
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alicia Checa-Fernandez | + 2437 word(s) | 2437 | 2021-06-17 06:09:12 | | | |
| 2 | Lindsay Dong | + 240 word(s) | 2677 | 2021-06-22 04:00:11 | | |
Video Upload Options
The Fenton process is an efficient treatment for removing many organics pollutants in aqueous systems at acidic pH (2.8-3.5). However, the in-situ application of this technology for soil remediation (where pHs around neutrality are required) presents important limitations, such as catalyst (iron) availability and oxidant (H2O2) stability. The addition of chelating agents (CAs) makes iron soluble at circumneutral pH by forming complexes with Fe, and thus, enabling Fenton reactions under these conditions. This strategy, called chelate-modified Fenton process (MF), can be employed to overcome the challenges identified in conventional Fenton.
1. Introduction
Soil and sediments contamination by organic compounds, resulting from industrial and municipal waste discharge and improper use of chemical fertilizers and pesticides, is a widespread problem worldwide due to its great harm to the ecological environment and public health [1][2][3]. Nowadays, the principal organic substances contributing to soil pollution are petroleum oil hydrocarbons (e.g., aliphatic, aromatic, polycyclic aromatic hydrocarbons (PAHs), BTEX (benzene, toluene, ethylbenzene, and xylenes), chlorinated hydrocarbons like polychlorinated biphenyls (PCBs), trichloroethylene (TCE), and perchloroethylene, nitroaromatic compounds, organophosphorus compounds), solvents, and pesticides [4].
Extensive work has been devoted to developing soil remediation techniques [5]. Advanced oxidation processes (AOPs) are powerful chemical methods with growing popularity for organic-contaminated soil remediation, being considered more effective than physical and biological approaches [6]. The oxidants used in AOPs include hydrogen peroxide (H2O2), persulfate (S2O82−), permanganate (MnO4−), and ozone (O3). One of the most frequently AOPs used is the Fenton process (H2O2 + Fe(II)), where H2O2 is the oxidant species and homogeneous Fe(II) acts as a catalyst for hydrogen peroxide decomposition [7][8].
Although the Fenton process has been proven to be a viable approach for remediating contaminated soils [9][10][11], there are various limitations associated with this treatment [10]. Fenton process displays its maximum OH• production and the subsequent pollutant oxidation activity under acidic pH [12][13][14]. However, subsurface systems are often buffered in the neutral pH range (pH 6–8), which greatly complicates the implementation of this process. Different techniques can be used to ensure the efficient presence of catalysis at neutral pH such as a) the employment of iron minerals naturally occurring in soils instead of soluble iron (Fe(II)) [15], and b) deliver a soluble inorganic or organic ligand (L) (also named chelating agent, CA) to maintain iron in the solution, enhancing the Fenton reactions. This process is called chelate-modified Fenton (MF) process. Inorganic and organic CAs form complexes with Fe(II)/Fe(III) at neutral pH, keep it soluble, and thus enhance the production of oxidative species and extend the applicability of Fenton oxidation to a wider range of pH [10][16][17]. The extraction of the transition metals of the soil is also enhanced by the addition of chelating agents [18]. Furthermore, some authors proposed that CAs can also improve the persistence of H2O2, allowing the radical species generated to flow through the soil, reaching the target contaminants [6].
In the simplified reaction scheme of chelate-modified Fenton (MF) it can be seen that the Fe-L complexes formed (Fe(II)-L and Fe(III)-L) would decompose H2O2 to generate radical species (hydroxyl and hydroperoxyl radicals) (Equations (14) and (15)), equivalent to classical Fenton reactions, being the catalytic regeneration (Equation (15)) the limiting stage of the process. However, in the presence of most organic CAs, it has been suggested that H2O2 was unlikely to reduce complexed iron, and the reduction of Fe(III)-L was mainly produced through O2•- generated (Equation (18)) rather than from the direct Fenton reactions with H2O2 (Equation (15)) [19][20][21][22][23][24]. In this way, the MF acceleration is attributed to the positive effect of O2•- during regeneration of Fe(II)-L from Fe(III)-L.
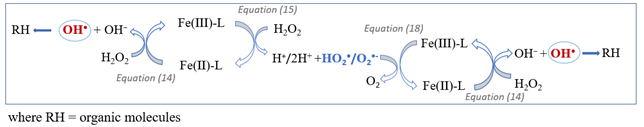
Figure 1. Simplified scheme summarizing the reactions involved in the chelate-modified Fenton process.
2. Chelating Agents Commonly Used in Modified Fenton Process for Soil Remediation
CAs used in soil remediation may be classified into inorganic and organic compounds. The most used inorganic compound is pyrophosphate (PPP). Among organic CAs, three main types may be considered according to their coordination sites: polycarboxylates (citrate (in the form of citric acid (CitrA) or its salt) and oxalate (in the form of oxalic acid (OA) or its salt), aminocarboxylates (ethylenediaminetetraacetic acid (EDTA), nitrilotriacetic acid (NTA), ethylenediamine-N,N’-disuccinic acid (EDDS), ethylenediamine-N,N’-bis(2-hydroxyphenyl)acetic acid (EDDHA)) and humic substances (humic (HA) and fulvic acids (FA)). The structure of the CAs most used for the remediation of soils contaminated with organic pollutants are summarized in Figure 2.
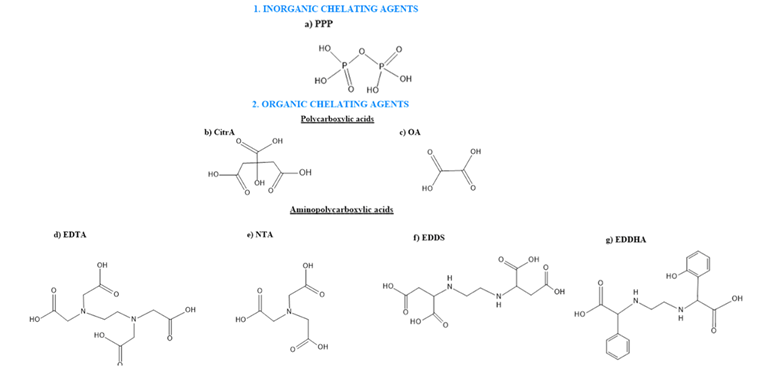
Figure 2. Structure of the investigated chelating agents: (1) inorganics; (a) pyrophosphate (PPP), and (2) organics; (b) citrate as citric acid (CitrA), (c) oxalate as oxalic acid (OA), (d) ethylenediaminetetraacetic (EDTA), (e) nitriloacetic acid (NTA), (f) ethylenediamine-N,N’-disuccinic acid (EDDS) and (g) ethylenediamine-N,N’-bis(2-hydroxyphenyl)acetic acid (EDDHA).
In addition to the CAs above-mentioned, other organics CAs less frequently used for soil remediation are catechol (CC), l-Ascorbic acid (l-AA), gallic acid (GA), picolinic acid (PA), sodium N,N′-bis(carboxymethyl) glutamic acid (GLDA), and cyclodextrins (CD).
2.1. Inorganic Chelating Agents: Pyrophosphate
Pyrophosphate (PPP) (Figure 2), in the form of sodium pyrophosphate (SP), is the most used inorganic CA for iron stabilization [25][26][27][28][29] and oxidant stabilization [25][27][30] in Fenton-type systems. Although some authors supported that the reduction of Fe(III)-PPP was limited, the delays in the rate of H2O2 decomposition, the increase in OH• production, and the amount of soluble iron lead to an increase in pollutant conversion when this ligand is used in soil remediation under near-neutral conditions [25][26][27][30]. Furthermore, the scavenging effect of this CA can be neglected due to the low reactivity of OH• towards P2O74-, compared to other organic CAs (see Table 1) [31]. In addition, PPP can serve as phosphorus fertilizer to plants and environmental microorganisms [30][32], facilitating a subsequent bioremediation treatment.
Table 1. Kinetic constants for the Fenton process in the presence of different CAs.
| Ligand (L) | ROS Involved in Pollutant Degradation | kFe(II)-L, H2O2 (M−1 s−1) Equation (14) |
kFe(III)-L, O2•− (M−1 s−1) Equation (18) |
kFe(II)-L/Fe(III)-L, OH• (M−1 s−1) Equation (23) |
kL, OH• (M−1 s−1) Equation (24) |
|---|---|---|---|---|---|
| - | OH• (acidic pH) or Fe(IV) (neutral pH) [33] | Equation (1): 40–80 [34][35] |
n.f. | Equation (4): Fe(II): 2.5–5 × 108 [34][35] |
- |
| PPP | OH• [36] | 1 × 105 [37][38][39] | n.f. | n.f. | 2.2 × 105 [40] 9 × 105 [41] |
| Citrate | OH• [42][36] | 3.6 × 103 [42] 4 × 103 [43] 4.9 × 103 [38] |
800 [43] | Fe(II)-L: 1.2 × 108 [43] Fe(III)-L: 1.2 × 108 * [44] |
5 × 107 [41][45] 1.2 × 108 (pH = 3), 2.4 × 108 (pH = 6), 3.2 × 108 (pH = 6.6) [44] |
| Oxalate | OH• [36] | 3.1 × 104 [46] | <1.0 × 106 [47] | Fe(II)-L: n.f. Fe(III)-L: 1 × 106[41] |
1.4 × 106 [48] 7.7 × 106 [47] 1 × 107 [44] |
| EDTA | OH• [36] | 3.2 × 103 [43] | 6 × 104 [43] 1.2 × 106 (pH = 7.3) [21] |
Fe(II)-L: 5 × 109 [41] Fe(III)-L: 7.0 × 108– 1.6 × 109 [41] |
4 × 108 (pH = 4) [48] 2 × 109 (pH = 9) [48][49] |
| NTA | OH• [19][36] | 9.7 × 103– 1.8 × 104 [50] |
n.f. | Fe(II)-L: 2.3–5 × 109 [41] Fe(III)-L: 4.8 × 108 [19] 1.6 × 108 [50][41] |
5.5 × 108 (pH = 6), 2.5 × 109 (pH = 9), 4.2 × 109 (pH = 10) [50] |
| EDDS | OH• (80%) and O2•− (20%) [24] | n.f. | n.f. | Fe(II)/(III)-L: 2.0-5.2 × 108 [24] |
2.5 × 109 [23] |
| EDDHA | OH• (50%) and O2•− (50%) [27] | n.f. | n.f. | n.f. | n.f. |
| HA | OH• [51] or O2•− [52] | n.f. | n.f. | n.f. | 1.4 × 104 LmgC–1 s–1 [51] |
n.f. = not found. * These authors supposed that kFe(III)-L, OH• was like kL, OH•.
2.2. Organic Chelating Agents
2.2.1. Polycarboxylic acids (PCAs)
Citrate and oxalate ligands are the most commonly used PCAs. Citrate ligand can be generated from citric acid (CitrA) or sodium salts. CitrA is an environmental-friendly ligand consisting of three carboxyl groups and one hydroxyl group (Figure 2). The high-rate constant of citrate and Fe-citrate complex towards OH• radicals leads to a significant scavenging effect at a high dose of this ligand. In this way, pH and citrate:Fe molar ratio have a decisive influence on the effectiveness of using this ligand in a MF process for soil remediation [53].
On the other hand, the ligand oxalate (in the form of oxalic acid ((C2O4)2−, OA) has been widely used to enhance the Fenton process for soil remediation [26][28][54][55]. The rate constant for the Fenton reaction in the absence of ligands is around 40–80 M−1s−1, whereas this value increases up to 3.1 × 104 M−1s−1 when the decomposition of H2O2 is catalyzed by Fe(II)-oxalate, which is 3 orders of magnitude higher [46][39], increasing process efficiency.
2.2.2. Aminopolycarboxylic Acids (APCAs)
-
Ethylenediaminetetraacetic Acid (EDTA) (Figure 2) is one of the most popular APCAs and has been widely used as a chelating agent for soil remediation [56][57]. EDTA can strongly combine Fe(II) or Fe(III) to form a stable metal complex in solution. Fe(III)–EDTA can be reduced to Fe(II)–EDTA by O2−. This radical is previously generated by a series of serial reactions initiated by the reaction between Fe(III)-EDTA and the oxidant (H2O2). Finally, Fe(II)–EDTA reacts with H2O2generating hydroxyl radicals [20][21]. Although EDTA enhances the Fenton effectiveness at near-neutral pH in soils [58][59][60][61], its use is limited due to its low biodegradability, contribution to heavy metal mobility/bioavailability, and persistence in the environment [62][63][64][65].
-
Nitrilotriacetic acid (NTA) is one of the environmentally friendly CAs used to replace EDTA [17]. However, although NTA is biodegradable, its usage is controversial because it is moderately toxic to humans and mammals [66]. In addition, significant scavenging of the hydroxyl radicals generated is expected by NTA due to the high-rate constant between this CA and OH, especially significant at high pH values [50].
-
Ethylenediaminedisuccinate (EDDS) has recently emerged as an alternative CA, this compound presenting properties similar to those of EDTA and readily biodegradable nature [24][63][67][68]. Since EDDS promotes the generation of superoxide radicals, the use of this new chelating agent in groundwater and soil remediation could be very effective [24]. Nevertheless, the application of EDDS should be limited by the fast reaction between EDDS and OH [23]. As mentioned above, apart from pH, the CA:Fe molar ratio greatly affects the efficiency of the process. It has been reported that EDDS showed a low ability to activate the oxidant at EDDS:Fe ratios higher than 1:1 [69].
-
Ethylenediamine-N,N′-bis(2-hydroxyphenyl)acetic Acid (EDDHA) is a biodegradable ligand with two phenolic groups substituting the carboxylates of EDTA (Figure 2), which highly increase its stability. the application of the Fe(II)/EDDHA/H2O2 system has been shown to efficiently degrade soils contaminated with organic pollutants [27].
2.2.3. Humic Substances (HS) and Soil Organic Matter (SOM)
Humic substances are the major constituents of the organic matter of soils and sediments. Humic substances are classified into humic and fulvic acids (HA and FA, respectively). Both are ubiquitous without potential toxicity, being considered “greener” amendments for the MF process [70]. The applicability of HA as a chelating agent in soil remediation greatly enhances the oxidation rate of organic compounds at neutral pH, the costs increase associated being negligible [55].
SOM, the organic fraction of the soil, which includes humic substances, can also develop an important role as CA. Xu et al. reported that SOM can combine with iron ions to form Fe-SOM, catalyzing the decomposition of H2O2 to produce OH• in the solid phase, which directly oxidizes the pollutant [71], as described in Figure 3a (adapted from Xu et al. [71]). This contrasted with the OH• production and the oil degradation in the aqueous phase in the absence of Fe-SOM (Figure 3b).
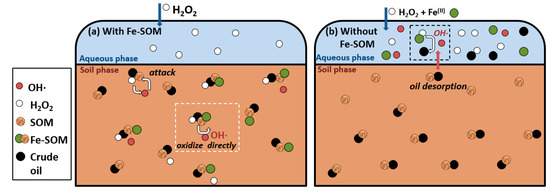
Figure 6. Schematic representation of different oxidation mechanisms in soil for crude oil degradation; (a) with Fe-SOM and (b) without Fe-SOM. Adapted from Xu et al. [71].
3. Application of Chelating Agents in Soil Remediation by Modified Fenton
3.1. Factors Affecting Contaminant Removal
An important aspect in the remediation of contaminated soils derives from the accessibility of the contaminants. Pollution aging leads to the migration of contaminants from easily accessible to difficult sites, becoming sequestrated in the soil matrix [72], which reduces the chemical remediation efficiency of hydrophobic organic pollutants [73]. In this sense, CAs can enhance the desorption of the contaminants [72][74][75], and therefore, favor the accessibility of the contaminant towards the oxidant.
Another limiting factor in the remediation of soils is the negative effect of the soil matrix. It has been reported that the soil type determines the concentration of soluble Fe(III) in soil slurry systems, probably through hydrolysis and adsorption [30]. Moreover, the stability of H2O2 seems to be related to the properties of the soil. In this way, the specific characteristics of the soil should be considered to properly select the CA for the MF process.
The soil texture and moisture also influence the remediation process. Loose soil texture can help the mass transfer of reagents, while dense soil leads to large consumption of reagents [6]. Moreover, the application of low doses of oxidant at low soil-moisture levels has shown to be the most effective option [76].
The adsorption and desorption of chemicals (CA and catalyst) onto the soil is a limiting factor that should be considered when applying the MF process to remediate polluted soils.
3.2. Results of Modified Fenton Obtained according to the Contaminant Type
The review of CAs application in the MF process will be carried out according to the main types of contaminants found in the literature for soil remediation. According to their characteristics, pollutants have been divided into the following groups:
-
BTEX and phenolic compounds
-
Polycyclic aromatic hydrocarbons (PAHs)
-
Total petroleum hydrocarbons (TPHs)
-
Unsaturated chlorinated compounds and pesticides
-
Saturated chlorinates compounds.
The reaction rate of each contaminant with the generated hydroxyl radicals (kcontaminant, OH•) will decisively influence the efficiency of its removal when applying the MF process. Therefore, to achieve an efficient pollutant degradation, the kinetic constant of the pollutant with OH• should be significantly higher than the kinetic constant of CA (and Fe-L) with these radicals. The kcontaminant, OH• values found in the literature have been summarized in Table 2.
Table 4. Kinetic constants of different organic contaminants with hydroxyl radicals.
| Group | Contaminant | kcontaminant, OH• (M−1s−1), (Equation (22)) |
Ref. |
|---|---|---|---|
| BTEX/ phenolic compounds |
Benzene | 6.6 × 108 | [77] |
| Toluene | 3.4 × 109 | [77] | |
| Ethylbenzene | 4.1 × 109 | [77] | |
| Xylene | 9.5 × 109 | [77] | |
| Phenol | 6.6 × 109 | [77] | |
| Bisphenol-A | 9.8 × 109 | [78] | |
| PAHs | Acenaphthene | 8.8 × 109 | [79] |
| Benzo[a]pyrene | 2.53 × 1010 | [79] | |
| Chrysene | 9.82 × 109 (20 °C, pH = 7) | [79] | |
| Fluorene | 2.8–9.9 × 109 | [79] | |
| Naphthalene | 0.5–1.2 × 1010 | [79] | |
| Phenanthrene | 1.34 × 1010 | [79] | |
| Unsaturated chlorinated compounds and pesticides | Polychlorinated biphenyls (PCBs) | 5 × 109 | [80] |
| Diuron | 4.8 × 109 | [41] | |
| Trichloroethylene (TCE) | 3–4 × 109 | [41][79] | |
| Tetrachloroethene (PCE) | 2.8 × 109 | [41] | |
| Pentachlorophenol (PCP) | 4 × 109 | [79] | |
| 1,1-dichloroethene | 6.8 × 109 | [41] | |
| Vinyl chloride | 1.2 × 1010 | [41] | |
| Atrazine, propazine, and terbuthylazine | 2.2–3.5 × 109 | [51] | |
| 1,2,3-trichlorobenzene | 6.1 × 109 | [79] | |
| 1,4-dichlorobenzene | 5.4 ×109 | [79] | |
| Saturated chlorinated compounds | γ-hexachlorocyclohexane (lindane) | 5.8 × 108 | [80] |
| Trichloromethane | 5.0 × 106 | [24] |
Extensive information on the use of different CAs for the remediation of soils contaminated with different types of organic pollutants has been summarized including the oxidation process and the reagents molar ratios selected as the most convenient, the type of soil treated (spiked or real), the type of experiments (batch or column), the pollutant and its concentration in the contaminated soils, the liquid to soil mass ratio (VL/W) and the system pH, the reaction time and, finally, the main results obtained concerning the pollutants, hydrogen peroxide, and CA conversions have been analyzed.
4. Potential Chelating Agents for Soil Remediation by Fenton Process
Apart from those mentioned above, other inorganics and organics CAs not considered in the review have shown promising results in removing organics pollutants from the aqueous phase, and they could be tested for soil remediation. Some of them are detailed below.
-
Polyoxometalates (POMs), such as PW12O403–and SiW12O404–, are inorganic CAs that form soluble complexes with Fe(III) under neutral pH conditions [81]. POM is biodegradable, non-toxic, and resistant to oxidation. Thus, its use presents significant advantages over most organic CAs [17].
-
Tripolyphosphate (TPP), one of the commonly used polyphosphates, had proved to be a promising inorganic CA for iron stabilization in Fe-based AOPs for the abatement of organics pollutants in aqueous systems [82][83].
-
Other organics CAs such as aspartic acid and glutamic acid have shown promising results [84]. The formation of Fe(II)-aspartate and Fe(II)-glutamate complexes has been proved to extend and improve the rate of p-nitrophenol degradation process to neutral pH conditions, making the application of these CAs a promising way to enhance the Fenton process [84]. Moreover, polyacrylic acids (PAAs) have been proven to be efficient CAs due to their multiple binding sites [85]. Applying the CAs mentioned above for the remediation of polluted soils has not been carried out, and further research is needed.
5. Conclusions
The addition of chelating agents (CAs) allows overcoming some of the main limitations of conventional Fenton in soil remediation, such as the possibility to operate at circumneutral pH, maintaining the catalyst (Fe) available, and increasing the oxidant (H2O2) stability. CAs, forming complexes with Fe, allow extending conventional Fenton pH to neutral or near-neutral pH. The selection of the CA is not trivial and several aspects should be considered, such as the pollutant type and its accessibility, the most convenient CA:Fe molar ratio to employ depending on the CA properties, the characteristics of the soil matrix (SOM and TOC content, presence of natural iron oxides and other metals, carbonates, etc.), and the future use of the soil, among others.
References
- TTeng, Y.; Wu, J.; Lu, S.; Wang, Y.; Jiao, X.; Song, L. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199.
- Chen, F.; Li, X.; Ma, J.; Qu, J.; Yang, Y.; Zhang, S. Remediation of soil co-contaminated with decabromodiphenyl ether (BDE-209) and copper by enhanced electrokinetics-persulfate process. J. Hazard. Mater. 2019, 369, 448–455.
- Song, B.; Zeng, G.; Gong, J.; Liang, J.; Xu, P.; Liu, Z.; Zhang, Y.; Zhang, C.; Cheng, M.; Liu, Y.; et al. Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals. Environ. Int. 2017, 105, 43–55.
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375.
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851.
- Zhang, T.; Liu, Y.; Zhong, S.; Zhang, L. AOPs-based remediation of petroleum hydrocarbons-contaminated soils: Efficiency, influencing factors and environmental impacts. Chemosphere 2020, 246, 125726.
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598.
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50.
- Zhu, C.; Zhu, F.; Wang, F.; Gao, J.; Fan, G.; Zhou, D.; Fang, G. Comparison of Persulfate Activation and Fenton Reaction in Remediating an Organophosphorus Pesticides-Polluted Soil. Pedosphere 2017, 27, 465–474.
- Usman, M.; Hanna, K.; Haderlein, S. Fenton oxidation to remediate PAHs in contaminated soils: A critical review of major limitations and counter-strategies. Sci. Total Environ. 2016, 569–570, 179–190.
- Venny; Gan, S.; Ng, H.K. Current status and prospects of Fenton oxidation for the decontamination of persistent organic pollutants (POPs) in soils. Chem. Eng. J. 2012, 213, 295–317.
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84.
- Pouran, S.R.; Aziz, A.A.; Daud, W.M.A.W. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69.
- Bello, M.M.; Abdul Raman, A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process. Saf. Environ. Prot. 2019, 126, 119–140.
- Watts, R.J.; Stanton, P.C.; Howsawkeng, J.; Teel, A. Mineralization of a sorbed polycyclic aromatic hydrocarbon in two soils using catalyzed hydrogen peroxide. Water Res. 2002, 36, 4283–4292.
- Ahile, U.J.; Wuana, R.; Itodo, A.U.; Sha’Ato, R.; Dantas, R.F. A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Sci. Total Environ. 2020, 710, 134872.
- Zhang, Y.; Zhou, M. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J. Hazard. Mater. 2019, 362, 436–450.
- Leštan, D.; Luo, C.-L.; Li, X.-D. The use of chelating agents in the remediation of metal-contaminated soils: A review. Environ. Pollut. 2008, 153, 3–13.
- Zhang, Y.; Klamerth, N.; El-Din, M.G. Degradation of a model naphthenic acid by nitrilotriacetic acid—Modified Fenton process. Chem. Eng. J. 2016, 292, 340–347.
- Gutteridge, J.M.C.; Maidt, L.; Poyer, L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem. J. 1990, 269, 169–174.
- Sutton, H.C. Efficiency of chelated iron compounds as catalysts for the Haber-Weiss reaction. J. Free. Radic. Biol. Med. 1985, 1, 195–202.
- Kwan, C.; Chu, W. The role of organic ligands in ferrous-induced photochemical degradation of 2,4-dichlorophenoxyacetic acid. Chemosphere 2007, 67, 1601–1611.
- Zhang, Y.; Klamerth, N.; Messele, S.A.; Chelme-Ayala, P.; El-Din, M.G. Kinetics study on the degradation of a model naphthenic acid by ethylenediamine-N,N′-disuccinic acid-modified Fenton process. J. Hazard. Mater. 2016, 318, 371–378.
- Huang, W.; Brigante, M.; Wu, F.; Mousty, C.; Hanna, K.; Mailhot, G. Assessment of the Fe(III)–EDDS Complex in Fenton-Like Processes: From the Radical Formation to the Degradation of Bisphenol A. Environ. Sci. Technol. 2013, 47, 1952–1959.
- Wang, X.; Brusseau, M. Effect of pyrophosphate on the dechlorination of tetrachloroethene by the Fenton reaction. Environ. Toxicol. Chem. 1998, 17, 1689–1694.
- Venny; Gan, S.; Ng, H.K. Inorganic chelated modified-Fenton treatment of polycyclic aromatic hydrocarbon (PAH)-contaminated soils. Chem. Eng. J. 2012, 180, 1–8.
- Ma, X.-H.; Zhao, L.; Dong, Y.-H.; Chen, H.; Zhong, M. Enhanced Fenton degradation of polychlorinated biphenyls in capacitor-oil-contaminated soil by chelating agents. Chem. Eng. J. 2018, 333, 370–379.
- Shih, Y.-J.; Binh, N.T.; Chen, C.-W.; Chen, C.-F.; Dong, C.-D. Treatability assessment of polycyclic aromatic hydrocarbons contaminated marine sediments using permanganate, persulfate and Fenton oxidation processes. Chemosphere 2016, 150, 294–303.
- Bennedsen, L.R.; Krischker, A.; Jørgensen, T.H.; Søgaard, E.G. Mobilization of metals during treatment of contaminated soils by modified Fenton’s reagent using different chelating agents. J. Hazard. Mater. 2012, 199–200, 128–134.
- Sun, Y.; Zhao, L.; Teng, Y. Effect of soil type on the degradation of polychlorinated biphenyls in a pyrophosphate-chelated Fenton-like reaction. Chem. Eng. J. 2020, 390, 124574.
- Richter, Y.; Fischer, B. Nucleotides and inorganic phosphates as potential antioxidants. JBIC J. Biol. Inorg. Chem. 2006, 11, 1063–1074.
- Khasawneh, F.; Hashimoto, I.; Sample, E. Reactions of ammonium ortho- and polyphosphate fertilizers in soil: II. Hydrolysis and reactions with soil. Soil Sci. Soc. Am. J. 1979, 43, 52–58.
- Winterbourn, C.C. Chapter One—The Biological Chemistry of Hydrogen Peroxide. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 3–25.
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572.
- Sychev, A.Y.; Isak, V.G. Iron compounds and the mechanisms of the homogeneous catalysis of the activation of O2 and H2O2 and of the oxidation of organic substrates. Russ. Chem. Rev. 1995, 64, 1105–1129.
- Wang, Z.; Qiu, W.; Pang, S.; Jiang, J. Effect of chelators on the production and nature of the reactive intermediates formed in Fe(II) activated peroxydisulfate and hydrogen peroxide processes. Water Res. 2019, 164, 114957.
- Rachmilovich-Calis, S.; Masarwa, A.; Meyerstein, N.; Meyerstein, D. The effect of pyrophosphate, tripolyphosphate and ATP on the rate of the Fenton reaction. J. Inorg. Biochem. 2011, 105, 669–674.
- Rush, J.; Maskos, Z.; Koppenol, W. Reactions of iron(II) nucleotide complexes with hydrogen peroxide. FEBS Lett. 1990, 261, 121–123.
- Park, J.S.B.; Wood, P.M.; Davies, M.J.; Gilbert, B.C.; Whitwood, A.C. A Kinetic and ESR Investigation of Iron(II) Oxalate Oxidation by Hydrogen Peroxide and Dioxygen as a Source of Hydroxyl Radicals. Free. Radic. Res. 1997, 27, 447–458.
- Melton, J.D.; Bielski, B.H. Studies of the kinetic, spectral and chemical properties of Fe(IV) pyrophosphate by pulse radiolysis. Int. J. Radiat. Appl. Instrumentation. Part. C Radiat. Phys. Chem. 1990, 36, 725–733.
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886.
- Zeng, B.; Zhang, P.; Zheng, M.; Xiao, N.; Han, J.; Wang, C.; Wang, Z.; Zhao, Z. Detection and identification of the oxidizing species generated from the physiologically important Fenton-like reaction of iron(II)-citrate with hydrogen peroxide. Arch. Biochem. Biophys. 2019, 668, 39–45.
- Miller, C.J.; Rose, A.L.; Waite, T.D. Importance of Iron Complexation for Fenton-Mediated Hydroxyl Radical Production at Circumneutral pH. Front. Mar. Sci. 2016, 3, 134.
- Zepp, R.G.; Faust, B.C.; Hoigne, J. Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron(II) with hydrogen peroxide: The photo-Fenton reaction. Environ. Sci. Technol. 1992, 26, 313–319.
- Shih, Y.-J.; Chen, K.-H.; Huang, Y.-H. Mineralization of organic acids by the photo-electrochemical process in the presence of chloride ions. J. Taiwan Inst. Chem. Eng. 2014, 45, 962–966.
- Jeong, J.; Yoon, J. pH effect on OH radical production in photo/ferrioxalate system. Water Res. 2005, 39, 2893–2900.
- Sedlak, D.L.; Hoigné, J. The role of copper and oxalate in the redox cycling of iron in atmospheric waters. Atmos. Environ. Part. A Gen. Top. 1993, 27, 2173–2185.
- Xue, X.; Hanna, K.; Despas, C.; Wu, F.; Deng, N. Effect of chelating agent on the oxidation rate of PCP in the magnetite/H2O2 system at neutral pH. J. Mol. Catal. A Chem. 2009, 311, 29–35.
- Lati, J.; Meyerstein, D. Oxidation of first-row bivalent transition-metal complexes containing ethylenediaminetetra-acetate and nitrilotriacetate ligands by free radicals: A pulse-radiolysis study. J. Chem. Soc. Dalton Trans. 1978, 1978, 1105–1118.
- De Laat, J.; Dao, Y.H.; El Najjar, N.H.; Daou, C. Effect of some parameters on the rate of the catalysed decomposition of hydrogen peroxide by iron(III)-nitrilotriacetate in water. Water Res. 2011, 45, 5654–5664.
- Lutze, H.V.; Bircher, S.; Rapp, I.; Kerlin, N.; Bakkour, R.; Geisler, M.; Von Sonntag, C.; Schmidt, T.C. Degradation of Chlorotriazine Pesticides by Sulfate Radicals and the Influence of Organic Matter. Environ. Sci. Technol. 2015, 49, 1673–1680.
- Yang, B.; Cheng, X.; Zhang, Y.; Li, W.; Wang, J.; Tian, Z.; Du, E.; Guo, H. Staged assessment for the involving mechanism of humic acid on enhancing water decontamination using H2O2-Fe(III) process. J. Hazard. Mater. 2021, 407, 124853.
- Katsumata, H.; Kaneco, S.; Suzuki, T.; Ohta, K.; Yobiko, Y. Photo-Fenton degradation of alachlor in the presence of citrate solution. J. Photochem. Photobiol. A Chem. 2006, 180, 38–45.
- Xu, J.; Xin, L.; Huang, T.; Chang, K. Enhanced bioremediation of oil contaminated soil by graded modified Fenton oxidation. J. Environ. Sci. 2011, 23, 1873–1879.
- Georgi, A.; Schierz, A.; Trommler, U.; Horwitz, C.; Collins, T.; Kopinke, F.-D. Humic acid modified Fenton reagent for enhancement of the working pH range. Appl. Catal. B Environ. 2007, 72, 26–36.
- Evangelou, M.W.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003.
- Sherwood, M.K.; Cassidy, D.P. Modified Fenton oxidation of diesel fuel in arctic soils rich in organic matter and iron. Chemosphere 2014, 113, 56–61.
- Ouriache, H.; Arrar, J.; Namane, A.; Bentahar, F. Treatment of petroleum hydrocarbons contaminated soil by Fenton like oxidation. Chemosphere 2019, 232, 377–386.
- Jorfi, S.; Rezaee, A.; Moheb-Ali, G.-A.; Jaafarzadeh, N.A. Pyrene removal from contaminated soils by modified Fenton oxidation using iron nano particles. J. Environ. Health Sci. Eng. 2013, 11, 17.
- Innocenti, I.; Verginelli, I.; Massetti, F.; Piscitelli, D.; Gavasci, R.; Baciocchi, R. Pilot-scale ISCO treatment of a MtBE contaminated site using a Fenton-like process. Sci. Total Environ. 2014, 485–486, 726–738.
- Piscitelli, D.; Zingaretti, D.; Verginelli, I.; Gavasci, R.; Baciocchi, R. The fate of MtBE during Fenton-like treatments through laboratory scale column tests. J. Contam. Hydrol. 2015, 183, 99–108.
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res. 2009, 43, 684–694.
- Xu, X.; Thomson, N.R. An evaluation of the green chelant EDDS to enhance the stability of hydrogen peroxide in the presence of aquifer solids. Chemosphere 2007, 69, 755–762.
- Oviedo, C.; Rodríguez, J. EDTA: The chelating agent under environmental scrutiny. Química Nova 2003, 26, 901–905.
- Rämö, J.; Sillanpää, M. Degradation of EDTA by hydrogen peroxide in alkaline conditions. J. Clean. Prod. 2001, 9, 191–195.
- Pinto, I.S.S.; Neto, I.F.F.; Soares, H. Biodegradable chelating agents for industrial, domestic, and agricultural applications—A review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906.
- Kołodyńska, D. Green complexing agent—EDDS in removal of heavy metal ions on strongly basic anion exchangers. Desalination 2011, 280, 44–57.
- Han, D.; Wan, J.; Ma, Y.; Wang, Y.; Huang, M.; Chen, Y.; Li, D.; Guan, Z.; Li, Y. Enhanced decolorization of Orange G in a Fe(II)-EDDS activated persulfate process by accelerating the regeneration of ferrous iron with hydroxylamine. Chem. Eng. J. 2014, 256, 316–323.
- Han, D.; Wan, J.; Ma, Y.; Wang, Y.; Li, Y.; Li, D.; Guan, Z. New insights into the role of organic chelating agents in Fe(II) activated persulfate processes. Chem. Eng. J. 2015, 269, 425–433.
- Zingaretti, D.; Lombardi, F.; Baciocchi, R. Soluble organic substances extracted from compost as amendments for Fenton-like oxidation of contaminated sites. Sci. Total Environ. 2018, 619–620, 1366–1374.
- Xu, J.; Fan, X.; Huang, F.; Li, X. Iron bound to soil organic matter catalyzes H2O2 to oxidize crude oil in soil. J. Hazard. Mater. 2017, 322, 516–524.
- Vicente, F.; Rosas, J.; Santos, A.; Romero, A. Improvement soil remediation by using stabilizers and chelating agents in a Fenton-like process. Chem. Eng. J. 2011, 172, 689–697.
- Jonsson, S.; Persson, Y.; Frankki, S.; van Bavel, B.; Lundstedt, S.; Haglund, P.; Tysklind, M. Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Contaminated Soils by Fenton’s Reagent: A Multivariate Evaluation of the Importance of Soil Characteristics and PAH Properties. J. Hazard. Mater. 2007, 149, 86–96.
- Rosas, J.; Vicente, F.; Santos, A.; Romero, A. Enhancing p-cresol extraction from soil. Chemosphere 2011, 84, 260–264.
- Vicente, F.; Santos, A.; Sagüillo, E.G.; Martínez-Villacorta, Á.M.; Rosas, J.; Romero, A. Diuron abatement in contaminated soil using Fenton-like process. Chem. Eng. J. 2012, 183, 357–364.
- Peluffo, M.; Mora, V.C.; Morelli, I.S.; Rosso, J.A. Persulfate treatments of phenanthrene-contaminated soil: Effect of the application parameters. Geoderma 2018, 317, 8–14.
- Han, L.; Siekmann, F.; Zetzsch, C. Rate Constants for the Reaction of OH Radicals with Hydrocarbons in a Smog Chamber at Low Atmospheric Temperatures. Atmosphere 2018, 9, 320.
- Rosenfeldt, E.J.; Linden, K.G. Degradation of Endocrine Disrupting Chemicals Bisphenol A, Ethinyl Estradiol, and Estradiol during UV Photolysis and Advanced Oxidation Processes. Environ. Sci. Technol. 2004, 38, 5476–5483.
- Mandal, S. Reaction Rate Constants of Hydroxyl Radicals with Micropollutants and Their Significance in Advanced Oxidation Processes. J. Adv. Oxid. Technol. 2018, 21, 178–195.
- Haag, W.R.; Yao, C.C.D. Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ. Sci. Technol. 1992, 26, 1005–1013.
- Lee, C.; Sedlak, D.L. A novel homogeneous Fenton-like system with Fe(III)–phosphotungstate for oxidation of organic compounds at neutral pH values. J. Mol. Catal. A Chem. 2009, 311, 1–6.
- Zhang, C.; Li, T.; Zhang, J.; Yan, S.; Qin, C. Degradation of p-nitrophenol using a ferrous-tripolyphosphate complex in the presence of oxygen: The key role of superoxide radicals. Appl. Catal. B Environ. 2019, 259, 118030.
- Deng, F.; Qiu, S.; Zhu, Y.; Zhang, X.; Yang, J.; Ma, F. Tripolyphosphate-assisted electro-Fenton process for coking wastewater treatment at neutral pH. Environ. Sci. Pollut. Res. 2019, 26, 11928–11939.
- Yehia, F.; Eshaq, G.; ElMetwally, A. Enhancement of the working pH range for degradation of p-nitrophenol using Fe2+–aspartate and Fe2+–glutamate complexes as modified Fenton reagents. Egypt. J. Pet. 2016, 25, 239–245.
- Li, Y.C.; Bachas, L.; Bhattacharyya, D. Kinetics Studies of Trichlorophenol Destruction by Chelate-Based Fenton Reaction. Environ. Eng. Sci. 2005, 22, 756–771.





