1. Introduction
Several drugs have shown activity in multiple myeloma (MM) and are available for clinical use. As a result, there are numerous regimens that use two or more of these drugs available for the treatment of newly diagnosed and refractory/relapsed MM. The major classes include alkylating agents (melphalan andcyclophosphamide), corticosteroids (dexamethasone andprednisone), immunomodulatory drugs (thalidomide, lenalidomide, and pomalidomide), and proteasome inhibitors (bortezomib, carfilzomib, andixazomib)
[1]. The treatment paradigm in MMis evolving with the introduction of various immunotherapies
[2]. First came the monoclonal antibodies (mAbs), which represented a paradigm shift in treating all MM stages
[3][4][5][6][7][3,4,5,6,7]. The initial mAbs included daratumumab, which targets CD38 on MM cells’ surface
[8][9][8,9], and elotuzumab, which targets SLAMF7
[10]. These new molecules were followed by isatuximab, which also targets CD38
[11]. The new agents have clearly shown significant activity and improved progression-free survival (PFS) in newly diagnosed and relapsed MM.
Other immunotherapy drugs are poised to alter the landscape of MM treatment going forward significantly. Antibody-Drug Conjugates (ADCs) represent a new platform
[12] and are best exemplified by belantamabmafodotin (BM)
[13]. BM is a mAb targeting B-cell maturation antigen (BCMA) linked to a microtubule-disrupting agent, monomethyl auristatin F (MMAF)
[14]. BCMA is a transmembrane glycoprotein and a member of the tumor necrosis factor (TNF) receptor superfamily, and it is preferentially expressed on mature B-cells and plasma cells in MM and plays an essential role in long-term plasma cell survival. The number of surface BCMA increases with the progression of the disease. Because of the localization to plasma cells and the expression on virtually no other cell type, BCMA can represent an ideal target for MM-specific cellular therapies
[15]. BM as a singleagent has been associated with a response rate >30% in patients with multiple previous lines of therapy, including those who do not respond to PIs, IMiDs, and mAbs. The drug is now being studied in combination with other agents in both upfront and relapsed settings. Besides, several other conjugate drugs targeting BCMA are under investigation in clinical trials
[16].
Another platform that has shown significant activity in patients with MM is bispecific antibodies (BiAbs)
[17]. BiAbs are designed to bring T-cells closer to the tumor cell by targeting both cell types simultaneously, promoting a more robust T-cell response and immune synapse formation to eliminate MM cells. With BiAbs, investigators reported few CRS rates, easy management, and very little neurotoxicity.
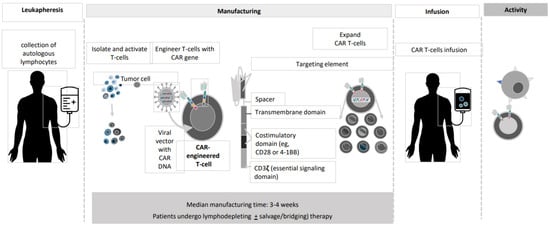
And last but not least, CAR T-cells. CAR T-cell transplantation technology has evolved dramatically during the past 25 years ()
[18][19][20][21][22][18,19,20,21,22]. The current version of BCMA-directed CAR T-cell therapy employs a construct with a single-chain variable fragment that recognizes the BCMA antigen, a spacer, an intracellular co-stimulatory signaling domain (often 4-1BB), and a CD3ζ intracellular domain to stimulate T-cell activation upon binding (). The initial wave of CAR T-cells that target BCMA has shown remarkable efficacy in MM.
Figure 1. CAR T manufacturing process.
Figure 2. BCMA CAR T-cell Contruct Design.
2. Anti-BCMACAR T-Cell Studies
Outcomes for patients with triple-exposed relapsed or refractory (R/R) MM have been poor, with a median PFS of only 3 to 4 months
[23]. Among R/R MM patients who have been exposed to IMIDs, PIs, mAbs, and have received multiple lines of therapy, there are no standard-of-care options. Often, the choice of treatment we offer is based on the availability of the utmost efficacious agent. Interest in CAR T-cell therapy in MM has been high, especially for products targeting BCMA. Several BCMA-targeted CAR T-cell therapies are in clinical development for patients with R/R MM, and trials’ results are published or presented at the most recent international congresses ( and ).
Table 1. Anti-BCMACAR T-Cell Studies: Main Patients’ characteristics.
| |
| Idecabtagene Vicleucel |
| (bb2121) |
|
| Ciltacabtagene Autoleucel |
| (JNJ-4528) |
|
| (JNJ-4528) |
|
| Orvacabtagene Autoleucel |
| (JCAR-H125) |
|
| Orvacabtagene Autoleucel |
| (JCAR-H125) |
|
| Idecabtagene Vicleucel |
| (ide-cel, bb2121) |
|
|
| Idecabtagene Vicleucel |
| (ide-cel, bb2121) |
|
|
| Author (year) |
|
| Munshi (2020) |
|
| Berdeja (2019) |
| Alsina (2020) |
|
| Berdeja (2020) |
| Madduri (2020) |
|
| Mailankody (2020) |
|
| Lin (2020) |
|
|
| Reference |
|
[24]
|
[25][26]
|
[25,26]
|
[27][28]
|
[27,28]
|
[29][30]
|
[29,30]
|
[31]
|
|
| Study Name |
|
| KarMMa |
|
[24]
| CRB-402 |
|
|
[25][26]
|
[25,26] |
| CARTITUDE-1 |
|
|
[27] |
| EVOLVE |
|
| CRB-401 |
|
[ |
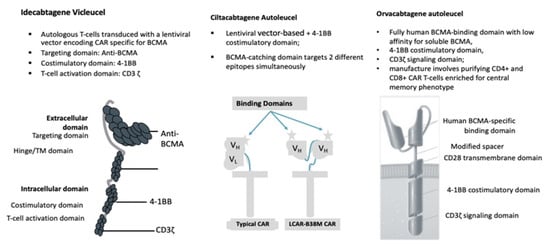
| Cells engineered with bb2121 construct are then ex vivo cultured with PI3K inhibitor bb007 |
|
| 2 BCMA-targeting single-domain antibodies to boost avidity plus a 4-1BB co-stimulatory domain |
|
| Comprising fully human BCMA-binding domain with low affinity for soluble BCMA, 4-1BB co-stimulatory domain, CD3ζ signaling domain |
|
| The same characteristics asthe KarMMa study |
|
|
| Median Age |
|
| 61 (range 33–78) |
|
| 62 (range, 33–74) |
|
| 61 (range, 43–78) |
|
| 61 (range, 33–77) |
|
| 61 |
|
|
| Cell Dose × 106 kg |
|
| 150 |
|
| 300 |
|
| 450 |
|
|
| Author (Year) |
|
| Munshi (2020) |
|
| Berdeja (2019) |
| Alsina (2020) |
|
| Berdeja (2020) |
| Madduri (2020) |
|
| Mailankody (2020) |
|
| Lin (2020) |
|
|
| Study Name |
|
| KarMMa |
|
| CRB-402 |
|
| CARTITUDE-1 |
|
| EVOLVE |
|
| CRB-401 |
|
28 | ] |
|
[27,28]
|
[29][30]
|
[29,30]
|
[31]
|
|
| Construct |
|
|
|
| Response Rate |
| The ide-cel CAR is comprised of a murine extracellular single-chain variable fragment (scFv) specific for recognizing BCMA, attached to a human CD8 α hinge and transmembrane domain fused to the T-cell cytoplasmic signaling domains of CD137 4-1BB and CD3-ζ chain, in tandem |
|
|
|
|
|
|
|
|
|
|
|
|
|
| ORR |
|
| 50% |
|
| 69% |
|
| 82% |
|
| 83% |
|
| 43% |
|
| 73% |
|
| 97% |
|
| 95% |
|
| 89% |
|
| 92% |
|
| 76(%) |
|
| 150 |
|
|
|
| CR |
|
| 25% |
| 300 |
|
| 29% |
|
| 39% |
| 450 |
|
| 0.75 |
|
| 18% |
| 300 |
|
|
| 450 |
|
| 67% |
|
| 37% |
|
| 42% |
|
| 29% |
|
| 39(%) |
| 600 |
|
| 50, 150, 450, or 800 × 106 in the dose-escalation phase 150 to 450 × 106 in the dose-expansion phase. |
|
|
| No. Patients |
|
|
|
| Median DoR |
| 4 |
|
| NR |
| 70 |
|
|
| 9.9 |
| 55 |
|
|
| 11.3 |
| 12 |
|
| 11.9 |
|
| 28 |
|
| NR |
| 20 |
|
| NR |
| 97 (29 phase Ib/68 phase II) |
|
|
| NR |
| 19 |
|
| 18 |
|
| NR |
| 7 |
|
| 21 patients dose-escalation phase; 41 dose-expansion phase. |
|
|
|
| 10.3(%) |
| Lymphodepletion |
| FLU + CY |
|
| FLU + CY |
|
|
| Reference |
|
|
| FLU + CY |
|
|
| FLU + CY |
|
| FLU + CY |
|
|
| Median Followup |
|
| 13.3 |
|
| 8.5 |
|
| 11.5 |
|
| 9.5 |
|
| 8.8 |
|
| 2.3 |
|
| 14.7 |
|
B-cell maturation antigen (BCMA); Lymhodepletion consisted of Fludarabine (FLU) 30 mg/m2 × 3 days + Cyclophosphamide (CY) 300 mg/m2 × 3 days
Table 2. anti-BCMA CAR T-Cell Studies: outcomes.
|
| Car t Cell Construct |
|
| Idecabtagene Vicleucel |
| (bb2121) |
|
| Ciltacabtagene Autoleucel |
|
|
Median PFS |
|
|
|
|
2.8 |
|
|
| 5.8 |
|
| 12.1 |
|
| NR |
|
| NR |
|
| NR |
|
| NR |
|
| 9.3 |
|
| NR |
|
| NR |
|
| 8.8 (%) |
|
|
| Evaluable for MRD |
|
| 4 |
|
| 70 |
|
| 54 |
|
| 7 |
|
| 6 |
|
| 4 |
|
| 57 |
|
| 11 |
|
| 11 |
|
| 3 |
|
| 37 |
|
|
| MRD- |
|
| 50% |
|
| 31% |
|
| 48% |
|
| 100% |
|
| 83.3% |
|
| 100% |
|
| 93% |
|
| 72.7% |
|
| 90.9% |
|
| 100% |
|
| 81% |
|
|
| CRS Event |
|
|
|
|
|
|
|
|
|
| All |
|
| 50% |
|
| 76% |
|
| 96% |
|
| 67% |
|
| 94.8% |
|
| 89% |
|
| 76(%) |
|
|
| Median Time to First Onset |
|
| 7 (2–12) |
|
| 2 (1–12) |
|
| 1 (1–10) |
|
| 3 (1–20) |
|
| 7 (1–12) |
|
| 2 (1–4) |
|
| Nk |
|
|
| Grade 3–4 |
|
| 0 |
|
| 4% |
|
| 6% |
|
| 2% |
|
| 4% |
|
| 3% |
|
| 6(%) |
|
|
| Grade 5 |
|
| 0 |
|
| 1% |
|
| 0 |
|
| 2% |
|
| 1% |
|
| 0 |
|
| 0 |
|
|
| Neurotoxicities |
|
|
|
|
|
|
|
|
|
| All |
|
| 0 |
|
| 17% |
|
| 20% |
|
| 22% |
|
| 20.6% |
|
| 13% |
|
| 44% |
|
|
| Median Time to First Onset |
|
| NA |
|
| 3 (1–10) |
|
| 2 (1–5) |
|
| 7 (3–24) |
|
| 8 (3–12) |
|
| 4 (1–6) |
|
| Nk |
|
|
| Grade 3–4 |
|
| 0 |
|
| 7% |
|
| 12% |
|
| 4% |
|
| 10.3% |
|
| 3% |
|
| 3% |
|
|
| Grade 5 |
|
| 0 |
|
| 0 |
|
| 0 |
|
| 2% |
|
| 0 |
|
| 0 |
|
| 0 |
|
B-cell maturation antigen (BCMA); median time to first onset expressed in days (range); median age expressed in years (range); median follow up and progression-free survival (PFS) expressed in months; overall response rate (ORR); complete remission (CR): median duration of response (DoR) expressed in months; not known (Nk).