development of oral mucositis, were reduced by an aqueous extract enriched with proanthocyanidins of the cinnamon fraction. This shows that the cinnamon presented in the study may be a promising agent in the alleviation of oral mucositis [52].
Other biomaterials or herbal products, such as Aloe vera, Acacia catechu, Chamomile, Hangeshashinto, indigowood root (Isatis indigotica Fort.), honey, Traumeel S, water grass decoction, and Weleda Ratanhia, also showed similar effects on the alleviation of OM via anti-inflammation activities [17][54][55][56][57][58][59][60][61][62][63]. The bioactive properties of the yarrow plant (Achillea mileofolium), honey, Callendula officinalis flowers, Hipophae rhamnoides L. plant, Chamomile, and Aloe vera were associated with antioxidant, anti-inflammatory, antibacterial, and wound healing effects of oral mucositis therapy (Table 3) [55][56][57][63][64][65][66][67][68]. Similar to the advantages of the medical-biological activities of these herbal agents, C. osmophloeum would provide an identical mechanism for relieving oral mucositis.
Table 3. Bioactive properties of natural agents for oral mucositis therapy.
| Natural Agents | Bioactivity | References |
|---|---|---|
| Yarrow Plant (Achillea millefolium) | Anti-bacterial and anti-inflammatory effect | Mirazandeh et al. (2014) [64] |
| Manuka Honey (Leptospermum scoparium) | Wound healing and anti-microbial | Hawley et al. (2013) [63] |
| Weleda Pflanzen-Zahngel and Weleda Ratanhia-Mundwasser | Anti-inflammatory, anti-bacterial, and lesion healing |
Tiemann et al. (2007) [61] |
| Calendula officinalis flowers | Anti-inflammatory, anti-bacterial, and anti-oxidant | Babaee et al. (2013) [65] |
| Honey and coffee | Antioxidant, anti-microbial, and anti-inflammatory |
Raeessi et al. (2014) [54] |
| Aloe vera | Anti-inflammatory, bactericidal, and wound healing | Sahebjamee et al. (2015) [62] |
| Hangeshashinto: Pinelliae tuber, Scutellariae Radix, Glycyrrhizae Radix, Zizyphi Fructus, Ginseng Radix, Zingiberis Processum rhizoma, and Coptidis rhizome | Anti-inflammatory | Aoyama et al. (2014) [58] |
| Indigowood Root (Isatis indigotica Fort.) | Anti-inflammatory | You et al. (2009) [60] |
| Topical Honey | Anti-inflammatory, anti-microbial, and wound healing | Khanal et al. (2010) [66] |
| Hippophae rhamnoides L. plant | Anti-oxidant, anti-ulcerogenic, anti-inflammatory, anti-microbial, and proinflammatory cytokine Antagonist |
Kuduban et al. (2016) [67] |
| Honey from the clover plant Trifolium alexandrenum | Anti-microbial | Rashad et al. (2009) [69] |
| Qingre Liyan decoction | Anti-oxidant and anti-inflammatory | Lambros et al. (2014) [70] |
| Hangeshashinto | Anti-inflammatory and anti-microbial | Kono et al. (2014) [56] |
| Chamomile | Anti-inflammatory, anti-bacterial, and antifungal |
Fidler et al. (1996) [55] |
| Rhodila algida | Anti-oxidant and immunostimulant | Loo et al. (2010) [71] |
| Qingre Liyan Decoction | Enhancing body immunity and promoting salivary EGF | Wu et al. (2007) [72] |
| Chamomile | Anti-inflammatory, anti-bacterial, and anti-fungal |
Pourdeghatkar et al. (2017) [57] |
| Pure Honey | Anti-bacterial and anti-inflammatory | Motallebnejad et al. (2008) [68] |
| Aloe vera | Anti-inflammatory, anti-bacterial, and anti-fungal |
Puataweepong et al. (2009) [73] |
| Aloe vera and vitamin E | Antioxidant, anti-inflammatory, and healing properties | Cuba et al. (2015) [74] |
| Traumeel S | Anti-inflammatory | Sencer et al. (2012) [75] |
| Chamomilla recutita | Anti-inflammatory | Braga et al. (2015) [76] |
| Wild chamomile (Matricaria recutita L.) | Anti-inflammatory, anti-bacterial, and anti-fungal |
Mazokopakis et al. (2003) [56] |
According to antibacterial mechanisms, several studies demonstrated bacterial changes due to radiotherapy and/or chemotherapy [76]. The bacteria were Gemella haemolysans, Streptococcus mitis [77], Escherichia coli, Pseudomonas aeruginosa, Enterobacter sp., Klebsiella pneumonia [78], Staphylococcus aureus, Staphylococcus epidermidis, Parvimonasmicra, Fusobacterium nucleatum, Treponema denticola, C. glabrata, C. kefyr [79], and Porphyromonas gingivalis [80]. Among these bacteria, C. osmophloeum has been confirmed to possess the antibacterial properties of Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis (Figure 1) [26].
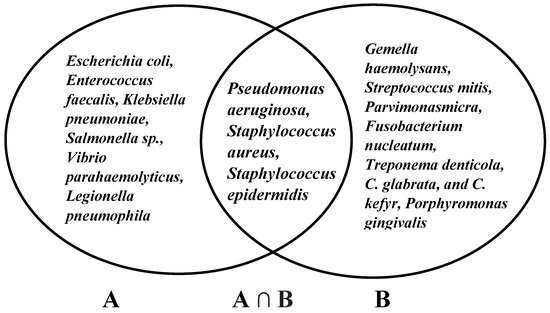
Figure 1. Antibacterial properties of C. osmophloeum (A) and bacterial infection of oral mucositis (B).
Flavanoid-rich fractions containing kaempferitin in Bauhinia forficata leaves have been investigated and shown to be effective ingredients for preventing the intestinal toxic effects of irinotecan chemotherapy. It was stated that kaempferitin, as one of the major active contents of C. osmophloeum, has been tested to prevent or reduce the severity of intestinal mucositis [18][81]. The chemotherapy drug 5-Fluororacil possesses side effects (i.e., induces mucositis manifestations in oral and gastrointestinal after chemotherapy). Chimonatus Nitens var. salicifolius aqueous extract contains three flavonoid contents: quercetin, kaempferol, and rutin, which might have an anti-inflammatory effect on gastrointestinal mucositis [82]. Kaempferol, cinamic acid, and nine other constituents obtained in mucoadhesive propolis agent have been proven to be effective in reducing radiation-induced oral mucositis. A clinical study of 24 patients revealed that mucositis only developed in two patients and each developed grade 1 mucositis and grade 2 mucositis, respectively; however, in the remaining 20 patients, mucositis did not develop [83].
Previous studies investigated the potential of C. osmophloeum to reduce oral mucositis. Nonetheless, C. osmopohloeum, which is a species of cinnamon, faces challenges in its use as an oral treatment. In addition, this review has several limitations. First, there are limited data on the medical-biological effects of C. osmophloeum and its potential use in oral mucositis therapy. Secondly, the reported events related to oral stomatitis allergy induced by cinnamon should be a concern [84][85][86]. In summary, C. osmophloeum and its constituent are anticipated to be effective and efficient in reducing the severity of OM by preventing secondary bacterial infection through their bactericidal activity, preventing the development of the second phase of OM (the primary damage response) or interrupting the third phase in which pro-inflammatory cytokines could enhance and accelerate the process of wound healing (Figure 2).
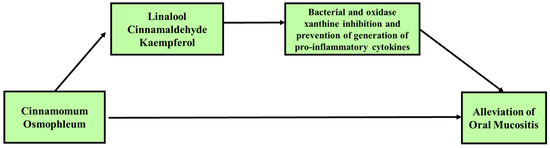
Figure 2. Proposed potential use of C. osmophloeum in the alleviation of oral mucositis (OM). C. osmophloeum ameliorated oxidative stress and pro-inflammation through its constituents. Several studies have investigated the anti-inflammation, antibacterial, and antioxidant activities. The review of medical-biological activities showed that C. osmophloeum and its constituents inhibit the pro-inflammatory response. C. osmophloeum potentially prevents the second phase of oral mucositis (the primary damage response) or may intercept in the third phase, with pro-inflammatory cytokines providing a positive reaction to enhance and accelerate the process of wound healing. C. osmophloeum was also confirmed to be bactericidal and inhibit bacteria which can reduce the severity of oral mucositis or secondary bacterial infection.
References
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; de Aquino Garcia Moura, L.; de Melo, N.R.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169.
- Wang, S.Y.; Yang, C.W.; Liao, J.W.; Zhen, W.W.; Chu, F.H.; Chang, S.T. Essential oil from leaves of Cinnamomum osmophloeum acts as a xanthine oxidase inhibitor and reduces the serum uric acid levels in oxonate-induced mice. Phytomedicine 2008, 15, 940–945.
- Lee, S.-C.; Xu, W.-X.; Lin, L.-Y.; Yang, J.-J.; Liu, C.-T. Chemical Composition and Hypoglycemic and Pancreas-Protective Effect of Leaf Essential Oil from Indigenous Cinnamon (Cinnamomum osmophloeum Kanehira). J. Agric. Food Chem. 2013, 61, 4905–4913.
- Chao, L.K.; Chang, W.T.; Shih, Y.W.; Huang, J.S. Cinnamaldehyde impairs high glucose-induced hypertrophy in renal interstitial fibroblasts. Toxicol Appl. Pharm. 2010, 244, 174–180.
- Huang, J.-S.; Lee, Y.-H.; Chuang, L.-Y.; Guh, J.-Y.; Hwang, J.-Y. Cinnamaldehyde and Nitric Oxide Attenuate Advanced Glycation End Products-Induced the JAK/STAT Signaling in Human Renal Tubular Cells. J. Cell. Biochem. 2015, 116, 1028–1038.
- Tung, Y.-T.; Huang, C.-C.; Ho, S.-T.; Kuo, Y.-H.; Lin, C.-C.; Lin, C.-T.; Wu, J.-H. Bioactive Phytochemicals of Leaf Essential Oils of Cinnamomum osmophloeum Prevent Lipopolysaccharide/d-Galactosamine (LPS/d-GalN)-Induced Acute Hepatitis in Mice. J. Agric. Food Chem. 2011, 59, 8117–8123.
- Yang, L.; Wu, Q.-Q.; Liu, Y.; Hu, Z.-F.; Bian, Z.-Y.; Tang, Q.-Z. Cinnamaldehyde attenuates pressure overload-induced cardiac hypertrophy. Int. J. Clin. Exp. Pathol. 2015, 8, 14345–14354.
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral mucositis. Oral Oncol. 2010, 46, 452–456.
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, Outcomes, and Costs of Radiation-Induced Oral Mucositis Among Patients With Head-and-Neck Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120.
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.L.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M.; et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy. Cancer 2008, 113, 2704–2713.
- Murphy, B.A.; Beaumont, J.L.; Isitt, J.; Garden, A.S.; Gwede, C.K.; Trotti, A.M.; Meredith, R.F.; Epstein, J.B.; Le, Q.-T.; Brizel, D.M.; et al. Mucositis-Related Morbidity and Resource Utilization in Head and Neck Cancer Patients Receiving Radiation Therapy With or Without Chemotherapy. J. Pain Symptom Manag. 2009, 38, 522–532.
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. North. Am. 2008, 52, 61-viii.
- Sonis, S.; Treister, N. Oral mucositis. In Oral complications of cancer and its management, 1st ed.; Davies, A., Epstein, J., Eds.; Oxford Universirty Press: Oxford, UK; New York, NY, USA, 2010; Volume 1, pp. 141–148.
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Supportive Oncol. 2007, 5, 3–11.
- Sonis, S.T. New thoughts on the initiation of mucositis. Oral Dis. 2010, 16, 597–600.
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284.
- Baharvand, M.; Jafari, S.; Mortazavi, H. Herbs in Oral Mucositis. J. Clin. Diagn Res. 2017, 11, ZE05–ZE11.
- Ku, W.-c.; Chang, Y.-l.; Wu, S.-f.; Shih, H.-n.; Tzeng, Y.-m.; Kuo, H.-r.; Chang, K.-m.; Agrawal, D.C.; Liu, B.-l.; Chang, C.-a.; et al. A comparative proteomic study of secretomes in kaempferitrin-treated CTX TNA2 astrocytic cells. Phytomedicine 2017, 36, 137–144.
- Tung, Y.-T.; Chua, M.-T.; Wang, S.-Y.; Chang, S.-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913.
- Tung, Y.T.; Yen, P.L.; Lin, C.Y.; Chang, S.T. Anti-inflammatory activities of essential oils and their constituents from different provenances of indigenous cinnamon (Cinnamomum osmophloeum) leaves. Pharm. Biol. 2010, 48, 1130–1136.
- Chao, L.K.; Hua, K.-F.; Hsu, H.-Y.; Cheng, S.-S.; Lin, I.F.; Chen, C.-J.; Chen, S.-T.; Chang, S.-T. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 2008, 46, 220–231.
- Fang, S.-H.; Rao, Y.K.; Tzeng, Y.-M. Inhibitory effects of flavonol glycosides from Cinnamomum osmophloeum on inflammatory mediators in LPS/IFN-γ-activated murine macrophages. Bioorganic Med. Chem. 2005, 13, 2381–2388.
- Lin, H.-Y.; Chang, S.-T. Kaempferol glycosides from the twigs of Cinnamomum osmophloeum and their nitric oxide production inhibitory activities. Carbohydr. Res. 2012, 364, 49–53.
- Lee, S.-C.; Wang, S.-Y.; Li, C.-C.; Liu, C.-T. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 2018, 26, 211–220.
- Chao, L.K.; Hua, K.-F.; Hsu, H.-Y.; Cheng, S.-S.; Liu, J.-Y.; Chang, S.-T. Study on the Antiinflammatory Activity of Essential Oil from Leaves of Cinnamomum osmophloeum. J. Agric. Food Chem. 2005, 53, 7274–7278.
- Chang, S.-T.; Chen, P.-F.; Chang, S.-C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127.
- Chang, C.W.; Chang, W.L.; Chang, S.T.; Cheng, S.S. Antibacterial activities of plant essential oils against Legionella pneumophila. Water Res. 2008, 42, 278–286.
- Cheng, S.-S.; Liu, J.-Y.; Hsui, Y.-R.; Chang, S.-T. Chemical polymorphism and antifungal activity of essential oils from leaves of different provenances of indigenous cinnamon (Cinnamomum osmophloeum). Bioresour. Technol. 2006, 97, 306–312.
- Wang, S.-Y.; Chen, P.-F.; Chang, S.-T. Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour. Technol. 2005, 96, 813–818.
- Chua, M.T.; Tung, Y.T.; Chang, S.T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresour Technol 2008, 99, 1918–1925.
- Yu, C.-W.; Li, W.-H.; Hsu, F.-L.; Yen, P.-L.; Chang, S.-T.; Liao, V.H.-C. Essential Oil Alloaromadendrene from Mixed-Type Cinnamomum osmophloeum Leaves Prolongs the Lifespan in Caenorhabditis elegans. J. Agric. Food Chem. 2014, 62, 6159–6165.
- Yeh, H.-F.; Luo, C.-Y.; Lin, C.-Y.; Cheng, S.-S.; Hsu, Y.-R.; Chang, S.-T. Methods for Thermal Stability Enhancement of Leaf Essential Oils and Their Main Constituents from Indigenous Cinnamon (Cinnamomum osmophloeum). J. Agric. Food Chem. 2013, 61, 6293–6298.
- Hsu, F.-L.; Li, W.-H.; Yu, C.-W.; Hsieh, Y.-C.; Yang, Y.-F.; Liu, J.-T.; Shih, J.; Chu, Y.-J.; Yen, P.-L.; Chang, S.-T.; et al. In Vivo Antioxidant Activities of Essential Oils and Their Constituents from Leaves of the Taiwanese Cinnamomum osmophloeum. J. Agric. Food Chem. 2012, 60, 3092–3097.
- Lin, T.-Y.; Liao, J.-W.; Chang, S.-T.; Wang, S.-Y. Antidyslipidemic Activity of Hot-water Extracts from Leaves of Cinnamomum osmophloeum Kaneh. Phytother. Res. 2011, 25, 1317–1322.
- Lin, G.M.; Chen, Y.H.; Yen, P.L.; Chang, S.T. Antihyperglycemic and antioxidant activities of twig extract from Cinnamomum osmophloeum. J. Tradit Complement. Med. 2016, 6, 281–288.
- Chen, T.H.; Huang, Y.H.; Lin, J.J.; Liau, B.C.; Wang, S.Y.; Wu, Y.C.; Jong, T.T. Cytotoxic lignan esters from Cinnamomum osmophloeum. Planta Med. 2010, 76, 613–619.
- Lee, M.-J.; Rao, Y.K.; Chen, K.; Lee, Y.-C.; Tzeng, Y.-M. Effect of flavonol glycosides from cinnamomum osmophloeum leaves on adiponectin secretion and phosphorylation of insulin receptor-β in 3t3-l1 adipocytes. J. Ethnopharmacol. 2009, 126, 79–85.
- Cheng, B.-H.; Sheen, L.-Y.; Chang, S.-T. Evaluation of anxiolytic potency of essential oil and s-(+)-linalool from cinnamomum osmophloeum ct. Linalool leaves in mice. J. Tradit. Complement Med. 2014, 5, 27–34.
- Lee, M.-G.; Kuo, S.-Y.; Yen, S.-Y.; Hsu, H.-F.; Leung, C.-H.; Ma, D.-L.; Wen, Z.-H.; Wang, H.-M.D. Evaluation of Cinnamomum osmophloeum Kanehira Extracts on Tyrosinase Suppressor, Wound Repair Promoter, and Antioxidant. Sci. World J. 2015, 2015, 7.
- Rao, Y.K.; Fang, S.-H.; Tzeng, Y.-M. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J. Ethnopharmacol. 2007, 114, 78–85.
- Cheng, B.H.; Sheen, L.Y.; Chang, S.T. Hypolipidemic effects of S-(+)-linalool and essential oil from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit Complement. Med. 2018, 8, 46–52.
- Lin, S.-S.C.; Lu, T.-M.; Chao, P.-C.; Lai, Y.-Y.; Tsai, H.-T.; Chen, C.-S.; Lee, Y.-P.; Chen, S.-C.; Chou, M.-C.; Yang, C.-C. In Vivo Cytokine Modulatory Effects of Cinnamaldehyde, the Major Constituent of Leaf Essential Oil from Cinnamomum osmophloeum Kaneh. Phytother. Res. 2011, 25, 1511–1518.
- Lee, S.-C.; Hsu, J.-S.; Li, C.-C.; Chen, K.-M.; Liu, C.-T. Protective Effect of Leaf Essential Oil from Cinnamomum osmophloeum Kanehira on Endotoxin-Induced Intestinal Injury in Mice Associated with Suppressed Local Expression of Molecules in the Signaling Pathways of TLR4 and NLRP3. PLoS ONE 2015, 10, e0120700.
- Theurer, M.; Shaik, N.; Lang, F. Stimulation of suicidal erythrocyte death by trans-cinnamaldehyde. Phytomedicine 2013, 20, 1119–1123.
- Huang, C.-Y.; Yeh, T.-F.; Hsu, F.-L.; Lin, C.-Y.; Chang, S.-T.; Chang, H.-T. Xanthine Oxidase Inhibitory Activity and Thermostability of Cinnamaldehyde-Chemotype Leaf Oil of Cinnamomum osmophloeum Microencapsulated with β-Cyclodextrin. Molecules 2018, 23, 1107.
- Lin, G.-M.; Lin, H.-Y.; Hsu, C.-Y.; Chang, S.-T. Structural characterization and bioactivity of proanthocyanidins from indigenous cinnamon (Cinnamomum osmophloeum). J. Sci. Food Agric. 2016, 96, 4749–4759.
- Curra, M.; Soares Junior, L.A.V.; Martins, M.D.; Santos, P.S.d.S. Chemotherapy protocols and incidence of oral mucositis. An integrative review. Einstein (Sao Paulo) 2018, 16, eRW4007.
- Köstler, W.J.; Hejna, M.; Wenzel, C.; Zielinski, C.C. Oral Mucositis Complicating Chemotherapy and/or Radiotherapy: Options for Prevention and Treatment. CA: A Cancer J. Clin. 2001, 51, 290–315.
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461.
- Molania, T.; Moghadamnia, A.A.; Pouramir, M.; Aghel, S.; Moslemi, D.; Ghassemi, L.; Motallebnejad, M. The effect of Cinnamaldehyde on mucositis and salivary antioxidant capacity in gamma-irradiated rats (a preliminary study). Daru 2012, 20, 89.
- Parkins, C.S.; Fowler, J.F.; Yu, S. A murine model of lip epidermal/mucosal reactions to X-irradiation. Radiother. Oncol. 1983, 1, 159–165.
- Veilleux, M.-P.; Grenier, D. Determination of the effects of cinnamon bark fractions on Candida albicans and oral epithelial cells. BMC Complement. Altern. Med. 2019, 19, 303.
- Feghali, K.; Feldman, M.; La, V.D.; Santos, J.; Grenier, D. Cranberry Proanthocyanidins: Natural Weapons against Periodontal Diseases. J. Agric. Food Chem. 2012, 60, 5728–5735.
- Raeessi, M.A.; Raeessi, N.; Panahi, Y.; Gharaie, H.; Davoudi, S.M.; Saadat, A.; Karimi Zarchi, A.A.; Raeessi, F.; Ahmadi, S.M.; Jalalian, H. “Coffee plus honey” versus “topical steroid” in the treatment of chemotherapy-induced oral mucositis: A randomised controlled trial. BMC Complement. Altern. Med. 2014, 14, 293.
- Fidler, P.; Loprinzi, C.L.; O’Fallon, J.R.; Leitch, J.M.; Lee, J.K.; Hayes, D.L.; Novotny, P.; Clemens-Schutjer, D.; Bartel, J.; Michalak, J.C. Prospective evaluation of a chamomile mouthwash for prevention of 5-FU-induced oral mucositis. Cancer 1996, 77, 522–525.
- Mazokopakis, E.E.; Vrentzos, G.E.; Papadakis, J.A.; Babalis, D.E.; Ganotakis, E.S. Wild chamomile (Matricaria recutita L.) mouthwashes in methotrexate-induced oral mucositis. Phytomedicine 2005, 12, 25–27.
- Pourdeghatkar, F.; Motaghi, M.; Darbandi, B.; BagherSalimi, A. The Effect of Chamomile Mouthwash on the Prevention of Oral Mucositis Caused by Chemotherapy in Children with Acute Lymphoblastic Leukemia. SSU 2017, 7, 76–81.
- Aoyama, T.; Nishikawa, K.; Takiguchi, N.; Tanabe, K.; Imano, M.; Fukushima, R.; Sakamoto, J.; Oba, M.S.; Morita, S.; Kono, T.; et al. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis. Cancer Chemother Pharm. 2014, 73, 1047–1054.
- Kono, T.; Kaneko, A.; Matsumoto, C.; Miyagi, C.; Ohbuchi, K.; Mizuhara, Y.; Miyano, K.; Uezono, Y. Multitargeted Effects of Hangeshashinto for Treatment of Chemotherapy-Induced Oral Mucositis on Inducible Prostaglandin E2 Production in Human Oral Keratinocytes. Integr. Cancer Ther. 2014, 13, 435–445.
- You, W.C.; Hsieh, C.C.; Huang, J.T. Effect of Extracts from Indigowood Root (Isatis indigotica Fort.) on Immune Responses in Radiation-Induced Mucositis. J. Altern. Complementary Med. 2009, 15, 771–778.
- Tiemann, P.; Toelg, M.; Ramos, F.M.H. Administration of Ratanhia-based herbal oral care products for the prophylaxis of oral mucositis in cancer chemotherapy patients: A clinical trial. Evid Based Complement. Altern. Med. 2007, 4, 361–366.
- Sahebjamee, M.; Mansourian, A.; Hajimirzamohammad, M.; Zadeh, M.T.; Bekhradi, R.; Kazemian, A.; Manifar, S.; Ashnagar, S.; Doroudgar, K. Comparative Efficacy of Aloe vera and Benzydamine Mouthwashes on Radiation-induced Oral Mucositis: A Triple-blind, Randomised, Controlled Clinical Trial. Oral Health Prev. Dent. 2015, 13, 309–315.
- Hawley, P.; Hovan, A.; McGahan, C.E.; Saunders, D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Supportive Care Cancer 2014, 22, 751–761.
- Miranzadeh, S.; Adib-Hajbaghery, M.; Soleymanpoor, L.; Ehsani, M. A New mouthwash for Chemotherapy Induced Stomatitis. Nurs. Midwifery Stud. 2014, 3, e20249.
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M.; et al. Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: A randomized controlled clinical study. Daru 2013, 21, 18.
- Khanal, B.; Baliga, M.; Uppal, N. Effect of topical honey on limitation of radiation-induced oral mucositis: An intervention study. Int. J. Oral Maxillofac. Surg. 2010, 39, 1181–1185.
- Kuduban, O.; Mazlumoglu, M.R.; Kuduban, S.D.; Erhan, E.; Cetin, N.; Kukula, O.; Yarali, O.; Cimen, F.K.; Cankaya, M. The effect of hippophae rhamnoides extract on oral mucositis induced in rats with methotrexate. J. Appl Oral Sci 2016, 24, 423–430.
- Motallebnejad, M.; Akram, S.; Moghadamnia, A.; Moulana, Z.; Omidi, S. The effect of topical application of pure honey on radiation-induced mucositis: A randomized clinical trial. J. Contemp. Dent. Pract. 2008, 9, 40–47.
- Rashad, U.M.; Al-Gezawy, S.M.; El-Gezawy, E.; Azzaz, A.N. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J. Laryngol. Otol. 2009, 123, 223–228.
- Lambros, M.P.; Kondapalli, L.; Parsa, C.; Mulamalla, H.C.; Orlando, R.; Pon, D.; Huang, Y.; Chow, M.S.S. Molecular signatures in the prevention of radiation damage by the synergistic effect of n-acetyl cysteine and qingre liyan decoction, a traditional chinese medicine, using a 3-dimensional cell culture model of oral mucositis. Evid. Based Complement Alternat. Med. 2015, 2015, 425760.
- Loo, W.T.Y.; Jin, L.J.; Chow, L.W.C.; Cheung, M.N.B.; Wang, M. Rhodiola algida improves chemotherapy-induced oral mucositis in breast cancer patients. Expert Opin. Investig. Drugs 2010, 19, S91–S100.
- Wu, M.-h.; Yuan, B.; Liu, Q.-f.; Wang, Q. Study of qingre liyan decoction (清热利咽汤) in treating and preventing acute radioactive oral mucositis. Chin. J. Integr. Med. 2007, 13, 280–284.
- Puataweepong, P.; Dhanachai, M.; Dangprasert, S.; Sithatani, C.; Sawangsilp, T.; Narkwong, L.; Puttikaran, P.; Intragumtornchai, T. The efficacy of oral aloe vera juice for radiation induced mucositis in head and neck cancer patients: A double-blind placebo-controlled study. Asian Biomedicine 2009, 3, 375–382.
- De Freitas Cuba, L.; Braga Filho, A.; Cherubini, K.; Salum, F.G.; Figueiredo, M.A.Z.d. Topical application of aloe vera and vitamin e on induced ulcers on the tongue of rats subjected to radiation: Clinical and histological evaluation. Support. Care Cancer 2016, 24, 2557–2564.
- Sencer, S.F.; Zhou, T.; Freedman, L.S.; Ives, J.A.; Chen, Z.; Wall, D.; Nieder, M.L.; Grupp, S.A.; Yu, L.C.; Sahdev, I.; et al. Traumeel s in preventing and treating mucositis in young patients undergoing sct: A report of the children’s oncology group. Bone Marrow Transplant 2012, 47, 1409–1414.
- Braga, F.T.M.M.; Santos, A.C.F.; Bueno, P.C.P.; Silveira, R.C.C.P.; Santos, C.B.; Bastos, J.K.; Carvalho, E.C. Use of chamomilla recutita in the prevention and treatment of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized, controlled, phase ii clinical trial. Cancer Nursing 2015, 38, 322–329.
- Napeñas, J.J.; Brennan, M.T.; Coleman, S.; Kent, M.L.; Noll, J.; Frenette, G.; Nussbaum, M.L.; Mougeot, J.-L.; Paster, B.J.; Lockhart, P.B.; et al. Molecular methodology to assess the impact of cancer chemotherapy on the oral bacterial flora: A pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 554–560.
- Sonalika, W.G.; Amsavardani Tayaar, S.; Bhat, K.G.; Patil, B.R.; Muddapur, M.V. Oral microbial carriage in oral squamous cell carcinoma patients at the time of diagnosis and during radiotherapy – A comparative study. Oral Oncol. 2012, 48, 881–886.
- Panghal, M.; Kaushal, V.; Kadayan, S.; Yadav, J.P. Incidence and risk factors for infection in oral cancer patients undergoing different treatments protocols. BMC Oral Health 2012, 12, 22.
- Laheij, A.M.G.A.; de Soet, J.J.; von dem Borne, P.A.; Kuijper, E.J.; Kraneveld, E.A.; van Loveren, C.; Raber-Durlacher, J.E. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Supportive Care Cancer 2012, 20, 3231–3240.
- Cechinel-Zanchett, C.C.; Boeing, T.; Somensi, L.B.; Steimbach, V.M.B.; Campos, A.; Krueger, C.d.M.A.; Schultz, C.; Sant’ana, D.d.M.G.; Cechinel-Filho, V.; Mota da Silva, L.; et al. Flavonoid-rich fraction of Bauhinia forficata Link leaves prevents the intestinal toxic effects of irinotecan chemotherapy in IEC-6 cells and in mice. Phytother. Res. 2019, 33, 90–106.
- Liu, Z.; Xi, J.; Schröder, S.; Wang, W.; Xie, T.; Wang, Z.; Bao, S.; Fei, J. Chimonanthus nitens var. salicifolius Aqueous Extract Protects against 5-Fluorouracil Induced Gastrointestinal Mucositis in a Mouse Model. Evid. -Based Complementary Altern. Med. 2013, 2013, 12.
- Vladimir, R.A.S.N.; Gustavo, S.A.; Rafael, T.G.; Samara, H.I.; Maralice, C.B.; Evandro, N.A.; Efigenia Ferreira e, F.; Ana Cristina Viana, C.; Alexandre, A.S.; Sheila, R.L.A.; et al. Mucoadhesive Propolis Gel for Prevention of Radiation-Induced Oral Mucositis. Curr. Clin. Pharmacol. 2014, 9, 359–364.
- Georgakopoulou, E.A. Cinnamon contact stomatitis. J. Derm. Case Rep. 2010, 4, 28–29.
- Tremblay, S.; Avon, S.L. Contact allergy to cinnamon: Case report. J. Can. Dent. Assoc. 2008, 74, 445–461.
- Calapai, G.; Miroddi, M.; Mannucci, C.; Minciullo, P.L.; Gangemi, S. Oral adverse reactions due to cinnamon-flavoured chewing gums consumption. Oral Dis. 2014, 20, 637–643.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
977
Revisions:
2 times
(View History)
Update Date:
24 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




