
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Claudio Cerchione | + 967 word(s) | 967 | 2021-06-04 08:05:35 | | | |
| 2 | Peter Tang | Meta information modification | 967 | 2021-06-17 04:40:44 | | |
Video Upload Options
Available data on anti-BCMA CART-cell therapy has demonstrated efficacy and manageable toxicity in heavily pretreated R/R MM patients. Chimeric antigen receptor (CAR) T-cell therapy targeting B-cell maturation antigen (BCMA) represents a new strategy for the treatment of relapsed/refractory MM (R/R).
1. Introduction
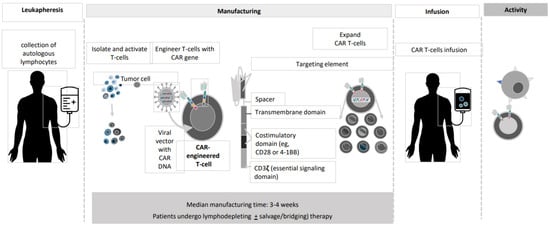
2. Anti-BCMACAR T-Cell Studies
|
Idecabtagene Vicleucel (bb2121) |
Ciltacabtagene Autoleucel (JNJ-4528) |
Orvacabtagene Autoleucel (JCAR-H125) |
Idecabtagene Vicleucel (ide-cel, bb2121) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author (year) |
Munshi (2020) |
Berdeja (2019) Alsina (2020) |
Berdeja (2020) Madduri (2020) |
Mailankody (2020) |
Lin (2020) |
||||||
|
Reference |
[24] |
[31] |
|||||||||
|
Study Name |
KarMMa |
CRB-402 |
CARTITUDE-1 |
EVOLVE |
CRB-401 |
||||||
|
Construct |
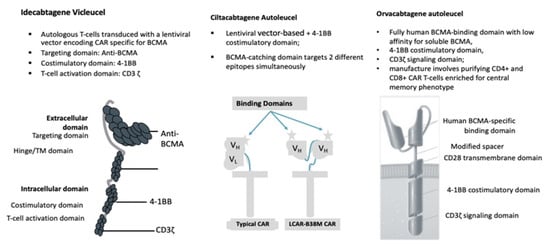
The ide-cel CAR is comprised of a murine extracellular single-chain variable fragment (scFv) specific for recognizing BCMA, attached to a human CD8 α hinge and transmembrane domain fused to the T-cell cytoplasmic signaling domains of CD137 4-1BB and CD3-ζ chain, in tandem |
Cells engineered with bb2121 construct are then ex vivo cultured with PI3K inhibitor bb007 |
2 BCMA-targeting single-domain antibodies to boost avidity plus a 4-1BB co-stimulatory domain |
Comprising fully human BCMA-binding domain with low affinity for soluble BCMA, 4-1BB co-stimulatory domain, CD3ζ signaling domain |
The same characteristics asthe KarMMa study |
||||||
|
Median Age |
61 (range 33–78) |
62 (range, 33–74) |
61 (range, 43–78) |
61 (range, 33–77) |
61 |
||||||
|
Cell Dose × 106 kg |
150 |
300 |
450 |
150 |
300 |
450 |
0.75 |
300 |
450 |
600 |
50, 150, 450, or 800 × 106 in the dose-escalation phase 150 to 450 × 106 in the dose-expansion phase. |
|
No. Patients |
4 |
70 |
55 |
12 |
28 |
20 |
97 (29 phase Ib/68 phase II) |
19 |
18 |
7 |
21 patients dose-escalation phase; 41 dose-expansion phase. |
|
Lymphodepletion |
FLU + CY |
FLU + CY |
FLU + CY |
FLU + CY |
FLU + CY |
||||||
|
Median Followup |
13.3 |
8.5 |
11.5 |
9.5 |
8.8 |
2.3 |
14.7 |
||||
B-cell maturation antigen (BCMA); Lymhodepletion consisted of Fludarabine (FLU) 30 mg/m2 × 3 days + Cyclophosphamide (CY) 300 mg/m2 × 3 days
|
Car t Cell Construct |
Idecabtagene Vicleucel (bb2121) |
Ciltacabtagene Autoleucel (JNJ-4528) |
Orvacabtagene Autoleucel (JCAR-H125) |
Idecabtagene Vicleucel (ide-cel, bb2121) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Author (Year) |
Munshi (2020) |
Berdeja (2019) Alsina (2020) |
Berdeja (2020) Madduri (2020) |
Mailankody (2020) |
Lin (2020) |
||||||
|
Study Name |
KarMMa |
CRB-402 |
CARTITUDE-1 |
EVOLVE |
CRB-401 |
||||||
|
Reference |
[24] |
[31] |
|||||||||
|
Response Rate |
|||||||||||
|
ORR |
50% |
69% |
82% |
83% |
43% |
73% |
97% |
95% |
89% |
92% |
76(%) |
|
CR |
25% |
29% |
39% |
18% |
67% |
37% |
42% |
29% |
39(%) |
||
|
Median DoR |
NR |
9.9 |
11.3 |
11.9 |
NR |
NR |
NR |
NR |
10.3(%) |
||
|
Median PFS |
2.8 |
5.8 |
12.1 |
NR |
NR |
NR |
NR |
9.3 |
NR |
NR |
8.8 (%) |
|
Evaluable for MRD |
4 |
70 |
54 |
7 |
6 |
4 |
57 |
11 |
11 |
3 |
37 |
|
MRD- |
50% |
31% |
48% |
100% |
83.3% |
100% |
93% |
72.7% |
90.9% |
100% |
81% |
|
CRS Event |
|||||||||||
|
All |
50% |
76% |
96% |
67% |
94.8% |
89% |
76(%) |
||||
|
Median Time to First Onset |
7 (2–12) |
2 (1–12) |
1 (1–10) |
3 (1–20) |
7 (1–12) |
2 (1–4) |
Nk |
||||
|
Grade 3–4 |
0 |
4% |
6% |
2% |
4% |
3% |
6(%) |
||||
|
Grade 5 |
0 |
1% |
0 |
2% |
1% |
0 |
0 |
||||
|
Neurotoxicities |
|||||||||||
|
All |
0 |
17% |
20% |
22% |
20.6% |
13% |
44% |
||||
|
Median Time to First Onset |
NA |
3 (1–10) |
2 (1–5) |
7 (3–24) |
8 (3–12) |
4 (1–6) |
Nk |
||||
|
Grade 3–4 |
0 |
7% |
12% |
4% |
10.3% |
3% |
3% |
||||
|
Grade 5 |
0 |
0 |
0 |
2% |
0 |
0 |
0 |
||||
B-cell maturation antigen (BCMA); median time to first onset expressed in days (range); median age expressed in years (range); median follow up and progression-free survival (PFS) expressed in months; overall response rate (ORR); complete remission (CR): median duration of response (DoR) expressed in months; not known (Nk).
References
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 1–10.
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.; Baljevic, M.; Campagnaro, E.; Castillo, J.J.; Chandler, J.C.; Costello, C.; Efebera, Y.; et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1685–1717.
- Bonello, F.; Grasso, M.; D’Agostino, M.; Celeghini, I.; Castellino, A.; Boccadoro, M.; Bringhen, S. The Role of Monoclonal Antibodies in the First-Line Treatment of Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma. Pharmaceuticals 2020, 14, 20.
- Park, S.; Byun, J.M.; Yoon, S.; Kim, K.; Jung, S.; Lee, J.; Min, C. Daratumumab monotherapy for relapsed/refractory multiple myeloma, focussed on clinical trial-unfit patients and subsequent therapy. Br. J. Haematol. 2021, 193, 101–112.
- Richter, J.; Thibaud, S. Anti-body building: The exercise of advancing immune based myeloma therapies. Blood Rev. 2020, 100789.
- Zamagni, Z.; Tacchetti, T.; Deias, D.; Patriarca, P. The Role of Monoclonal Antibodies in Smoldering and Newly Diagnosed Transplant-Eligible Multiple Myeloma. Pharmaceuticals 2020, 13, 451.
- Morè, S.; Petrucci, M.T.; Corvatta, L.; Fazio, F.; Offidani, M.; Olivieri, A. Monoclonal Antibodies: Leading Actors in the Relapsed/Refractory Multiple Myeloma Treatment. Pharmaceuticals 2020, 13, 426.
- Chong, L.L.; Soon, Y.Y.; Soekojo, C.Y.; Ooi, M.; Chng, W.J.; de Mel, S. Daratumumab-based induction therapy for multiple myeloma: A systematic review and meta-analysis. Crit. Rev. Oncol. 2021, 159, 103211.
- Richard, S.; Jagannath, S.; Cho, H.J.; Parekh, S.; Madduri, D.; Richter, J.; Chari, A. A comprehensive overview of daratumumab and carfilzomib and the recently approved daratumumab, carfilzomib and dexamethasone regimen in relapsed/refractory multiple myeloma. Expert Rev. Hematol. 2021, 14, 31–45.
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Špička, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 1–10.
- Richardson, P.G.; Beksaç, M.; Špička, I.; Mikhael, J. Isatuximab for the treatment of relapsed/refractory multiple myeloma. Expert Opin. Biol. Ther. 2020, 20, 1395–1404.
- McMillan, A.; Warcel, D.; Popat, R. Antibody-drug conjugates for multiple myeloma. Expert Opin. Biol. Ther. 2020, 12, 1–13.
- Sheikh, S.; Lebel, E.; Trudel, S. Belantamab mafodotin in the treatment of relapsed or refractory multiple myeloma. Future Oncol. 2020, 16, 2783–2798.
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13, 125.
- Carpenter, R.O.; Evbuomwan, M.O.; Pittaluga, S.; Rose, J.J.; Raffeld, M.; Yang, S.; Gress, R.E.; Hakim, F.T.; Kochenderfer, J.N. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple mye- loma. Clin. Cancer Res. 2013, 19, 2048–2060.
- Verkleij, C.; Frerichs, K.A.; Broekmans, M.; Absalah, S.; Maas-Bosman, P.; Kruyswijk, S.; Nijhof, I.S.; Mutis, T.; Zweegman, S.; van de Donk, N. T-cell redirecting bispecific antibodies targeting BCMA for the treatment of multiple myeloma. Oncotarget 2020, 11, 4076–4081.
- Palma, B.; Marchica, V.; Catarozzo, M.T.; Giuliani, N.; Accardi, F. Monoclonal and Bispecific Anti-BCMA Antibodies in Multiple Myeloma. J. Clin. Med. 2020, 9, 3022.
- Wudhikarn, K.; Mailankody, S.; Smith, E.L. Future of CAR T cells in multiple myeloma. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 272–279.
- Roex, G.; Timmers, M.; Wouters, K.; Campillo-Davo, D.; Flumens, D.; Schroyens, W.; Chu, Y.; Berneman, Z.N.; Lion, E.; Luo, F.; et al. Safety and clinical efficacy of BCMA CAR-T-cell therapy in multiple myeloma. J. Hematol. Oncol. 2020, 13, 164.
- Rodríguez-Otero, P.; Prósper, F.; Alfonso, A.; Paiva, B.; San Miguel, J.F. CAR T-Cells in Multiple Myeloma Are Ready for Prime Time. J. Clin. Med. 2020, 9, 3577.
- Rana, J.; Biswas, M. Regulatory T cell therapy: Current and future design perspectives. Cell Immunol. 2020, 356, 104193.
- Nadeem, O.; Tai, Y.T.; Anderson, K.C. Immunotherapeutic and Targeted Approaches in Multiple Myeloma. Immunotargets Ther. 2020, 9, 201–215.
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients withmultiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275.
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Jagannath, S.; Berdeja, J.G.; Lonial, S.; Raje, N.S.; Siegel, D.S.; Lin, Y.; Oriol, A.; et al. Idecabtagenevicleucel, a BCMA-targeted CAR T-cell therapy, in patients with relapsed and refractory multiple myeloma: Initial KarMMa results. J. Clin. Oncol. 2020, 38 (Suppl. S15), 8503.
- Berdeja, J.G.; Alsina, M.; Shah, N.; Siegal, D.S.; Jagannath, S.; Madduri, D.; Kaufman, J.L.; Munshi, N.C.; Rosenblatt, J.; Jasielec, J.K.; et al. Updated Results from an Ongoing Phase 1 Clinical Study of bb21217 Anti-Bcma CAR T Cell Therapy. Blood 2019, 134 (Suppl. 1), 927.
- Alsina, M.; Shah, N.; Raje, N.S.; Jagannath, S.; Madduri, D.; Kaufman, J.L.; Siegel, D.S.; Munshi, N.C.; Rosenblatt, J.; Lin, Y.; et al. Updated Results from the Phase I CRB-402 Study of Anti-Bcma CAR-T Cell Therapy bb21217 in Patients with Relapsed and Refractory Multiple Myeloma: Correlation of Expansion and Duration of Response with T Cell Phenotypes. Blood 2020, 136 (Suppl. 1), 25–26.
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Singh, I.; Zudaire, E.; Yeh, T.M.; Allred, A.J.; Olyslager, Y.; Banerjee, A.; Goldberg, J.D.; et al. Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen-directed CAR-T-cell therapy, in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020, 38 (Suppl. S15), 8505.
- Madduri, D.; Berdeja, J.G.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.; Stewart, A.K.; Hari, P.; Htut, M.; O’Donnell, E.; et al. CARTITUDE-1: Phase 1b/2 study of ciltacabtagene autoleucel, a B-cell maturation antigen–directed chimeric antigen receptor T cell therapy, in relapsed/refractory multiple myeloma. Blood 2020, 136 (Suppl. S1), 22–25.
- Mailankody, S.; Htut, M.; Lee, K.P.; Bensinger, W.; Devries, T.; Piasecki, J.; Ziyad, S.; Blake, M.; Byon, J.; Jakubowiak, A. JCARH125, anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: Initial proof of concept results from a phase 1/2 multicenter study (EVOLVE). Blood 2018, 132 (Suppl. S1), 957.
- Mailankody, S.; Jakubowiak, A.J.; Htut, M.; Costa, L.J.; Lee, K.; Ganguly, S.; Kaufman, J.L.; Siegel, D.S.; Bensinger, W.; Cota, M.; et al. Orvacabtageneautoleucel (orva-cel), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): Update of the phase 1/2 EVOLVE study (NCT03430011). J. Clin. Oncol. 2020, 38 (Suppl. S15), 8504.
- Lin, Y.; Raje, N.S.; Berdeja, J.G.; Siegel, D.S.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Massaro, M.; et al. Idecabtagene Vicleucel (ide-cel, bb2121), a BCMA-Directed CAR T Cell Therapy, in Patients with Relapsed and Refractory Multiple Myeloma: Updated Results from Phase 1 CRB-401. Blood 2020, 136 (Suppl. S1), 26–27.




