
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos Rial | + 8122 word(s) | 8122 | 2021-06-15 08:48:28 | | | |
| 2 | Carlos Rial | -2449 word(s) | 5673 | 2021-06-16 18:44:41 | | | | |
| 3 | Conner Chen | Meta information modification | 5673 | 2021-06-17 11:20:33 | | | | |
| 4 | Conner Chen | Meta information modification | 5673 | 2021-06-17 11:28:16 | | | | |
| 5 | Conner Chen | Meta information modification | 5673 | 2021-06-17 11:29:20 | | |
Video Upload Options
Aminophenoxazinones are degradation products resulting from the metabolism of different plant species, which comprise a family of natural products well known for their pharmacological activities. Aminophenoxazinones are tricyclic structures with double bonds in aromatic systems containing oxygen and nitrogen atom, which facilitates the development of synthetic derivatives to enhance the properties of these molecules. Aminophenoxazinones possess a number of promising properties like anticarcinogenic, antifungal, antiparasitic, antibacterial or antimicrobial activities.
1. Aminophenoxazinones as Degradation Products of Benzoxazinones
Some plant species, mainly those from the Gramineae family, which comprises prominent crops such as wheat, rye or maize, produce benzoxazinones like the cyclic hydroxamic acids 2,4-dihydroxy-1,4-benzoxazolin-3-one (DIBOA) and 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) (Figure 1). Those, together with different degradation metabolites, are well known for their biological activities[1][2]. In fact, the potential benefits of the intake of food containing benzoxazinones would be in agreement with the time that they remain inside the human body, with dose-dependent absorption (peak in plasma after 3 h) and rapid washout[3]. Some examples of these highly interesting compounds with these structures are 2-hydroxy-1,4-benzoxazin-3-one (HBOA), which is proposed as the biosynthetic precursor of DIBOA and DIMBOA[4], as well as 2-hydroxy-7-methoxy-1,4-benzoxazin-3-one (HMBOA), a degradation product of DIMBOA exuded by wheat [5]. A study of a diet based on rye bread revealed that the 2-β glycosylated derivatives of HBOA and DIBOA are the major benzoxazinoids in plasma and urine[6]. The concentrations measured for all the detected compounds in this study would provide key information for understanding their bioavailability, absorption and metabolism. In fact, DIBOA has shown inhibitory effects on the growth of cancer lines like DU145 (prostate), which motivated the development of in vivo studies on human and the intake of enriched food[7].
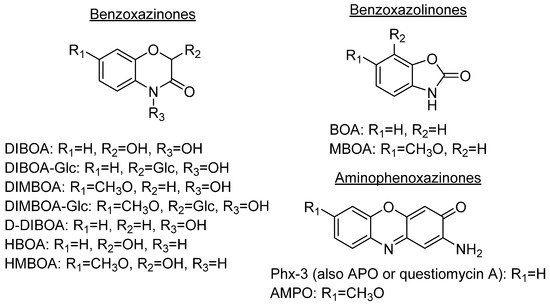
Figure 1. Prominent benzoxazinones and some of their degradation products.
Because of the diversity of products that can be obtained from the degradation of hydroxylated benzoxazinones depending on their cultivation soil [8] or the extraction method, which could be either by submerging them into aqueous solutions[9] or by putting them in contact with certain microorganisms[10], they have always been a focus of interest.
DIBOA and DIMBOA can be detected inside plants as their β-D-glucosides DIBOA-Glc and DIMBOA-Glc. β-D-Glucosides are transformed into DIBOA and DIMBOA when plants are damaged, by enzymatic mechanisms catalyzed by a β-glucosidase (Scheme 1). The latter compound has also been detected as the major benzoxazinone in Triticum aestivum L., whereas DIBOA-Glc is mainly concentrated in S. cereale L.[11]. DIMBOA is unstable in soil and spontaneously transforms into 6-methoxybenzoxazolin-2-one (MBOA), and subsequently turns into 2-amino-7-methoxy-3H-phenoxazin-3-one (AMPO) through the action of edaphic microorganisms when submerged into aqueous solutions[12]. This fact could explain the higher resistance of AMPO to biodegradation. A similar case occurs with the analogous DIBOA and its benzoxazin-2(3H)-one (BOA) that turns into to Phx-3 (also known as APO or questiomycin A). However, DIBOA has a high persistence and could have a more important role in chemical defence mechanisms than DIMBOA in plants that produce large amounts of hydroxamic acids[9].
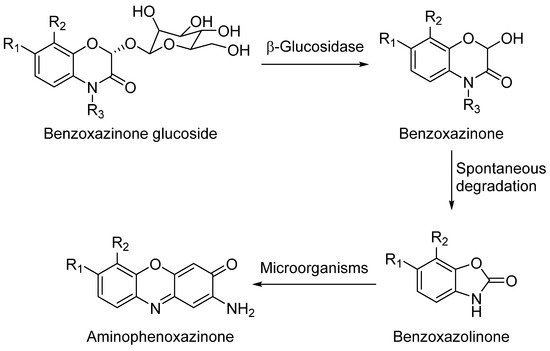
Scheme 1. General degradation process of benzoxazinones.
In comparison to their precursors with benzoxazinone structures, aminophenoxazinones like Phx-3 are much stable and easier to extract. These advantages, which have enabled the development of numerous studies on their biological activities[11] and modes of action[1] have also facilitated their production for commercial purposes. In the case of Phx-3, Venturelli et al. demonstrated that its phytotoxic effects against the growth of certain plants (like the model species Arabidopsis thaliana) are related to the inhibition of histone deacetylases, which provokes locus-specific alterations in histone acetylation processes[13]. In certain agronomical contexts, some studies like this or the conducted by Macías et al. reflect how lower concentrations of Phx-3 successfully achieve high phytotoxicity levels[14]. Another interesting phytotoxic-related compound is D-DIBOA, which has been proposed as an excellent candidate for the development of new herbicides[15].
The degradation process of benzoxazinones functionalized with hydroxamic acid groups leads to benzoxazolinones[9]. BOA and DIBOA, from benzoxacinone-producer plants like maize[16], wheat[17] or rye[18] are the most representative isolated compounds obtained until now.
Aminophenoxazines are an interesting family of tricyclic compounds related to the previously mentioned benzoxazinones and benzoxazolinones. They are originated as a result of the decomposition of benzoxazolinones into their corresponding 2-aminophenol and its subsequent dimerization.
A wide variety of aminophenoxazines has been developed because of their pharmacological interest (and also agronomical). Their antiviral[19], antitumoral[20] and antibiotic[21] activities have been studied by those methods that apply the dimerization of two phenolic units into a tricyclic compound. A process that depends not only on the phenol itself, but also on the reaction conditions. In the case of Phx-3, its dimerization was accomplished through a hydrometanolic solution[22] combined with the use of photochemistry[23]. Different aminophenoxazines have been obtained by applying this method to alkyl, halo or nitroaminophenols.
The chemistry of phenoxazines (Figure 2), the scaffold of aminophenoxazines, has attracted the interest of researchers for more than a century because of their wide range of applications in the manufacturing industry and in medicine. Phenoxazine was first prepared in 1887 by thermal condensation of o-aminophenol with catechol[24]. Since then, several studies have developed new derivatives of phenoxazine, proving their effectiveness as drug compounds. The study of Prinz et al. is a recent example, where a new family of N-heterocyclic (4-phenyl-piperazin-1-yl)methanones derivatives showed excellent inhibition growth activities against different tumor cell lines, being remarked the activity levels of compound 1 (Figure 2)[25].
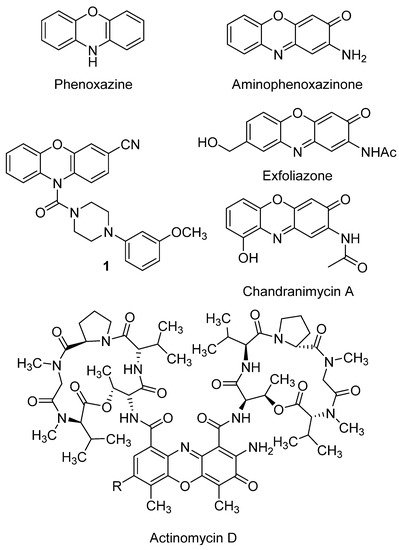
Figure 2. Phenoxazine and aminophenoxazinone structures, and some molecules of their different derivatives, like the drug actinomycin D.
It is worth highlighting the great number of articles published in recent years about new phenoxazine derivatives with applicability for detecting specific compounds related to diseases. For example, new fluorophores containing two molecules of phenoxazine (or phenothiazine, where the oxygen atom is replaced by a sulfur one) have been developed for in vivo tumor detection by formation of fluorescent polymer nanoparticles[26]. Similarly, BC-2 and BC-3 are compounds based on a phenoxazine scaffold, which have been successfully tested in vivo to detect hypochlorous acid, compound whose overproduction is related with diseases like atherosclerosis or rheumatoid arthritis[27].
Aminophenoxazines (Figure 2) were first applied as industrial colorants and pigments. Further properties and their corresponding applications such as antioxidants[28], polymerization stabilizers, pesticides, insecticides[29], biological markers[30], acid-base indicators[31] and, with more relevance, drugs[29] were gradually found.
The increasing number of the studies that demonstrated the pharmacological properties of aminophenoxazines made the main focus of attention to move from its use as a dye to the biological field. In fact, these tricyclic compounds are the structural base of several pharmacotherapeutic agents with a wide range of biological properties.
A recent study on the biological activities of certain synthetic aminophenoxazinones rendered highly promising results regarding the development of new drugs with antibacterial, antifungal, anticancer, reversal resistance as well as anti-inflammatory, antiviral or antifilarials[29]. Moreover, aminophenoxazine is the base of actinomycin D, a well-known antibiotic obtained from Streptomyces antibioticus[32]. This antibiotic can present potent high anticancer activity, but also has some side effects that must be taken into account, such as myelosuppression or renal cytotoxicity[33]. Actinomycin D currently continues being studied. As an example of recent positive result, the increase of effectiveness for ovarian cancer treatment found for its synergic use with photodynamic therapy can be mentioned[34].
The study of structure-activity relationships (SAR) makes possible to define optimal structural characteristics to improve the properties of the molecules. That is the case of the natural product chandrananimycin A (Figure 2), whose hydroxyl group was related with the better cytotoxicity levels of the compound against HeLa cell line (IC50 = 8.87 ± 3.49 µM)[35]. A second example of SAR is the conducted by Pasceri et al. which proved that the electron-withdrawing carboxylate group is associated to a moderate activity of a new a family of synthetized Phx-3 derivatives (including the natural products chandrananimycin A and exfoliazone, Figure 2) on the inhibition on indoleamine 2,3-dioxygenase (an enzyme that is associated to the suppression of the immune response in some forms of cancer)[36]. More in depth studies focused on SAR are required to provide firm conclusions, being usual that the SAR conclusions provided in the bibliography might be supported by additional studies which evaluate a wider number of related structural derivatives.
2. Anticancer Activity
In general terms, cancer is an uncontrolled cellular division process that provokes the aggregation of cells and, subsequently, the formation of tumors. A number of additional factors such as genetic mutations, improper diet, smoking or the ingestion of heavy metals and other contaminants may also have some influence on this process by altering the signaling pathways[37][38]. The following sections explain in detail the effect of aminophenoxazinones on different types of cancer. The compounds Phx-1, Phx-2 and Phx-3 (Figure 3) have been the most studied aminophenoxazines. Their use as anticancer drug aims at the generation of apoptosis of cancer cells, by altering or generating specific mechanisms.
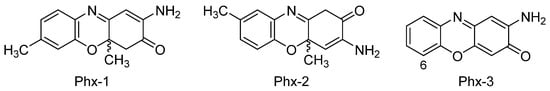
Figure 3. Most studied aminophenoxazinones: 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazin-3-one (Phx-1), 3-amino-1,4α-dihydro-4α,8-dimethyl-2H-phenoxazin-2-one (Phx-2) and 2-aminophenoxazine-3-one (Phx-3).
2.1. Gastric and Colon Cancer
Gastric cancer is the third cause of cancer-related death worldwide and a common pathology with a high incidence rate[39], being nowadays a great clinical challenge. Three treatment methods are commonly performed: surgery, endoscopic resection and chemotherapy[40]. Unfortunately, these treatments do not guarantee a satisfactory level of effectiveness, even if their results are rather promising, for example due to the risk of metastasis. On the other hand, the use of different types of synthetic drugs have shown unpredictable side effects. Despite the advances in the understanding of the pathophysiological mechanisms and in the treatment of gastric cancer in recent years, no significant changes have been achieved yet[41] because of no significant alternatives have been established, especially for patients with delayed diagnoses[42].
The intracellular pH (pHi) varies in accordance with the acidification of the extracellular medium as homeostatic response. However, cancer cells do not decrease this pHi as expected, despite of tumors lead to the acidification of the extracellular pH. The regulatory system commonly concluded to be related is the membrane protein Na+/H+ exchanger called NHE-1[43]. Several studies have suggested that a decrease in pHi may precede apoptotic events in cancer cells. Therefore, drugs with ability to induce these reductions are considered optimal candidates to treat cancer by triggering programmed death of the cells[44]. Some examples of such bioactive compounds are etoposide, cycloheximide or camptothecin. However, the three of them have significant adverse effects on the host[45][46].
2-Aminophenoxazin-3-one (Phx-3) in particular, stands out according to the numerous studies that have proven its effectiveness to change the pHi of different cancer cell lines[47]. In the study of Che et al., the treatment of KB-31 (squamous carcinoma) and K562 (chronic myeloid leukemia) human cell lines (both with a pHi much higher than normal cells) with Phx-3 significantly decreased the pHi (up to 0.9 units) after 20 min. Phx-1 also generated a pHi decrease, but less marked. Authors have compared the activity of both aminophenoxazinones with that of camptothecin, being this a positive result due to the low or null adverse effects found for Phx-1 or Phx-3 in different studies[48][49]. However, the mechanism that causes the pHi decrease in these cells lines when Phx-3 or Phx-1 are administrated is not clear. Therefore, it becomes necessary to determine if a pHi decrease precedes the apoptosis of cancer cells and its relation with the activity of NHE-1.
Kasuga et al.[50] determined that the growth of the gastric cancer cells MKN45 and KATO III is inhibited by treatment with Phx-1 or Phx-3, whereas the apoptotic mechanism would be associated with a caspase-independent pathway. The changes of pHi caused by Phx-3 in gastric cancer cells were not investigated until 2011[51], when the effect of Phx-3, associated with NHE-1, on gastric cancer cells was investigated in terms of how they affected pHi, as well as with regard to the possible causal correlation between a decrease in pHi and the appearance apoptotic events. The method of Litman et al.[52] was used to estimate the pHi values by fluorescence. The results confirmed that the treatment of cancer cells with Phx-3 produces a rapid decrease in pHi depending on the dose administered: by 1.65 units for MKN45 cells and by 1.3 units for MKN74 cells after the administration of a 100 µM solution of Phx-3. This dose also suppressed NHE-1. These results suggested that Phx-3 provokes a decrease in pHi that precedes the apoptotic events, whereas the mechanism for inhibiting NHE-1 was associated with the interaction of Phx-3 with the lipid layers by altering the lipid properties of the cells’ membranes. Moreover, Phx-3 causes a significant depolarization of mitochondria, which activates caspases and influences apoptotic events in cancer cells. For further insight on Phx-3 mode of action against gastric cancer (as well as against colon cancer, presented hereunder), please, see the review by Tomoda et al. [53].
Colorectal cancer (colon and rectal altogether cancer) is the third most frequent cancer and the second leading overall cause of cancer deaths[54][41]. The in vitro activity of Phx-3 in human colon cancer cell lines and specifically on COLO201, DLDI and PMCO1 lines has been determined[55]. The results demonstrated that this aminophenoxazinone causes high levels of cytotoxicity at low doses (IC50 value between 6–12 μM) and pro-apoptotic effects in these lines. Phx-3 also generates cytotoxic effects on HT-29 (IC50 = 16.7 μM)[56] and LoVo-1 lines (IC50 = 20.03 ± 4.98 μM)[57]. In the same studies, Phx-1 has also shown activity against all colon cancer lines mentioned, although to a lesser extent.
The review of Tomoda et al.[53] gathered information about indirect anticancer activity of Phx-3, being worth highlighting at this point the high suppressive activity found in vitro[58] to generate the anion superoxide in neutrophils. The overproduction of this anion is related with ulcerative colitis, a disease associated with colon cancer.
Thus, studies like the ones detailed in this section support that Phx-3 would be an accurate agent itself to treat gastric and colon cancers. Regarding in vivo evaluations of adverse effects, experiments in which mice were treated with (orally administered) Phx-3 showed that, in addition, this compound causes low adverse effects, since they did not show gastrointestinal lesions or diarrhea during 4 weeks of administration[59].
Even though the Phx family (mainly Phx-3) has been the most studied, it is possible to find evaluations of other derivatives. This is the case of N-(2-hydroxyphenyl)-2-phenazinamine, known as NHP (Figure 4), a secondary metabolite isolated for the first time in 2012 from a marine actinomycete[60]. This molecule possesses the tricyclic scaffold of Phx-3, bonded to a fourth aromatic ring (a phenol). Besides, in comparison to Phx-3, NHP contains an additional nitrogen atom in the second ring (in substitution of the oxygen), and lacks of the carbonyl group. This new molecule showed high cytotoxic activity against the colon adenocarcinoma line HCT116 (IC50 = 27.82 µg/mL). Moreover, NHP was active against other human lines: COC1 (ovarian cancer, IC50 = 28.11 µg/mL), HepG2 (liver hepatocellular carcinoma, IC50 = 40.33 µg/mL) and A549 (lung adenocarcinoma, IC50 = 38.53 µg/mL). At comparing the activity of NHP with that found for Phx-3 against two of these lines in other studies, it is worth highlighting that the corresponding IC50 value of NHP is significantly higher than that shown by Phx-3 against A549 (5.48 ± 0.38 µg/mL), whereas a value of 6.58 ± 0.61 µM was obtained for the HepG2 line. On the other hand, the value of NHP against A549 improves that of the aminophenoxazinone Phx-1 (78.29 ± 5.11 µg/mL) [57][61]. The comparison of these results with the structure of the three compounds (NHP, Phx-1 and Phx-3) suggests that a deep structural-activity relationships study would be necessary to determine which structural characteristics provokes the differences between the mentioned activity levels.
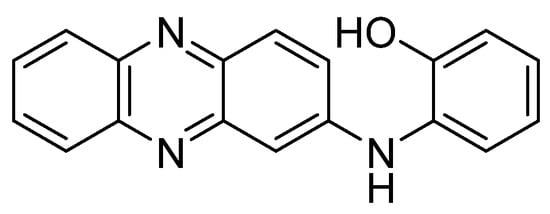
Figure 4. Structure of N-(2-hydroxyphenyl)-2-phenazinamine (NHP).
2.2. Glioblastoma
Glioblastoma is the most common type of aggressive brain tumor in adults, whose treatment is considered a high challenge for oncology since surgery, chemotherapy or radiotherapy are not enough to prevent the tumor progression. One of the main difficulties is achieving the cross of sufficient doses of the chemotherapeutic agents through the blood-brain barrier[62]. The ability of some phenoxazine and phenothiazine derivatives to cross this blood-brain barrier was discovered[63]. This represents an invaluable potential reason for these compounds to be used as anticancer drugs for the treatment of glioblastoma. The anti-tumor activity of phenothiazines was lower, though it should be noted the effectiveness of these compounds as drugs for the treatment of psychotic disorders[64].
Glioblastoma is a complex disorder that appears as a response to a particular form of cellular damage by which cells’ signaling pathways get altered. Cell differentiation is then affected, and as different cell growth factors are over-expressed, the glioblastoma cells get activated and the oncogenesis process, the process by which a normal cell becomes a carcinogen, is promoted[65]. Two of the signaling pathways most related to growth factors, and along with to oncogenesis, are the phosphatidylinositol 3 kinase (PI3K/AKT) and the RAS/RAF/MAPK (ERK) [66]. AKT is a protein kinase, frequently hyperactivated in tumors, with the capacity to deactivate certain proapoptotic factors (like caspase-9) while favoring the expression of anti-apoptotic genes. Moreover, it deactivates certain tumor suppressor genes through phosphorylations, which makes glioblastoma tumors susceptible to AKT[67]. In the case of ERK, its activation is associated with cell survival, proliferation, and death[68]. The protein c-jun N-terminal quinase (JNK) is also involved in pro-apoptotic. It is activated when subjected to different types of stress such as the exposure to anticancer drugs, osmotic stress or radiation[69].
Focusing on the aminophenoxazinone Phx-3, Che et al.[70] studied its apoptosis-inducing effect on LN229 glioblastome cell line. The cell growth was significantly inhibited by Phx-3 at 1 µM, being strongly inhibited at concentrations above 2 µM. The IC50 values were 2.602 ± 0.087 µM (24 h) and 1.655 ± 0.093 µM (48 h), which confirms the potent inhibitory effect of Phx-3 at low concentrations against LN229 cell line, being one of the lowest values ever measured until 2013. As an example of the previous study on the effect of Phx-3 on glioblastoma cell lines, let us point out the remarkable cell growth inhibition confirmed for U251MG cell[71].
Che et al. also clarified the molecular mechanisms of the apoptosis induced by Phx-3, by attending to the ERK and JNK signaling pathways. The levels of these phosphorylated pathways increased significantly (6 h) after treatment with Phx-3, being maintained for 20 h. The activation of JNK was suggested to be mediated by the generation of reactive oxygen species (ROS). On the contrary, the phosphorylation of AKT and mTOR (protein kinase that acts as a signal transducer) were suppressed. All these effects would contribute with the apoptosis induced by Phx-3, and the relevance of their roles was studied by the evaluation of the activity of Phx-3 in the presence or absence of specific inhibitors. The results showed as the phosphorylation of ERK would not represent a major pathway for the induction of apoptosis, whereas AKT would act under ERK signaling. In the case of JNK, its activation would play a key role in the induction of apoptosis. Long exposure to Phx-3 would activate alternative apoptotic mechanisms by mitochondrial demoralization, like the inhibition of mTOR activity.
Previous studies confirmed that treatment of the lung adenocarcinoma cell line A549 with Phx-3 resulted in the depolarization of the mitochondria, causing a decrease of pHi and the generation of ROS[72]. The suppression of the pathway of interest, JNK, would significantly protect against the induction of apoptosis by ROS. Therefore, the generation of ROS by mitochondria in LN229 cells was also examined by Che et al.[70], in the presence or absence of ROS scavengers (melatonin or Nac). Fluorescence values increased after 3 h of exposure to Phx-3 in a dose-dependent manner, whereas the ROS production was significantly inhibited in the presence a scavenger. Melatonin almost completely prevented the induction of apoptosis by Phx-3, and deactivated the JNK pathway. Regarding the other pathways, the application of Phx-3 in presence of a ROS scavenger restored the phosphorylation state of ERK and AKT to the control levels, and inhibited the phosphorylation of mTOR.
In summary, all the information gathered indicates that Phx-3 depolarizes mitochondria and decreases pHi, which causes the generation of ROS in mitochondria. Then, ROS activates the JNK pathway, which is a signaling pathways highly related to glioblastoma, and also the main generator of apoptosis in cancer cells by caspase cascade. The long exposure to Phx-3 would activate alternative mechanisms.
2.3. Melanoma
The incidence of melanoma has increased at a higher rate than other solid tumors, being currently the third most common skin cancer. When at advanced stages it represents a serious threat when it spreads to internal organs, which is the major cause of death in skin-cancer patients. This disease may be due to different causes, including both genetic and environmental factors. Several factors have already been identified as significant influencers on both the incidence and the clinical and oncogenic characteristics of this disease. These factors are UV exposure, use of tanning beds, a family history of melanoma, and phenotypic characteristics of fair skin and hair color[73][74].
Phx-3 has been considered as effective for inhibiting the proliferation of malignant B16 melanoma cells in mice in vitro and in vivo, causing few adverse effects [49]. In the in vitro assay, B16 cells were transplanted into mice with the presence of Phx-3, and the inhibition of the proliferation of cells was achieved in a time- and dose-dependent manner. In presence of this aminophenoxazinone (concentration of 41.5 µM), the inhibition was almost 80% after 48 h, and over 90% after 72 h. Regarding the in vivo effects of Phx-3 on cells, no tumor formation was observed (cutaneous or subcutaneous) after the transplantation of melanoma cells into the flank of mice, by using a very low concentration of Phx-3 (0.5 mg/kg). Besides, this dose of Phx-3 did not increase the tumor size. The different effective concentrations between the in vitro and in vivo activities was suggested to be related with the in vivo synergy of Phx-3 with cytokines, proteins involved in cell signaling.
No weight loss was observed in mice after the treatment with Phx-3. In addition, no pathological changes were observed in the liver and kidneys, or in serum levels of relevant blood biochemical parameters.
In a subsequent study[75], it was found that Phx-3 inhibits the expression of tyrosinase (rate-limiting melanogenic enzyme), as well as the transcription factor that regulates its expression.
From the results mentioned in this section, Phx-3 could be proposed as suitable candidate drug for the treatment or prevention of melanoma, including its cosmetic use to treat hyperpigmentation and to prevent melanogenesis.
3. Activity of aminophenoxazinones on other cell lines
3.1. 2-Aminophenoxazine-3-one (Phx-3)
First of all, we should highlight the study that Che et al. (2011) carried out to evaluate the cytotoxicity of Phx-3 on different types of cancer cells, such as MCF-7, A431, KCP-4, A549, KLM-1, MIA PaCa-2, ACHN, LoVo-1, U251MG and Y-79 lines [57]. In the case of ACHN (renal carcinoma), Liu et al. (2008) had already reported modest cytotoxic activity generated by Phx-3, as well as for a methylated and acetate derivative of this aminophenoxazinone [76]. Attending at IC50 values, the sensitivity of the mentioned cancer cell lines after 72 h of treatment with Phx-3 showed how almost all of them were vulnerable to Phx-3 at 10 µM (IC50 lower than 8 µM). KLM-1, Lovo-1 and Y79 lines were the exceptions (IC50 close to 20 µM). The normal cell lines HEL (embryonic pulmonary fibroblast) and HUVEC (umbilical cord) were also tested, being obtained high IC50 values (over 50 µM and 16 µM, respectively), which indicates that these cells are less sensitive to Phx-3 than cancer cells.Regarding the pHi decrease, significant results were obtained for all cancer cell lines (reduction of 0.22-1.00 units at 20 µM, and 0.64-1.20 units at 100 µM), being comparable to those of the normal lines. The decrease was proved dose-dependent for MCF-7 (breast cancer) and A431 (skin cancer) cell lines for 30 min and ranging between 0 and 100 µM. Therefore, it can be concluded that the use of Phx-3 causes drastic acidification of cancer cells, which in turn induces their apoptosis [77]. Phx-3 would be a suitable drug for the treatment of cancer, as it causes drastic decreases in pHi (by more than 0.6), and induces apoptosis and cytotoxic effects on cancer cells without significant adverse effects.MCF-7 and A431 lines were further evaluated to provide conclusions of the mechanism of action of Phx-3, attending at the reduction of the mitochondrial membrane potential (first and irreversible step towards apoptosis). The population of both MCF-7 and A431 lines, determined as their decrease in mitochondrial potential, increased as a direct function of both time and concentration of Phx-3. According to these data, and considering the reduction of the pHi value in both cell lines, it can be concluded that the apoptosis of MCF-7 and A431 cells could be preceded by the early acidification caused by Phx-3.In other study, the cytotoxic and pro-apoptotic effects of Phx-3 on hepatocellular carcinoma dRLh-84 (rat) and HepG2 (human) cell lines, and the normal hepatocellular RLN-10 (rat) cell line have been studied [61]. Phx-3 reduced the number of viable cells in the three lines by a dose-dependent degree, being 2 µM enough to induce apoptosis by nuclear condensation and cell shrinkage. Moreover, Phx-3 combined with 2-deoxyglucose significantly enhanced apoptosis, but, on the other hand, some adverse effects were observed on normal liver cells. More recently, the combined treatment of Phx-3 with sorafenib was demonstrated to suppress the formation of hepatocellular carcinoma on in vivo studies. Phx-3 was reported as suppressor of the expression of GRP78, target protein directly related with different cancer cell lines, in HepG2 cells [78]. It must be noted that Phx-3 is named as questiomycin A in this last article.The list of cancer lines with cell whose growth can be inhibited by Phx-3 may be completed with HeLa (cervical cancer, IC50 = 12.09 ± 3.29 µM) [35], U266 (myeloma), HL-60 (acute myeloid leukemia) and A549 (lung adenocarcinoma) lines[71]. The study of Moriya et al. (2011) was also focused on the pro-apoptotic transcription factor CHOP, being suggested that the regulation of its expression could represent a major target for treatments. So, in the case of U266, the activity of Phx-3 was enhanced by its combined application together with an inhibitor of NF-κB, a transcription factor related to the inhibition of CHOP.
3.2. Amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazin-3-one (Phx-1)
Phx-1 (Figure 3) is an aminophenoxazinone with anticancer activity, obtained from the reaction of 2-amino-5-methylphenol with bovine hemoglobin [79]. The in vitro studies that have been conducted on this compound are similar to those previously described for Phx-3 [57].Regarding the pHi reduction in MCF-7 and A431 cancer cell lines, similar or rather close results as those from Phx-3 were observed at the lowest concentrations (5-10 µM) were applied. However, Phx-1 proved to be significantly less active than Phx-3 at 50 µM, and concentration had to be increased up to 100 µM for any major differences to appear in the A431 line.The study of Phx-1 on diverse cell lines (the same previously mentioned in the first paragraph of section 3.1), revealed that this compound achieves significant pHi reductions, but not as high as those attained by Phx-3. Thus, Phx-1 reached 0.01-0.16 at 20 µM and 0.11-0.59 at 100 µM, whereas Phx-3 achieved 0.22-1.00 at 20 µM and 0.64-1.20 at 100 µM. In both cases, the normal cell lines tested (HEL and HUVEC) suffered an equally significant decrease in pHi, comparable to that suffered by cancer cells.The data obtained in relation to the cytotoxic effects of Phx-1, compared with those of Phx-3, revealed that the cytotoxicity generated by Phx-1 on all the tested cell lines was much lower (the most sensitive cancer line was MCF-7). These results are in agreement with the pHi changes undergone by the cells treated either Phx-1 or Phx-3 that have been summarized in the previous paragraph. This could represent a certain advantage regarding cancer treatment, since healthy cells would suffer a lesser damage.Although the IC50 value of Phx-1 for Y-79 cells (retinoblastoma, eye cancer) is the highest, previous studies[80] showed in vivo antitumor effects in cells transplanted into mice whose strain suffers from a genetic mutation that causes the deterioration or lack of the thymus (organ where T cells mature). Phx-1 induced in vivo apoptosis to Y-79 in mice without any type of adverse effect even at high doses. These favorable signs make of Phx-1 a suitable candidate for the development of drugs for the treatment of retinoblastoma. In general, although Phx-1 is not highly effective, it could facilitate the induction of apoptosis and exert a cytotoxic effect on cancer cells.It is worth highlighting that Phx-1 inhibited the proliferation and induced the apoptosis of diverse human leukemia cell lines (K562, HL-60 and HAL-01) in a dose-dependent manner. This study also proved that Phx-1 reduced in vivo the tumor growth rate in mice, whereas just few adverse effects were found on weight loss and white blood cell [81].Once confirmed the ability of Phx-1 and Phx-3 to induce the apoptosis of diverse cancer cell lines, Tabuchi et al. (2011) studied the effects of these compounds to induce apoptotic cell death in human neutrophils, as this kind of cells are related with the mitochondrial depolarization and reduction of pHi. Both aminophenoxazines caused apoptosis or the loss of the morphology of neutrophils, while lymphocytes and monocytes did not undergo this process. These results would suggest that Phx-1 and Phx-3 are specific drugs to induce apoptotic cell death of neutrophils, being potential preventive anti-inflammatory drugs[58].The antitumoral activity of Phx-1 and Phx-3 on NB-1 (neuroblastoma cell line) was demonstrated by another study. It was thereby confirmed that both aminophenoxazinones induced apoptosis and necrosis, being the IC50 value of Phx-3 much lower (0.5 µM vs. 20 µM)[82].From the structural point of view, the differences in the bioactivity exhibited by Phx-1 and Phx-3 would be associated to the methyl group in Phx-1, even this premise is still to be confirmed. Molecular dynamics simulations could cast some light on the solubility of these compounds in cell membranes, which would allow to determine how much their structural differences affect cross-membrane processes and, thereby, explain the differences in their activity levels. In this line, certain recent studies have proven that the replacement of hydroxyl groups by fluorinated esters (in the eudesmanolide structure), improves the cytotoxicity of these compounds to cancer cells (HeLa, cervical cancer)[83].
3.3. Phenoxazine-indole conjugates
Recent studies have tested the activity by different derivatives with an indole group on different cancer cell lines. Indole groups are heterocyclic compounds formed by a benzene atom linked to a pyrrole compound, that have a pair of free electrons in the nitrogen atom of their aromatic ring. It is a fairly common component in perfumes, drug candidates and hormones (like melatonin)[84]. Some natural indole alkaloids, such as vincristine (Figure 5), has been accepted in USA by the Food and Drug Administration (FDA) as antitumor drugs[85].
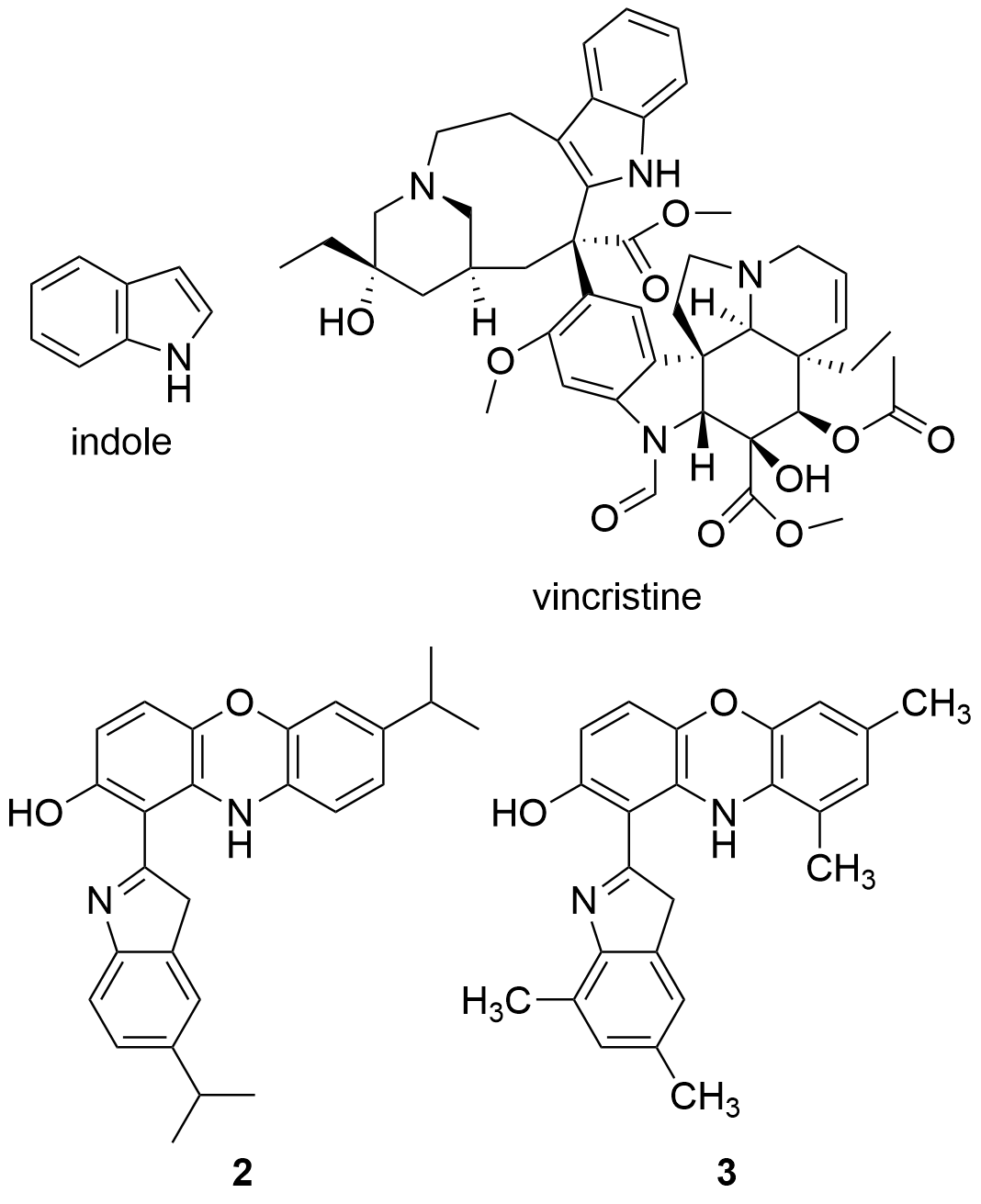
Figure 5. Structures of indole, the anticancer drug vincristine and the most active indole derivatives.
Taking into account the potential pharmacological effects of indoles, as well as those already recognized of phenoxazines and the synergistic effects exhibited by some pharmacophoric hybrids[86], Nunewar et al. (2020)[87] suggested that certain hybrids formed by two of these groups would possess some proliferative effect by operating as DNA exchangers. The general proposed mechanism consists of a first transfer of the molecule to the hydrophobic space between two adjacent DNA base pairs. As consequence, DNA would undergo conformational changes, in order for the molecule to accommodate itself between the base pairs. The resulting complex prevents DNA replication, which would lead to the death of the cell, being these results especially useful for the treatment of rapidly growing cancer cells.
Therefore, the cytotoxicity of certain indol derivatives was tested by Nunewar et al. (2020) on A549 (lung), MG-63 (bone), BT-474 (breast), Hep G2 (liver) and HCT116 (colon) human cancer cell lines, along with a normal line of lung epithelial tissue (L-132). All the derivatives tested showed remarkable IC50 values. The most inhibited cell line was A549, by the derivative 2 (IC50 = 3.71 ± 0.57 µM), characterized by two isopropyl substituents (Figure 5), and followed by compound 3 (IC50 = 4.43 ± 0.64 µM). In addition, 2 was the only derivative with the previously explained DNA intercalation capacity. These results confirm that hybrid compounds are a promising alternative for the search of new anticancer drugs.
3.4. Pyridophenoxazinone derivatives conjugated to L-lysine
Also in 2020 a number of studies were conducted on a series of pyridophenoxazinones (which possess intercalating capacity, and for generating free radicals that induce cell death by oxidative-stress) conjugated with the amino acid L-lysine, which were designed and synthesized with the aim of developing novel drug compounds with anticancer potential. Similarly to those describe in the previous section, these studies focused on the ability of the compounds to intercalate between the base pairs of nucleic acids. Thus, synthetized derivatives contained a basic side chain of L-lysine in the positions 9 or 10, and the N-terminal group of L-lysine was linked to the chromophore through an amide bond (Figure 6).
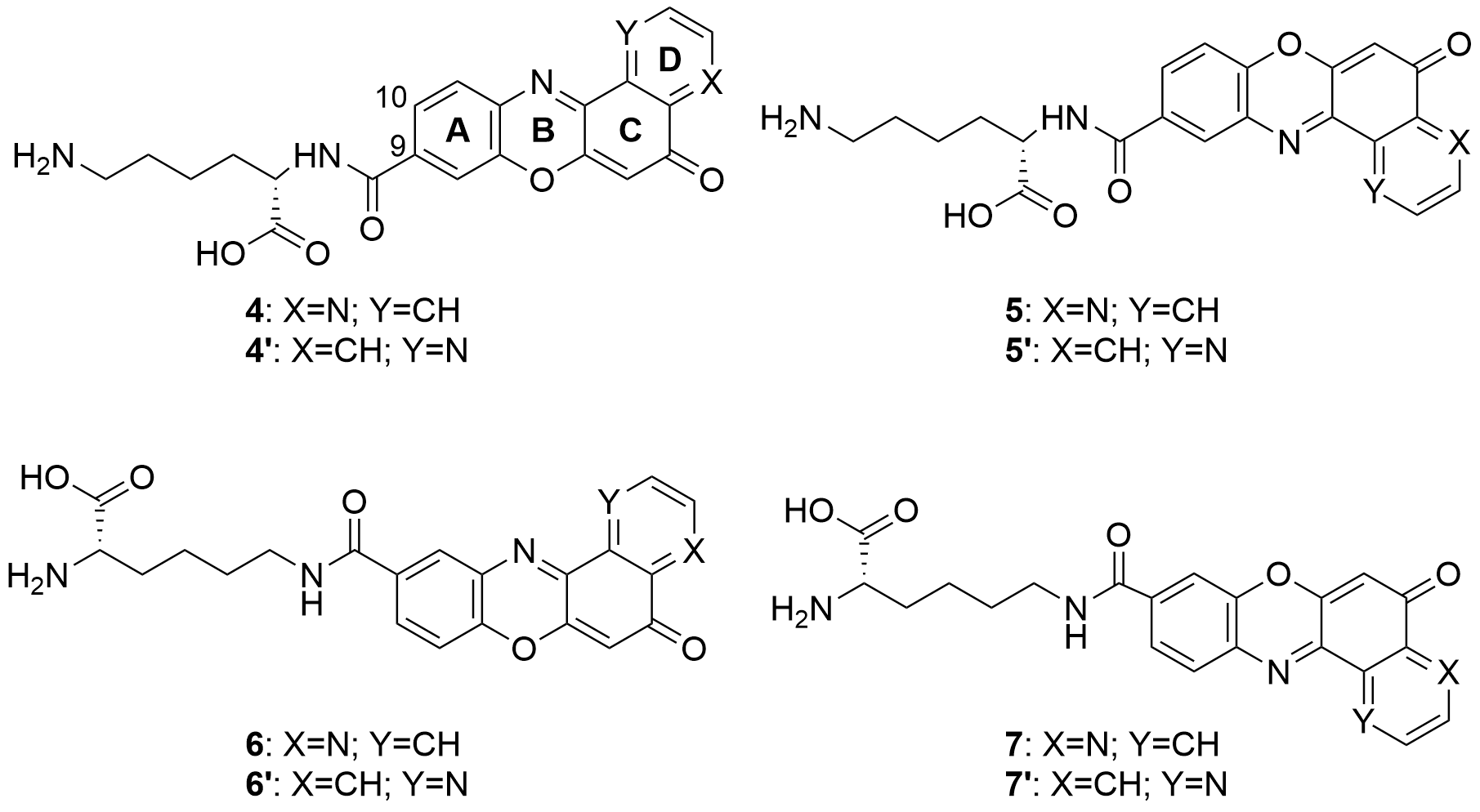
Figure 6. Structure of the pyridophenoxazinone derivatives conjugated to L-lysine.
Products 4-7 and 4’-7’ were tested on cancer cell lines of leukemia (CCRF-CEM, CCRF-SB and MT-4), colon (HT-29), breast cancer (MCF-7), cervical cancer (HeLa), papillary renal cell carcinoma (ACHN), and melanoma (SKMEL-28 and G-361). All the products inhibited the proliferation of a panel of human liquid and solid neoplastic cell lines, the latter being more sensitive to antiproliferative effects (only compound 7 showed similar activity in both states). The IC50 values of the derivatives with the L-lysine side chain attached to C-10 were higher than their corresponding derivative functionalized at C-9. The IC50 values of 4-7 were comparable to that of AMD and even lower than those of Doxo and VP-16 (commercial drugs). It is worth highlighting the lowest values of IC50, all in the range of 0.001-0.007 µM, achieved by 4 and 7 for HT-29, SKMEL-28, MCF-7 and G-361 lines. Authors proved that both compounds are strong DNA intercalators, and possess the capacity to selectively target the topoisomerase Topo IIα.
The difference in activity between the two series of derivatives was related to structure-activity correlations. Thus, the position of the nitrogen atom in D-ring would play an important role in the antiproliferative activity, whereas the position of the L-lysine side chain and the type of amine groups affect the cytotoxic activity both between the two series and within each series.
All the mentioned properties indicate that this new series of pyridophenoxazinones conjugated to L-lysine have a great antitumor therapeutic potential. Products 4 and 7 in particular, provide really interesting opportunities for the development of new DNA-targeting anticancer drugs.
References
- Sánchez-Moreiras, A.M.; Coba de la Peña, T.; Martínez, A.; González, L.; Pellisier, F.; Reigosa, M.J. Mode of action of the hydroxamic acid BOA and other related compounds. In Allelopathy; Macías, F.A., Galindo, J.C.G., Molinillo, J.M.G., Cutler, H.G., Eds.; CRC Press, Boca Raton, FL., 2004; pp. 239–252.
- Hector R. Bravo; Waldo Lazo; Antialgal and Antifungal Activity of Natural Hydroxamic Acids and Related Compounds. Journal of Agricultural and Food Chemistry 1996, 44, 1569-1571, 10.1021/jf950345e.
- Bettina M. Jensen; Khem Adhikari; Heidi J. Schnoor; Nanna Juel-Berg; Inge S. Fomsgaard; Lars K. Poulsen; Quantitative analysis of absorption, metabolism, and excretion of benzoxazinoids in humans after the consumption of high- and low-benzoxazinoid diets with similar contents of cereal dietary fibres: a crossover study. European Journal of Nutrition 2015, 56, 387-397, 10.1007/s00394-015-1088-6.
- Shuyun Peng; William Scott Chilton; Biosynthesis of dimboa in maize using deuterium oxide as a tracer. Phytochemistry 1994, 37, 167-171, 10.1016/0031-9422(94)85018-6.
- Praveen Kumar; Donald E. Moreland; William Scott Chilton; 2H-1,4-benzoxazin-3(4H)-one, an intermediate in the biosynthesis of cyclic hydroxamic acids in maize. Phytochemistry 1994, 36, 893-898, 10.1016/s0031-9422(00)90458-8.
- Khem Adhikari; Helle N. Lærke; Anne G. Mortensen; Inge S. Fomsgaard; Plasma and Urine Concentrations of Bioactive Dietary Benzoxazinoids and Their Glucuronidated Conjugates in Rats Fed a Rye Bread-Based Diet. Journal of Agricultural and Food Chemistry 2012, 60, 11518-11524, 10.1021/jf301737n.
- Stine Krogh Steffensen; Hans A. Pedersen; Khem B. Adhikari; Bente B. Laursen; Claudia Jensen; Søren Høyer; Michael Borre; Helene H. Pedersen; Mette Borre; David Edwards; et al.Inge Sindbjerg Fomsgaard Benzoxazinoids in Prostate Cancer Patients after a Rye-Intensive Diet: Methods and Initial Results. Journal of Agricultural and Food Chemistry 2016, 64, 8235-8245, 10.1021/acs.jafc.6b03765.
- Praveen Kumar; Ronald W. Gagliardo; William Scott Chilton; Soil transformation of wheat and corn metabolites mboa and DIM2BOA into aminophenoxazinones. Journal of Chemical Ecology 1993, 19, 2453-2461, 10.1007/bf00980682.
- Francisco A. Macias; Alberto Oliveros-Bastidas; David Marín; Diego Castellano; Ana M. Simonet; José M. G. Molinillo; Degradation Studies on Benzoxazinoids. Soil Degradation Dynamics of (2R)-2-O-β-d-Glucopyranosyl-4-hydroxy-(2H)- 1,4-benzoxazin-3(4H)-one (DIBOA-Glc) and Its Degradation Products, Phytotoxic Allelochemicals from Gramineae. Journal of Agricultural and Food Chemistry 2005, 53, 554-561, 10.1021/jf048702l.
- Inge S. Fomsgaard; Anne G. Mortensen; Sandra C.K. Carlsen; Microbial transformation products of benzoxazolinone and benzoxazinone allelochemicals––a review. Chemosphere 2004, 54, 1025-1038, 10.1016/j.chemosphere.2003.09.044.
- Francisco A. Macías; David Marín; Alberto Oliveros-Bastidas; José María González Molinillo; Rediscovering the bioactivity and ecological role of 1,4-benzoxazinones. Natural Product Reports 2009, 26, 478-489, 10.1039/b700682a.
- Francisco A. Macías; Alberto Oliveros-Bastidas; David Marín; Diego Castellano; Ana M. Simonet; José María González Molinillo; Degradation Studies on Benzoxazinoids. Soil Degradation Dynamics of 2,4-Dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA) and Its Degradation Products, Phytotoxic Allelochemicals from Gramineae. Journal of Agricultural and Food Chemistry 2004, 52, 6402-6413, 10.1021/jf0488514.
- Sascha Venturelli; Regina G. Belz; Andreas Kämper; Alexander Berger; Kyra Von Horn; André Wegner; Alexander Böcker; Gérald Zabulon; Tobias Langenecker; Oliver Kohlbacher; et al.Fredy BarnecheDetlef WeigelUlrich M. LauerMichael BitzerClaude Becker Plants Release Precursors of Histone Deacetylase Inhibitors to Suppress Growth of Competitors. The Plant Cell 2015, 27, 3175-3189, 10.1105/tpc.15.00585.
- Francisco A. Macías; Alberto Oliveros-Bastidas; David Marín; Nuria Chinchilla; Diego Castellano; José María González Molinillo; Evidence for an Allelopathic Interaction Between Rye and Wild Oats. Journal of Agricultural and Food Chemistry 2014, 62, 9450-9457, 10.1021/jf503840d.
- Nuria Chinchilla; David Marín; Alberto Oliveros-Bastidas; José M. G. Molinillo; Francisco A. Macías; Soil biodegradation of a benzoxazinone analog proposed as a natural products-based herbicide. Plant and Soil 2015, 393, 207-214, 10.1007/s11104-015-2485-6.
- Gierl, A.; Gruen, S.; Genschel, U.; Huettl, R.; Frey, M. Evolution of indole and benzoxazinone biosynthesis in Zea mays. In Recent Advances in Phytochemistry; Elsevier, Ed.; 2004; pp. 69–83.
- Mireia Farres; Marta Villagrasa; Ethel Eljarrat; Damià Barceló; Romà Tauler; Chemometric evaluation of different experimental conditions on wheat (Triticum aestivum L.) development using liquid chromatography mass spectrometry (LC–MS) profiles of benzoxazinone derivatives. Analytica Chimica Acta 2012, 731, 24-31, 10.1016/j.aca.2012.04.017.
- Margot Schulz; Adriano Marocco; Vincenzo Tabaglio; Francisco A. Macias; José María González Molinillo; Benzoxazinoids in Rye Allelopathy - From Discovery to Application in Sustainable Weed Control and Organic Farming. Journal of Chemical Ecology 2013, 39, 154-174, 10.1007/s10886-013-0235-x.
- Tomoda, A., Yamaguchi, T., Sato, K., & Iwata, A. (2004). Antiviral agents containing aminophenoxazines. Jpn. Patent, 2004143101.
- Bitzer, M.; Lauer, U.M.; Venturelli, S.; Armeanu, S. Aminophenoxazinone compounds as antitumor and antiinflammatory agents 2009
- Komala Pandurangan; Shane Gallagher; Grace G. Morgan; Helge Müller-Bunz; Francesca Paradisi; Structure and antibacterial activity of the silver(i) complex of 2-aminophenoxazine-3-one. Metallomics 2010, 2, 530-534, 10.1039/c003515g.
- F. Kehrmann; Ueber Oxydationsproducte vono-Aminophenolen. European Journal of Inorganic Chemistry 1906, 39, 134-138, 10.1002/cber.19060390127.
- Zoltan Szeverényi; Elena R. Milaeva; Lászlò I. Simándi; Kinetics of the oxidation of 2-aminophenol by dioxygen in the presence of tetrakis(3,5-di-t-butyl-4-hydroxyphenyl)-dodecachlorophthalocyaninatocobalt(II). Journal of Molecular Catalysis 1991, 67, 251-258, 10.1016/0304-5102(91)85050-c.
- S. Gabriel; Ueber eine Darstellungsweise primärer Amine aus den entsprechenden Halogenverbindungen. European Journal of Inorganic Chemistry 1887, 20, 2224-2236, 10.1002/cber.18870200227.
- Helge Prinz; Ann-Kathrin Ridder; Kirsten Vogel; Konrad J. Böhm; Igor Ivanov; Jahan B. Ghasemi; Elham Aghaee; Klaus Müller; N-Heterocyclic (4-Phenylpiperazin-1-yl)methanones Derived from Phenoxazine and Phenothiazine as Highly Potent Inhibitors of Tubulin Polymerization. Journal of Medicinal Chemistry 2017, 60, 749-766, 10.1021/acs.jmedchem.6b01591.
- Shun Li; Tianyuan Cheng; Changfeng Yin; Sensen Zhou; Quli Fan; Wei Wu; Xiqun Jiang; Phenothiazine versus Phenoxazine: Structural Effects on the Photophysical Properties of NIR-II AIE Fluorophores. ACS Applied Materials & Interfaces 2020, 12, 43466–43473, 10.1021/acsami.0c12773.
- Wubin Zheng; Jiajia Yang; Yang Shen; Yusi Yao; Guanglei Lv; Shiyou Hao; Chunxia Li; The near-infrared fluorescent probes based on phenoxazine for the rapid detection of hypochlorous acid. Dyes and Pigments 2020, 179, 108404, 10.1016/j.dyepig.2020.108404.
- Luke Farmer; Evan A. Haidasz; Markus Griesser; Derek A. Pratt; Phenoxazine: A Privileged Scaffold for Radical-Trapping Antioxidants. The Journal of Organic Chemistry 2017, 82, 10523-10536, 10.1021/acs.joc.7b02025.
- Efeturi A. Onoabedje; Samuel A. Egu; Mercy A. Ezeokonkwo; Uchechukwu C. Okoro; Highlights of molecular structures and applications of phenothiazine & phenoxazine polycycles. Journal of Molecular Structure 2019, 1175, 956-962, 10.1016/j.molstruc.2018.08.064.
- Carla M.A. Alves; Sarala Naik; Paulo Coutinho; M. Sameiro T. Gonçalves; Novel DNA fluorescence probes based on N-[5-(11-functionalised-undecylamino)-9H-benzo[a]phenoxazin-9-ylidene]propan-1-aminium chlorides: synthesis and photophysical studies. Tetrahedron Letters 2011, 52, 112-116, 10.1016/j.tetlet.2010.10.165.
- Wei-Jin Zhu; Jin-Yun Niu; Dan-Dan He; Ru Sun; Yu-Jie Xu; Jian-Feng Ge; Near-infrared pH probes based on phenoxazinium connecting with nitrophenyl and pyridinyl groups. Dyes and Pigments 2018, 149, 481-490, 10.1016/j.dyepig.2017.09.059.
- Agata Jaszczyszyn; Kazimierz Gąsiorowski; Piotr Świątek; Wiesław Malinka; Katarzyna Cieślik-Boczula; Joanna Petrus; Bogusława Czarnik-Matusewicz; Chemical structure of phenothiazines and their biological activity. Pharmacological Reports 2012, 64, 16-23, 10.1016/s1734-1140(12)70726-0.
- Mei Peng; Yiling Ding; Ling Yu; Yali Deng; Weisi Lai; Yun Hu; Hongwen Zhang; Xianqing Wu; Hong Fan; Hui Ding; et al.Yilin WuGuangshi Tao Tegafur Substitution for 5-Fu in Combination with Actinomycin D to Treat Gestational Trophoblastic Neoplasm. PLOS ONE 2015, 10, e0143531, 10.1371/journal.pone.0143531.
- Layla Mohammad Hadi; Elnaz Yaghini; Alexander J. MacRobert; Marilena Loizidou; Synergy between Photodynamic Therapy and Dactinomycin Chemotherapy in 2D and 3D Ovarian Cancer Cell Cultures. International Journal of Molecular Sciences 2020, 21, 3203, 10.3390/ijms21093203.
- Jonas Kühlborn; Matthias Konhäuser; Jonathan Groß; Peter R. Wich; Till Opatz; Xylochemical Synthesis of Cytotoxic 2-Aminophenoxazinone-Type Natural Products Through Oxidative Cross Coupling. ACS Sustainable Chemistry & Engineering 2019, 7, 4414-4419, 10.1021/acssuschemeng.8b06353.
- Pasceri, R.; Siegel, D.; Ross, D.; Moody, C.J.; Aminophenoxazinones as inhibitors of indoleamine 2,3-dioxygenase (IDO). Synthesis of exfoliazone and chandranimycin A. J. Med. Chem. 2013, 56, 3310–3317, 10.1021/jm4000049z.
- Song Wu; Sarah E Powers; Wenquan Zhu; Yusuf A. Hannun; Substantial contribution of extrinsic risk factors to cancer development. Nature 2015, 529, 43-47, 10.1038/nature16166.
- А. Romaniuk; M. Lyndin; V. Sikora; Y. Lyndina; K. Sikora; Heavy metals effect on breast cancer progression. Journal of Occupational Medicine and Toxicology 2017, 12, 32-32, 10.1186/s12995-017-0178-1.
- Mattiuzzi, C.; Lippi, G.; Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222.
- Japanese Gastric Cancer Association; Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2020, 24, 1-21, 10.1007/s10120-020-01042-y.
- Yan Zhao; Qinhao Guo; Jiejing Chen; Jun Hu; Shuwei Wang; Yueming Sun; Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: A clinical and in vitro investigation. Oncology Reports 2013, 31, 358-364, 10.3892/or.2013.2850.
- William Waddingham; Stella A V Nieuwenburg; Sean Carlson; Manuel Rodriguez-Justo; Manon Spaander; Ernst J Kuipers; Marnix Jansen; David G Graham; Matthew Banks; Recent advances in the detection and management of early gastric cancer and its precursors. Frontline Gastroenterology 2020, flgastro-2018, 101089, 10.1136/flgastro-2018-101089.
- Christian Stock; Stine Falsig Pedersen; Roles of pH and the Na + /H + exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective?. Seminars in Cancer Biology 2017, 43, 5-16, 10.1016/j.semcancer.2016.12.001.
- Dolores Pérez-Sala; Dolores Collado-Escobar; Faustino Mollinedo; Intracellular Alkalinization Suppresses Lovastatin-induced Apoptosis in HL-60 Cells through the Inactivation of a pH-dependent Endonuclease. Journal of Biological Chemistry 1995, 270, 6235-6242, 10.1074/jbc.270.11.6235.
- Yue Song; Zhao Wang; Zengping Hao; Lihong Li; Junli Lu; Hongjun Kang; Yanping Lu; Yanqin You; Lijuan Li; Qingyun Chen; et al.Bo Chen Requirement for etoposide in the treatment of pregnancy related hemophagocytic lymphohistiocytosis: a multicenter retrospective study. Orphanet Journal of Rare Diseases 2019, 14, 50, 10.1186/s13023-019-1033-5.
- Kaufmann, S.H.; nduction of Endonucleolytic DNA Cleavage in Human Acute Myelogenous Leukemia Cells by Etoposide, Camptothecin, and Other Cytotoxic Anticancer Drugs: A Cautionary Note. Cancer Res. 1989, 49, 5870–5878.
- Xiao-Fang Che; Shin-Ichi Akiyama; Akio Tomoda; Suppression of the proliferation of cancer cell lines, KB-3-1 and K562 cells preceded by a decrease in intracellular pH caused by phenoxazine derivatives.. Oncology Reports 2008, 19, 1253-1258, 10.3892/or.19.5.1253.
- Hiroshi Mori; Katsuaki Honda; Ryoji Ishida; Tomoyoshi Nohira; Akio Tomoda; Antitumor activity of 2-amino-4,4α-dihydro-4α, 7-dimethyl-3H-phenoxazine-3-one against Meth A tumor transplanted into BALB/c mice. Anti-Cancer Drugs 2000, 11, 653-657, 10.1097/00001813-200009000-00010.
- Naoko Miyano-Kurosaki; Kunihiko Kurosaki; Michiko Hayashi; Hiroshi Takaku; Masaaki Hayafune; Ken Shirato; Teruhiko Kasuga; Takahiko Endo; Akio Tomoda; 2-Aminophenoxazine-3-one Suppresses the Growth of Mouse Malignant Melanoma B16 Cells Transplanted into C57BL/6Cr Slc Mice. Biological and Pharmaceutical Bulletin 2006, 29, 2197-2201, 10.1248/bpb.29.2197.
- Teruhiko Kasuga; Takafumi Tabuchi; Ken Shirato; Kazuhiko Imaizumi; Akio Tomoda; Caspase-independent cell death revealed in human gastric cancer cell lines, MKN45 and KATO III treated with phenoxazine derivatives.. Oncology Reports 2007, 17, 409-415, 10.3892/or.17.2.409.
- Akio Tomoda; Hiroyuki Nagata; Xiao-Fang Che; Keisuke Miyazawa; Masato Konishi; Hideyuki Ubukata; Takafumi Tabuchi; Rapid decrease of intracellular pH associated with inhibition of Na+/H+ exchanger precedes apoptotic events in the MNK45 and MNK74 gastric cancer cell lines treated with 2-aminophenoxazine-3-one. Oncology Reports 2011, 25, 341-346, 10.3892/or.2010.1082.
- Thomas Litman; Stine F. Pedersen; Birte Kramhøft; Torben Skovsgaard; Else K. Hoffmann; pH regulation in sensitive and multidrug resistant Ehrlich ascites tumor cells.. Cellular Physiology and Biochemistry 1998, 8, 138-150, 10.1159/000016277.
- Akio Tomoda; Keisuke Miyazawa; Takafumi Tabuchi; Prevention of Carcinogenesis and Development of Gastric and Colon Cancers by 2-Aminophenoxazine-3-one (Phx-3): Direct and Indirect Anti-Cancer Activity of Phx-3. International Journal of Molecular Sciences 2013, 14, 17573-17583, 10.3390/ijms140917573.
- Mattiuzzi, C.; Lippi, G.; Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222.
- Akio Tomoda; Takeshi Nakachi; Takafumi Tabuchi; Akira Takasaki; Sadao Arai; Keisuke Miyazawa; Anticancer activity of phenoxazines produced by bovine erythrocytes on colon cancer cells. Oncology Reports 2010, 23, 1517-1522, 10.3892/or_00000790.
- Junfeng Wang; Weijun He; Xiaochu Qin; Xiaoyi Wei; Xinpeng Tian; Li Liao; Shengrong Liao; Bin Yang; Zhengchao Tu; Bo Chen; et al.Fazuo WangXiaojiang ZhouYonghong Liu Three new indolyl diketopiperazine metabolites from the antarctic soil-derived fungus Penicillium sp. SCSIO 05705. RSC Advances 2015, 5, 68736-68742, 10.1039/c5ra10828d.
- Xiao-Fang Che; Chun-Lei Zheng; Shin-Ichi Akiyama; Akio Tomoda; 2-Aminophenoxazine-3-one and 2-amino-4,4.ALPHA.-dihydro-4.ALPHA.,7-dimethyl-3H-phenoxazine-3-one cause cellular apoptosis by reducing higher intracellular pH in cancer cells. Proceedings of the Japan Academy, Series B 2011, 87, 199-213, 10.2183/pjab.87.199.
- Takanobu Tabuchi; Xiao-Fang Che; Katsuya Hiraishi; Masakazu Adachi; Kei Miyano; Hideki Sumimoto; Takafumi Tabuchi; Keisuke Miyazawa; Akio Tomoda; Selectively Induced Apoptosis in Human Neutrophils in the Presence of Oxidative Phenoxazines, 2-Amino-4,4α-dihydryo-4α-7H-phenoxazine-3-one and 2-Aminophenoxazine-3-one, Preceded by Decrease of Intracellular pH, Depolarization of the Mitochondria, and Inhibition of Superoxide Generation. Journal of Pharmacological Sciences 2011, 117, 139-148, 10.1254/jphs.11134fp.
- Keizo Kohno; Masaki Miyake; Osamu Sano; Mari Tanaka-Kataoka; Shigeto Yamamoto; Satomi Koya-Miyata; Norie Arai; Mitsukiyo Fujii; Hikaru Watanabe; Shimpei Ushio; et al.Kanso IwakiShigeharu Fukuda Anti-inflammatory and Immunomodulatory Properties of 2-Amino-3H-phenoxazin-3-one. Biological and Pharmaceutical Bulletin 2008, 31, 1938-1945, 10.1248/bpb.31.1938.
- Xiaochun Gao; Yuanyuan Lu; Yingying Xing; Yihua Ma; Jiansheng Lu; Weiwei Bao; Yiming Wang; Tao Xi; A novel anticancer and antifungus phenazine derivative from a marine actinomycete BM-17. Microbiological Research 2012, 167, 616-622, 10.1016/j.micres.2012.02.008.
- Akio Tomoda; Akira Takemura; Xiao-Fang Che; Takafumi Tabuchi; Shota Moriya; Keisuke Miyazawa; Enhancement of cytotoxic and pro-apoptotic effects of 2-aminophenoxazine-3-one on the rat hepatocellular carcinoma cell line dRLh-84, the human hepatocellular carcinoma cell line HepG2, and the rat normal hepatocellular cell line RLN-10 in combination with 2-deoxy-D-glucose. Oncology Reports 2011, 27, 347-355, 10.3892/or.2011.1531.
- Dani S. Bidros; Michael A. Vogelbaum; Novel drug delivery strategies in neuro-oncology. Neurotherapeutics 2009, 6, 539-546, 10.1016/j.nurt.2009.04.004.
- M Azuine; Cancer chemopreventive effect of phenothiazines and related tri-heterocyclic analogues in the 12-O-tetradecanoylphorbol-13-acetate promoted Epstein-Barr virus early antigen activation and the mouse skin two-stage carcinogenesis models. Pharmacological Research 2004, 49, 161-169, 10.1016/j.phrs.2003.07.014.
- Kenia, H.; Shivkumar, B.; Kotnal, R.B.; Ramesha, A.; Devadiga, P.; Simpi, C.C.; Chandrashekar, V.M.; Synthesis and Evaluation of Phenothiazine Derivatives. IOSR J. Pharm. 2020, 10, 54–62.
- Mitsutoshi Nakada; Daisuke Kita; Takuya Watanabe; Yutaka Hayashi; Lei Teng; Ilya V. Pyko; Jun-Ichiro Hamada; Aberrant Signaling Pathways in Glioma. Cancers 2011, 3, 3242-3278, 10.3390/cancers3033242.
- Deepa Soni; James A.J. King; Andrew Henry Kaye; Christopher M. Hovens; Genetics of glioblastoma multiforme: mitogenic signaling and cell cycle pathways converge. Journal of Clinical Neuroscience 2005, 12, 1-5, 10.1016/j.jocn.2004.04.001.
- Marek Los; Subbareddy Maddika; Bettina Erb; Klaus Schulze-Osthoff; Switching Akt: from survival signaling to deadly response. BioEssays 2009, 31, 492-495, 10.1002/bies.200900005.
- Gray Pearson; Fred Robinson; Tara Beers Gibson; Bing-E Xu; Mahesh Karandikar; Kevin Berman; Melanie H. Cobb; Mitogen-Activated Protein (MAP) Kinase Pathways: Regulation and Physiological Functions*. Endocrine Reviews 2001, 22, 153-183, 10.1210/edrv.22.2.0428.
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. In Inflammatory Processes: Molecular Mechanisms and Therapeutic Opportunities; Letts, L.G., Morgan, D.W., Eds.; Birkhäuser Basel: Basel, 2000; pp. 13–21.
- Xiao-Fang Che; Shota Moriya; Chun-Lei Zheng; Akihisa Abe; Akio Tomoda; Keisuke Miyazawa; 2-Aminophenoxazine-3-one-induced apoptosis via generation of reactive oxygen species followed by c-jun N-terminal kinase activation in the human glioblastoma cell line LN229. International Journal of Oncology 2013, 43, 1456-1466, 10.3892/ijo.2013.2088.
- Keisuke Miyazawa; Shota Moriya; Tomohiro Kawaguchi; Xiao-Fang Che; Akio Tomoda; Involvement of endoplasmic reticulum stress-mediated CHOP (GADD153) induction in the cytotoxicity of 2-aminophenoxazine-3-one in cancer cells. International Journal of Oncology 2011, 39, 981-988, 10.3892/ijo.2011.1072.
- Akio Tomoda; Chun-Lei Zheng; Xiao-Fang Che; Shin-Ichi Akiyama; Keisuke Miyazawa; 2-Aminophenoxazine-3-one induces cellular apoptosis by causing rapid intracellular acidification and generating reactive oxygen species in human lung adenocarcinoma cells. International Journal of Oncology 2010, 36, 641-650, 10.3892/ijo_00000540.
- Lindsey Goddard; Lauren Yorozuya; Jane Hirokane; Art of prevention: The importance of melanoma surveillance. International Journal of Women's Dermatology 2020, 6, 257-259, 10.1016/j.ijwd.2020.01.003.
- Jarmila Čelakovská; Josef Bukač; Lenka Čáková; Marie Šimková; Eva Jandová; Epidemiology of Melanoma in the Czech Republic in East Bohemia in the Period 2002–2017 and the Effect of the Annual Sunshine Exposure. Acta Medica (Hradec Kralove, Czech Republic) 2020, 63, 10-17, 10.14712/18059694.2020.10.
- Masaki Miyake; Shigeto Yamamoto; Osamu Sano; Mitsukiyo Fujii; Keizo Kohno; Shimpei Ushio; Kanso Iwaki; Shigeharu Fukuda; Inhibitory Effects of 2-Amino-3H-phenoxazin-3-one on the Melanogenesis of Murine B16 Melanoma Cell Line. Bioscience, Biotechnology, and Biochemistry 2010, 74, 753-758, 10.1271/bbb.90795.
- Wei-Hong Liu; Ming-Gang Li; Yi-Qing Li; Jiang-Yuan Zhao; Zhang-Gui Ding; Pei-Wen Yang; Xiao-Long Cui; Meng-Liang Wen; Cytotoxic metabolites of Streptimonospora salina. Chemistry of Natural Compounds 2008, 44, 503-505, 10.1007/s10600-008-9102-3.
- Tannock, I.F.; Rotin, D.; Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384.
- Kayo Machihara; Hidenori Tanaka; Yoshihiro Hayashi; Ichiro Murakami; Takushi Namba; Questiomycin A stimulates sorafenib-induced cell death via suppression of glucose-regulated protein 78. Biochemical and Biophysical Research Communications 2017, 492, 33-40, 10.1016/j.bbrc.2017.08.042.
- Akio Tomoda; Sadao Arai; Ryoji Ishida; Takashi Shimamoto; Kazuma Ohyashiki; An improved method for the rapid preparation of 2-amino-4,4a-dihydro-4a,7-dimethyl-3H-phenoxazine-3-one, a novel antitumor agent.. Bioorganic & Medicinal Chemistry Letters 2001, 11, 1057-1058, 10.1016/s0960-894x(01)00153-6.
- Keisuke Kimura; Yoshihiko Usui; Takaaki Hattori; Naohiko Yamakawa; Hiroshi Goto; Masahiko Usui; Shinya Okada; Ken Shirato; Akio Tomoda; Phenoxazine derivative, 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazine-3-one suppresses growth of human retinoblastoma cell line Y79 in vitro and in vivo. Oncology Reports 2008, 19, 3-10, 10.3892/or.19.1.3.
- Shimamoto, T.; Tomoda, A.; Ishida, R.; Ohyashiki, K.; Antitumor effects of a novel phenoxazine derivative on human leukemia cell lines through activation of caspase-3 and telomerase. Clin. Cancer Res. 2001, 7, 704–708.
- Ken Shirato; Kazuhiko Imaizumi; Akihisa Abe; Akio Tomoda; Phenoxazine Derivatives 2-Amino-4,4.ALPHA.-dihydro-4.ALPHA.-phenoxazine-3-one and 2-Aminophenoxazine-3-one-Induced Apoptosis through a Caspase-Independent Mechanism in Human Neuroblastoma Cell Line NB-1 Cells. Biological and Pharmaceutical Bulletin 2007, 30, 331-336, 10.1248/bpb.30.331.
- Francisco J. R. Mejías; Alexandra G. Durán; Jesús G. Zorrilla; Rosa M. Varela; José M. G. Molinillo; Manuel M. Valdivia; Francisco A. Macías; Acyl Derivatives of Eudesmanolides To Boost their Bioactivity: An Explanation of Behavior in the Cell Membrane Using a Molecular Dynamics Approach. ChemMedChem 2020, 16, 1297-1307, 10.1002/cmdc.202000783.
- Mehak Chauhan; Anjali Saxena; Biswajit Saha; An insight in anti-malarial potential of indole scaffold: A review. European Journal of Medicinal Chemistry 2021, 218, 113400, 10.1016/j.ejmech.2021.113400.
- Navriti Chadha; Om Silakari; Indoles as therapeutics of interest in medicinal chemistry: Bird's eye view. European Journal of Medicinal Chemistry 2017, 134, 159-184, 10.1016/j.ejmech.2017.04.003.
- Kamaldeep Paul; Shweta Bindal; Vijay Luxami; Synthesis of new conjugated coumarin–benzimidazole hybrids and their anticancer activity. Bioorganic & Medicinal Chemistry Letters 2013, 23, 3667-3672, 10.1016/j.bmcl.2012.12.071.
- Saiprasad N. Nunewar; Naveen Kotla; Jaya Lakshmi Uppu; Apoorva Dixit; Venkatesh Pooladanda; Chandraiah Godugu; Neelima D. Tangellamudi; Synthesis of 1-(Indol-2-yl)-phenoxazine hybrids from quinacetophenone precursors and their biological evaluation as DNA intercalating agents. Journal of Molecular Structure 2020, 1217, 128311, 10.1016/j.molstruc.2020.128311.





