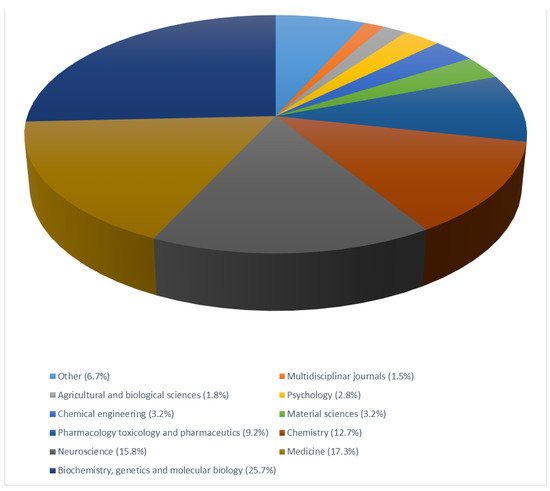
Copper is an essential trace metal controlling human physiology. Brain cells use copper during development, and it is indispensable in vital processes such as respiration, energy production, formation of the myelin sheath around neurons, synthesis of neurotransmitters, immune system responses, collagen and pigment synthesis, and wound healings. We surveyed the number of papers in the medicine database on the biological connection between AD and copper. The search on ‘Scopus’ with the terms “Alzheimer’s disease and copper” provided 3009 document results ().
The stratification by ‘subject area’ of the Scientific Journals of publication can help depict the large areas of scientific articles published on this topic that enclose.
1.1. Copper Connection with Alzheimer’s Disease: Biochemistry Literature[1]
This literature includes Inorganic and Coordination Chemistry and is mostly focused on the interaction of Aβ and the Aβ precursor protein (APP, encoded by the
APP gene) with copper. APP is a copper protein
[2]. Consolidate knowledge indicates that the APP/Aβ system is central for AD pathogenesis, and a recent view proposes that the APP/Aβ system is centrally involved in neuronal copper transport at the synapses and in processes of learning and memory
[3][4][5]. Loosely bound copper, as a transition metal, actively facilitates oxidative stress via Fenton like and Haber Weiss reactions. These reactions have been demonstrated to result in Aβ oligomer formation and their precipitation within plaques along with lipid peroxidation
[6][7][8][9]. Established evidence demonstrated that: APP is a copper protein that binds and reduces copper from Cu(II) to Cu(I)
[2], facilitating copper-induced toxicity in cell cultures and oxidative stress through the production of H
2O
2 [10]; that Aβ and metals are packed together in the brain Aβ plaques
[2][11][12]; that Aβ plaques can be dissolved by chelating agents, which sequester the copper
[11]. Overload of Cu(II)-Aβ molecules are also probable, particularly at the synapses where both are released
[3]: copper ions are released from synaptic vesicles reaching concentrations as high as 15 μmol/L in a form of labile copper, not bound to proteins
[13]. These conditions facilitate Cu-Aβ formation. At the same time, cell-associated copper into neurons could be decreased
[14].
1.2. Copper Connection with Alzheimer’s Disease: Medicine Literature
Most of these studies focused on the comparison of copper levels in diverse organ tissues or biological matrices (e.g., serum, plasma, cerebrospinal fluid, brain, hair, nails). Most of these findings have been evaluated through meta-analyses that reported excess copper in general circulation
[15][16][17] and copper deficiency in the brain
[18]. The picture provided supports the evidence that patients with AD fail to maintain a correct metabolic balance and distribution of copper in the body
[5]. In line with these findings, a recent meta-analysis has attributed the copper excess found in AD serum to the expansion of the fraction of exchangeable Cu
2+ defined as copper not bound to ceruloplasmin (non-ceruloplasmin copper, also referred to as ‘free’ copper)
[16]. This serum fraction is composed of copper loosely bound to albumin, α2 macroglobulin, peptides and amino acids and exchanged among them
[16]. Non-ceruloplasmin copper has been already identified as a marker of Wilson disease, a rare inborn error of copper metabolism and a paradigm of non-ceruloplasmin copper toxicosis and accumulation
[5]. Overall, existing meta-analyses in AD provided results of decreased levels of copper in the brain
[18], along with a non-ceruloplasmin copper increase in serum/plasma
[15][16][17][19][20], that accounts for copper excess in the bloodstream
[21], in line with a number of clinical studies ().
Table 1. Clinical studies analyzing the copper and ATP7B link to Alzheimer’s disease risk and the association with the subjects’ clinical status.
| |
Subjects |
Risk (OR 1, RR 2, HR 3) |
CI 4 95% |
p Value |
| Serum Copper and Risk for Alzheimer’s Disease |
|
|
|
|
| Copper level was higher in subjects with AD than in control subjects and correlated with poor neuropsychological performance and medial temporal lobe atrophy [22] |
76 AD vs. 79 healthy subjects |
1.8 |
1.36–2.43 |
p < 0.05 |
| Copper level was higher in subjects with AD than in patients with vascular dementia subjects (VaD) [23] |
48 AD vs. 20 VaD |
2.06 |
1.28–3.31 |
p < 0.003 |
| Non-ceruloplasmin copper was higher in AD than in healthy controls and VaD and correlated with poor neuropsychological performance [24] |
47 AD vs. 44 healthy subjects and 24 VaD subjects |
|
|
p < 0.001 |
| Non-ceruloplasmin copper was higher in AD than in healthy controls; Cerebrospinal (CSF) β-amyloid and H-Tau correlated with serum non-ceruloplasmin copper; copper in the CSF was partially dependent on the serum Non-ceruloplasmin copper (t = 2.2, p = 0.04). Mini-Mental State Examination (MMSE) and verbal memory scores correlated positively with β-amyloid (r = 0.46, p = 0.002) and inversely with nonceruloplasmin-Cu (= 0.45, p = 0.003) [25] |
28 AD vs. 25 healthy subjects |
|
|
p < 0.001 |
| Non-ceruloplasmin copper predicted the annual change in MMSE; when the annual change in MMSE was divided into <3 or ≥3 points, Non-ceruloplasmin copper was the only predictor of a more severe decline [26] |
81 AD subjects, 1 year longitudinal study |
1.23 |
1.03–1.47 |
p < 0.022 |
| Non-ceruloplasmin copper was higher in MCI than in healthy subjects [27] |
83 MCI subjects, 100 healthy subjects |
1.22 |
1.05–1.41 |
p < 0.01 |
| Copper level showed a significant increase in the serum of AD and MCI compared to control (p = 0.038) [28] |
36 AD, 18 MCI vs. 25 healthy subjects |
|
|
p < 0.05 |
| Non-ceruloplasmin copper increased the risk of having AD; when combined in an algorithm with sex, APOE, Cp/Tf, TAS, the ability to discriminate AD patients vs. controls was high (ROC 5, AUC 6 = 0.9) [29] |
93 AD, 45 VaD, 48 healthy subjects |
3.21 |
1.53–6.71 |
p < 0.002 |
| Non-ceruloplasmin copper was a predictor of conversion to AD: MCI subjects with nonceruloplasmin-Cu levels > 1.6 µmol/L had a hazard conversion rate (50% conversion in 4 years) that was ~3 higher than those with values ≤ 1.6 µmol/L (< 20% in 4 years) [30] |
131 MCI subjects, 6 years longitudinal study |
3.3 |
1.21–9.24 |
p = 0.02 |
| Non-ceruloplasmin copper levels higher in MCI and AD with respect to control (p < 0.0001) [31] |
44 AD and 36 MCI vs. 28 healthy subjects |
|
|
p < 0.001 |
| Non-ceruloplasmin copper and Cu:Cp resulted higher in AD and in Wilson disease (WD) than in healthy controls; while nCp-Cu was similar between AD and WD, Cu:Cp was higher in WD. 24 h urinary copper excretion in AD patients (12.05 μg/day) was higher than in healthy controls (4.82 μg/day); 77.8% of the AD patients under D-penicillamine treatment had a 24 h urinary excretion higher than 200 μg/day, suggestive of a failure of copper control [21] |
385 AD, 9 WD, 336 healthy subjects |
|
|
p < 0.0001 |
| Non-ceruloplasmin copper does not change in frontotemporal lobar degeneration (FTLD) [23] |
85 FTLD, 55 healthy subjects |
|
|
p < 0.001 |
| ATP7B Gene Variants and Risk for Alzheimer’s Disease |
|
|
|
|
| Specific genetic variants in the ATP7B gene, namely rs1801243 (OR = 1.52, 95% CI = 1.10–2.09), rs2147363 (OR = 1.58, 95% CI = 1.11–2.25), rs1061472 (OR = 1.73, 95% CI = 1.23–2.43), and rs732774 (OR = 2.31, 95% CI = 1.41–3.77) increased the risk of having AD [32] |
285 AD vs. 230 healthy subjects |
2.3 |
1.41–3.77 |
p < 0.001 |
| Wilson disease-causing variant rs7334118 in linkage disequilibrium with the intronic rs2147363 (associated with AD risk) was detected in two AD patients but in no healthy individuals. However, this Wilson disease mutation did not explain the observed genetic association of rs2147363. Conversely, in silico analyses of rs2147363 functionality highlighted that this variant is located in a binding site of a transcription factor and is associated with regulatory functions [33] |
286 AD vs. 283 healthy subjects |
1.3 |
1.06–1.69 |
p = 0.015 |
| Haplotype TGC in specific genetic variants in the ATP7B gene, namely rs1801243, rs1801249, rs1801244, and rs1801243 was associated with an increased risk of having AD [34] |
120 AD vs. 111 healthy subjects |
5.16 |
2.54–10.5 |
p < 0.001 |
Clinical studies also provided evidence of copper association with the severity of the disease in terms of performance in neuropsychological test batteries
[25][35], disease stage
[22][27][30][31] and electroencephalography (EEG) brain rhythms alterations, atrophic and cerebrovascular burden
[3][36].
In the AD brain, the progressive increase of the labile copper pool is consistent with the parallel presence of an expanded pool of non-ceruloplasmin copper in the blood
[25][37]. Copper disturbance in AD can be described by a loss of functional copper from protein-bound pools that reduces energy production and oxidative stress control, and a gain of redox-toxic function that is described by a bigger pool of copper loosely bound to proteins
[3]. Non-ceruloplasmin copper increases the susceptibility to AD approximately threefold
[29][30] and it is also associated with a higher frequency of specific variants of the
ATP7B gene
[32][33][34][38][39][40][41][42][43][44][45][46][47][48] (). The gene variants rs1061472 and rs732774 of
ATP7B are single nucleotide polymorphisms (SNPs) that modify properties of ATPase7B protein and are associated with a higher risk of AD and with the presence of a higher fraction of non-ceruloplasmin copper in serum
[25][42][45].
1.3. Copper Connection with Alzheimer’s Disease: Neuroscience
Several scientific articles evaluating measurements of electrical activities in cell culture models have been included in this category
[4][49]. A bulk of evidence in this category comes from studies in experimental models focused on the investigation of the causative correlation of copper and non-ceruloplasmin copper in AD development and progression ()
[50][51].
Table 2. Experimental models focused on the investigation of the causative correlation of copper and non-ceruloplasmin copper in Alzheimer’s disease (AD) development and progression.
| Authors, Year |
Animal Model |
Dose and Route |
Duration |
Effects |
| Animal Models of Copper Neurotoxicity Induced by Altered Diet |
| Sparks and Schreurs, 2003 [52] |
New Zealand white rabbits |
12 mg/L copper in DW 1 + 2% cholesterol − oral |
10 weeks |
Accumulation of Aβ in brain; deficit in complex memory acquisition |
| Sparks et al., 2006 [53] |
Beagle dogs |
200 mg/L CuSO4 in DW + high fat diet − oral |
4 months |
Extracellular Aβ deposits |
| Lu et al., 2006 [54] |
Kumming strain mice |
0.21 mg/L copper in DW + 2% cholesterol − oral |
8 weeks |
Cognitive deficits; neuronal apoptosis |
| Arnal et al., 2013 [55] |
Wistar rat |
3 mg/L copper in tap water + 2% cholesterol − oral |
2 months |
Increased oxidative stress in brain; increased non-ceruloplasmin copper in hippocampus; increased Aβ (1–42)/ Aβ (1–40) in cortex and hippocampus |
| Arnal et al., 2013 [55] |
Wistar rat |
3 mg/L copper in tap water + 2% cholesterol − oral |
8 weeks |
Slight nut noticeable change in visuo-spatial memory |
| Yao et al., 2018 [56] |
Tg2567 mouse |
0.1 mg/L copper in drinking water and 2% cholesterol in the food |
3 months |
Significant deposit of Aβ and senile-plaque formation in hippocampus and temporal cortex regions |
| Abolaji et al., 2020 [57] |
D. melanogaster flies |
Cu2+ (1 mM) |
7 days |
reduced survival |
| Lamtai et al., 2020 [58] |
Rat |
CuCl2 (0.25 mg/kg, 0.5 mg/kg and 1 mg/kg) injected intraperitoneally |
8 weeks |
Working memory, spatial learning and memory were significantly impaired in rats treated with Cu at dose of 1 mg/kg |
| Models of Copper Neurotoxicity in Genetically Compromised Animals |
| Sparks et al., 2006 [53] |
Watanable rabbits |
0.13 mg/L copper in DW − oral |
10 weeks |
Accumulation of Aβ in superior temporal cortex and hippocampus |
| Sparks et al., 2006 [53] |
PS1/APP transgenic mice |
0.12 mg/L Cu in DW − oral |
6 weeks |
Deposition of Aβ |
| Singh et al., 2013 [59] |
APP sw/0 mice |
0.13 mg/L copper in DW − oral |
90 days |
Increase brain Aβ production; increased neuroinflammation; memory impairment; increased Cu levels in brain capillaries and parenchyma |
| Yu et al., 2014 [60] |
3xTg-AD |
250 mg/L CuSO4 in drinking water |
6 months |
memory impairment |
Huat et al.
[61], in a recent review, stated that “considering the robust evidence for copper’s essential roles in the brain, it is not surprising that many studies have proposed that an imbalance in its homeostasis is associated with neurodegenerative disorders”.
In a seminal study published in 2003, Sparks and Schreurs demonstrated that in a cholesterol-fed rabbit model of AD, adding trace amounts of 0.12 mg/L copper to distilled drinking water resulted in significantly enhanced cognitive waning. It also exacerbated Aβ plaque deposition to that of control animals
[52]. The study by Singh et al. provided a specific emphasis on the causative role that non-ceruloplasmin copper might play in AD onset and progression
[59]. Singh et al.
[59] studied normal mice (wild type) and a mouse model of AD (AβPP transgenic mice) exposed to 0.13 mg/L of copper sulfate for 90 days levels of copper via drinking water, which doubled plasma concentrations of non-ceruloplasmin copper. This fact caused either a reduction of CSF Aβ clearance across the blood brain barrier in wild-type mice or an identical effect, along with an increase in Aβ production in the transgenic mice. Thus, proposing the concept that non-ceruloplasmin copper is a causative risk factor for AD.
Thus, the relationship between copper and AD has been extensively researched in recent years.
Chronic exposure to copper and its dyshomeostasis has been linked to accelerate cognitive decline and potentially to increase the risk of AD
[4][21][62]. However, copper ions due to their redox ability have been considered to be the main potential therapeutic targets in AD, and a considerable number of ligands have been developed in order to modulate the toxicity associated with copper in this context, via disruption of the Aβ-copper interaction
[63].
2.1. Copper Used in Plant Disease Management
Copper has been used in agriculture as a fertilizer and in the management of plant diseases. Organic agriculture is very dependent on copper as a fungicide. Several fungicides have copper in their formulation. The first fungicide to be used in all cropping systems worldwide and most famous there is the Bordeaux mixture (25% CuSO
4). The Bordeaux mixture and, consequently, copper has been used in agriculture for more than 160 years in the management of plant diseases
[64]. Fishel
[65] stated that, during the 1850s in the Bordeaux region in France, a vineyard farmer was having trouble with people stealing grapes from his vines. He applied a mixture of copper and lime to part of his vineyards to make the grapes unattractive. The result was that in the plants where the copper-lime mix was applied, there was no plant disease incidence.
Nowadays copper is mostly used to control the following plant diseases: Grape downy mildew, caused by the
Plasmopara viticola, which is a highly damaging disease for grapes, particularly in oceanic climates; Apple scab, caused by the
Venturia inaequalis; Potato late blight, caused by the
Phytophthora infestans, responsible for a severe disease affecting potato production. In tropical regions, there is an occurrence of the Coffee Rust Disease caused by
Hemileia vastatri and the cocoa Witches’ Broom Disease, caused by
Crinipellis perniciosa [66][67][68].
The Bordeaux mixture is widely used in organic agriculture worldwide since it is considered to have low toxicity for humans and the environment. Also, other fungicides containing copper in the forms of hydroxide, oxychloride, oxide, and octanoate, can be used in Organic Agriculture. However, they need authorization from the certifiers of organic products to minimize the accumulation of copper in the soil
[69]. In Brazil, the recommendation follows specific legislation similar to that proposed by FAO
[70][71][72].
In Europe, during the 1950s, copper in quantities of 20 to 30 kg ha
−1 year
−1, and sometimes even more than 80 kg ha
−1 year
−1, was applied to crops to protect the plants. In Germany, between 2010 and 2015, on average, in organic farming of hops, grapes, potatoes, apples, squash, and pears copper amounting to 3.1, 2.2, 1.5, 1.5, 1.4, and 1.3 kg ha
−1 yr
−1, respectively, was used. In that country, the application of copper is restricted to 3 kg ha
−1 yr
−1 (4 kg ha
−1 yr
−1 for hops)
[73]. In 2013, a survey investigating copper use in Germany
[74] revealed that the amounts of copper used per hectare in conventional grape (0.8 kg ha
−1), hop (1.7 kg ha
−1), and potato-farming (0.8 kg ha
−1) were well below those used in organic farming for the same crops (2.3, 2.6, and 1.4 kg ha
−1, respectively).
The United States Department of Agriculture (USDA)
[75] included several copper-based substances in ‘The National List of Allowed and Prohibited Substances’ in organic agriculture in the United States of America (USA). For instance, copper sulfate is as an algicide in aquatic rice systems and used as tadpole shrimp control in aquatic rice production. Its application is limited to one application per field during any 24 months, and application rates are limited to those which do not increase baseline soil test values for copper over a timeframe agreed upon by the producer and the accredited certifying agent. The USA’s legislation indicates that copper-based materials must be used in a manner that minimizes accumulation in the soil and shall not be used as herbicides.
According to Brazilian law
[70][71][72] and FAO recommendation
[69], the maximum amount of copper to be applied in organic agriculture is 6 kg ha
−1 yr
−1. According to Motta
[76], in Brazil, some certifiers limit the use of the element to 3 kg ha
−1 yr
−1. On the other hand, in East Africa, 8 kg ha
−1 yr
−1 is the maximum allowed the annual copper application in areas with organic agriculture
[77][78].
Formulation of Bordeaux mixture, for use in fruit trees, contains 2–10 g/L of copper sulfate and the same amount of lime (Ca(OH)
2) diluted in water. The application is sprayed from the vegetative phase until the fruit maturation, with intervals of 10 to 15 days between applications
[79]. Natural adhesive spreaders, such as sugar (10–15 g) or skim milk (200 mL), can be used for better adherence to plant leaves
[76].
For the grapevine, for example, there is no fixed volume of Bordeaux mixture to be used per hectare, which can vary between 150–700 L ha
−1. This volume varies according to several factors, such as the type of sprayer, the size of the plants, the distance between rows of plants, the climatic conditions, the disease to be controlled, and the vegetative stage of the plant
[80]. Thus, considering that the smallest application volume (150 L ha
−1) has the highest copper content (10 g/L), the highest volume (700 L ha
−1) and the lowest content (2 g/L), a maximum of 16 applications per year can be performed in Brazil, so that the maximum annual copper dose of 6 kg ha
−1 yr
−1 is not exceeded, as recommended by FAO
[69].
2.2. Copper Accumulation in Soil and Water
In general, the copper ion is very immobile in soil. Therefore, continuous copper spraying results in the accumulation of this element in the topsoil, reaching toxic levels, possibly causing plant stress, decreasing the soil microbiota biodiversity, and reducing soil fertility
[81][82][83]. That is the main reason why organic farmers try to minimize copper use
[73].
Sacristán and Carbó
[84] studied Spanish and Australian agricultural soils cropped with lettuce. They found that soils with higher pH and higher levels of organic matter and clay result in lower copper mobility in-depth, and therefore copper accumulates in the topsoil layers. However, in general, the toxic effect of copper in plants increases as pH values decrease, due to a rise in the copper bioavailability. On the other hand, in a study conducted in an agricultural region of Haining County in southeast China, Wu et al.
[85] found that the copper availability ratio and available copper concentration were decreased as a function of decreasing pH in acid soils (pH < 6.5), and increased with increasing pH in alkali soils (pH > 7.5). In Chile, Ávila et al.
[86] found that copper toxicity for earthworms was lower in soils with a higher than 3.5% organic matter (OM) content, likely due to the change in bioavailable copper ions. The mitigating effect of OM was exact for soils with up to 500 mg kg
−1 of copper.
The copper exists in soils mainly (60%) water soluble, exchangeable and sorbed forms of total copper in the upper part of soil profiles and the percentage decreases with increasing depth
[87]. In Australia, total copper content in soil of Victorian vineyards is five to fifty times as high as that found in natural soils. Moreover, copper content in soil decreases with increasing distance from vines
[87].
Soil copper concentration of 100 mg kg
−1 influences rice growth, and 10% or more of grain yield, straw weight, and root weight were lost
[88]. Besides copper use for plant protection, the use of pig slurry can contribute to copper accumulation in the soil. The tillage system determines the distribution of copper in the soil layers and can be used as a tool to avoid accumulation in the topsoil
[89].
Copper release into water occurs through soil erosion, industrial discharge, sewage-treatment, and antifouling paints. Thus, erosion of soils that contain soil particles with adsorbed copper can result in increased copper concentrations in rivers and lakes
[90]. Certainly, there is generally no contamination of the groundwater table due to copper applications in agriculture. Copper has a low mobility in the soil and its mobility decreases with increasing clay or organic matter content
[84].
At present, the main concern is the increase of copper content during the distribution of drinking water, because many pipes and plumbing fixtures contain copper, which can leach into the drinking water
[90]. Certainly in the last few years the use of PVC pipes instead of copper pipes has reduced the concentration of copper in the water. However, this is a problem that still persists in old houses that have copper plumbing for heated water, because this water is often used to cook food.