
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Albert Adell | + 3800 word(s) | 3800 | 2021-06-10 08:36:00 | | | |
| 2 | Vivi Li | Meta information modification | 3800 | 2021-06-10 11:56:10 | | |
Video Upload Options
N-methyl-D-aspartate (NMDA) receptor antagonists such as phencyclidine (PCP), dizocilpine (MK-801) and ketamine have long been considered a model of schizophrenia, both in animals and humans. However, ketamine has been recently approved for treatment-resistant depression, although with severe restrictions. Interestingly, the dosage in both conditions is similar, and positive symptoms of schizophrenia appear before antidepressant effects emerge. Here, we describe the temporal mechanisms implicated in schizophrenia-like and antidepressant-like effects of NMDA blockade in rats, and postulate that such effects may indicate that NMDA receptor antagonists induce similar mechanistic effects, and only the basal pre-drug state of the organism delimitates the overall outcome.
1. Introduction
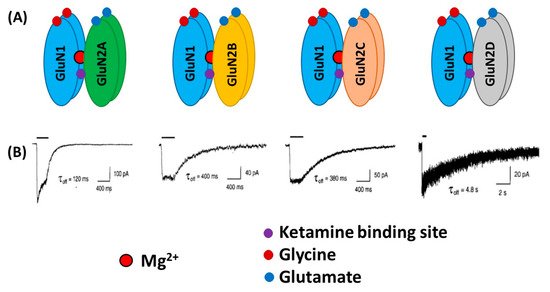
2. NMDA Receptors in Schizophrenia
2.1. Clinical Evidence
2.1.1. Neurophysiology
2.1.2. Post-Mortem Studies
2.2. Preclinical Evidence
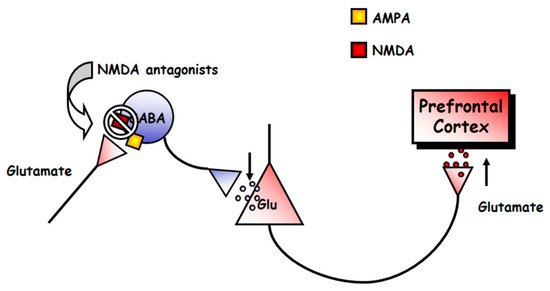
2.2.1. Neurophysiology
2.2.2. Animal Models
3. NMDA Receptors in Depression
3.1. Clinical Evidence
Post-Mortem Studies
3.2. Preclinical Evidence
References
- Pittenger, C.; Sanacora, G.; Krystal, J.H. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol. Disord. Drug Targets 2007, 6, 101–115.
- Hall, J.; Trent, S.; Thomas, K.L.; O’Donovan, M.C.; Owen, M.J. Genetic risk for schizophrenia: Convergence on synaptic pathways involved in plasticity. Biol. Psychiatry 2015, 77, 52–58.
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109.
- Collingridge, G.L.; Kehl, S.J.; McLennan, H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. 1983, 334, 33–46.
- Malenka, R.C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell 1994, 78, 535–538.
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496.
- Ulbrich, M.H.; Isacoff, E.Y. Rules of engagement for NMDA receptor subunits. Proc. Natl. Acad. Sci. USA 2008, 105, 14163–14168.
- Monyer, H.; Burnashev, N.; Laurie, D.J.; Sakmann, B.; Seeburg, P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 1994, 12, 529–540.
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105.
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61.
- Chergui, K. Dopamine induces a GluN2A-dependent form of long-term depression of NMDA synaptic responses in the nucleus accumbens. Neuropharmacology 2011, 60, 975–981.
- Mullasseril, P.; Hansen, K.B.; Vance, K.M.; Ogden, K.K.; Yuan, H.; Kurtkaya, N.L.; Santangelo, R.; Orr, A.G.; Le, P.; Vellano, K.M.; et al. A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat. Commun. 2010, 1, 90.
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414.
- Le Meur, K.; Galante, M.; Angulo, M.C.; Audinat, E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J. Physiol. 2007, 580, 373–383.
- Lozovaya, N.A.; Grebenyuk, S.E.; Tsintsadze, T.; Feng, B.; Monaghan, D.T.; Krishtal, O.A. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J. Physiol. 2004, 558, 451–463.
- Thomas, C.G.; Miller, A.J.; Westbrook, G.L. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 2006, 95, 1727–1734.
- Janssen, W.G.; Vissavajjhala, P.; Andrews, G.; Moran, T.; Hof, P.R.; Morrison, J.H. Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp. Neurol. 2005, 191, S28–S44.
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308.
- Krystal, J.H.; Karper, L.P.; Seibyl, J.P.; Freeman, G.K.; Delaney, R.; Bremner, J.D.; Heninger, G.R.; Bowers, M.B.J.; Charney, D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994, 51, 199–214.
- Malhotra, A.K.; Pinals, D.A.; Weingartner, H.; Sirocco, K.; Missar, C.D.; Pickar, D.; Breier, A. NMDA receptor function and human cognition: The effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996, 14, 301–307.
- Newcomer, J.W.; Farber, N.B.; Jevtovic-Todorovic, V.; Selke, G.; Melson, A.K.; Hershey, T.; Craft, S.; Olney, J.W. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 1999, 20, 106–118.
- Malhotra, A.K.; Adler, C.M.; Kennison, S.D.; Elman, I.; Pickar, D.; Breier, A. Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: A study with ketamine. Biol. Psychiatry 1997, 42, 664–668.
- Lahti, A.C.; Weiler, M.A.; Michaelidis, T.; Parwani, A.; Tamminga, C.A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 2001, 25, 455–467.
- Stone, J.M.; Erlandsson, K.; Arstad, E.; Squassante, L.; Teneggi, V.; Bressan, R.A.; Krystal, J.H.; Ell, P.J.; Pilowsky, L.S. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: A [123I] CNS-1261 SPET study. Psychopharmacology (Berl) 2008, 197, 401–408.
- Pilowsky, L.S.; Bressan, R.A.; Stone, J.M.; Erlandsson, K.; Mulligan, R.S.; Krystal, J.H.; Ell, P.J. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol. Psychiatry 2006, 11, 118–119.
- Kumari, V.; Soni, W.; Mathew, V.M.; Sharma, T. Prepulse inhibition of the startle response in men with schizophrenia: Effects of age of onset of illness, symptoms, and medication. Arch. Gen. Psychiatry 2000, 57, 609–614.
- Parwani, A.; Duncan, E.J.; Bartlett, E.; Madonick, S.H.; Efferen, T.R.; Rajan, R.; Sanfilipo, M.; Chappell, P.B.; Chakravorty, S.; Gonzenbach, S.; et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol. Psychiatry 2000, 47, 662–669.
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001, 156, 234–258.
- Duncan, E.J.; Madonick, S.H.; Parwani, A.; Angrist, B.; Rajan, R.; Chakravorty, S.; Efferen, T.R.; Szilagyi, S.; Stephanides, M.; Chappell, P.B.; et al. Clinical and sensorimotor gating effects of ketamine in normals. Neuropsychopharmacology 2001, 25, 72–83.
- Shelley, A.M.; Silipo, G.; Javitt, D.C. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: Implications for theories of cortical dysfunction. Schizophr. Res. 1999, 37, 65–79.
- Jessen, F.; Fries, T.; Kucharski, C.; Nishimura, T.; Hoenig, K.; Maier, W.; Falkai, P.; Heun, R. Amplitude reduction of the mismatch negativity in first-degree relatives of patients with schizophrenia. Neurosci. Lett. 2001, 309, 185–188.
- Michie, P.T.; Innes-Brown, H.; Todd, J.; Jablensky, A.V. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol. Psychiatry 2002, 52, 749–758.
- Umbricht, D.; Koller, R.; Vollenweider, F.X.; Schmid, L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol. Psychiatry 2002, 51, 400–406.
- Matsuura, M.; Yoshino, M.; Ohta, K.; Onda, H.; Nakajima, K.; Kojima, T. Clinical significance of diffuse delta EEG activity in chronic schizophrenia. Clin. Electroencephalogr. 1994, 25, 115–121.
- Winterer, G.; Coppola, R.; Goldberg, T.E.; Egan, M.F.; Jones, D.W.; Sanchez, C.E.; Weinberger, D.R. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am. J. Psychiatry 2004, 161, 490–500.
- Waberski, T.D.; Norra, C.; Kawohl, W.; Thyerlei, D.; Hock, D.; Klostermann, F.; Curio, G.; Buchner, H.; Hoff, P.; Gobbelé, R. Electrophysiological evidence for altered early cerebral somatosensory signal processing in schizophrenia. Psychophysiology 2004, 41, 361–366.
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113.
- Sun, Y.; Farzan, F.; Barr, M.S.; Kirihara, K.; Fitzgerald, P.B.; Light, G.A.; Daskalakis, Z.J. Gamma oscillations in schizophrenia: Mechanisms and clinical significance. Brain Res. 2011, 1413, 98–114.
- Buzsáki, G.; Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225.
- Tada, M.; Nagai, T.; Kirihara, K.; Koike, S.; Suga Maraki, T.; Kobayashi, T.; Kasai, K. Differential alterations of auditory gamma oscillatory responses between pre-onset high-risk individuals and first-episode schizophrenia. Cereb. Cortex 2016, 26, 1027–1035.
- Hirano, Y.; Oribe, N.; Kanba, S.; Onitsuka, T.; Nestor, P.G.; Spencer, K.M. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry 2015, 72, 813–821.
- Cardin, J.A.; Carlen, M.; Meletis, K.; Knoblich, U.; Zhang, F.; Deisseroth, K.; Tsai, L.H.; Moore, C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009, 459, 663–667.
- Sohal, V.S.; Zhang, F.; Yizhar, O.; Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459, 698–702.
- Gonzalez-Burgos, G.; Lewis, D.A. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008, 34, 944–961.
- Hashimoto, T.; Arion, D.; Unger, T.; Maldonado-Aviles, J.G.; Morris, H.M.; Volk, D.W.; Mimics, K.; Lewis, D.A. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 2008, 13, 147–161.
- Moyer, C.E.; Delevich, K.M.; Fish, K.N.; Asafu-Adjei, J.K.; Sampson, A.R.; Dorph-Petersen, K.A.; Lewis, D.A.; Sweet, R.A. Reduced glutamate decarboxylase 65 protein within primary auditory cortex inhibitory boutons in schizophrenia. Biol. Psychiatry 2012, 72, 734–743.
- Homayoun, H.; Moghaddam, B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007, 27, 11496–11500.
- Pinault, D. N-methyl D-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol. Psychiatry 2008, 63, 730–735.
- Hakami, T.; Jones, N.C.; Tolmacheva, E.A.; Gaudias, J.; Chaumont, J.; Salzberg, M.; O’Brien, T.J.; Pinault, D. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS ONE 2009, 4, e6755.
- Kocsis, B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol. Psychiatry 2012, 71, 987–995.
- Sullivan, E.M.; Timi, P.; Hong, L.E.; O’Donnell, P. Reverse translation of clinical electrophysiological biomarkers in behaving rodents under acute and chronic NMDA receptor antagonism. Neuropsychopharmacology 2015, 40, 719–727.
- Baldeweg, T.; Spence, S.; Hirsch, S.R.; Gruzelier, J. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet 1998, 352, 620–621.
- Lee, K.H.; Williams, L.M.; Haig, A.; Gordon, E. “Gamma (40 Hz) phase synchronicity” and symptom dimensions in schizophrenia. Cogn. Neuropsychiatry 2003, 8, 57–71.
- Spencer, K.M.; Nestor, P.G.; Perlmutter, R.; Niznikiewicz, M.A.; Klump, M.C.; Frumin, M.; Martha, E.; Shenton McCarley, R.W. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc. Natl. Acad. Sci. USA 2004, 101, 17288–17293.
- Brenner, C.A.; Krishnan, G.P.; Vohs, J.L.; Ahn, W.Y.; Hetrick, W.P.; Morzorati, S.L.; O’Donnell, B.F. Steady state responses: Electrophysiological assessment of sensory function in schizophrenia. Schizophr. Bull. 2009, 35, 1065–1077.
- Woo, T.U.; Spencer, K.; McCarley, R.W. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv. Rev. Psychiatry 2010, 18, 173–189.
- Plourde, G.; Baribeau, J.; Bonhomme, V. Ketamine increases the amplitude of the 40-Hz auditory steady-state response in humans. Br. J. Anaesth. 1997, 78, 524–529.
- Hong, L.E.; Summerfelt, A.; Buchanan, R.W.; O’Donnell, P.; Thaker, G.K.; Weiler, M.A.; Lahti, A.C. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 2010, 35, 632–640.
- Kocsis, B.; Brown, R.E.; McCarley, R.W.; Hajos, M. Impact of ketamine on neuronal network dynamics: Translational modeling of schizophrenia-relevant deficits. CNS Neurosci. Ther. 2013, 19, 437–447.
- Callicott, J.H.; Bertolino, A.; Mattay, V.S.; Langheim, F.J.; Duyn, J.; Coppola, R.; Goldberg, T.E.; Weinberger, D.R. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex 2000, 10, 1078–1092.
- Manoach, D.S.; Gollub, R.L.; Benson, E.S.; Searl, M.M.; Goff, D.C.; Halpern, E.; Saper, C.B.; Rauch, S.L. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol. Psychiatry 2000, 48, 99–109.
- Dienel, S.J.; Enwright, J.F., III; Hoftman, G.D.; Lewis, D.A. Markers of glutamate and GABA neurotransmission in the prefrontal cortex of schizophrenia subjects: Disease effects differ across anatomical levels of resolution. Schizophr. Res. 2020, 217, 86–94.
- Lahti, A.C.; Holcomb, H.H.; Medoff, D.R.; Tamminga, C.A. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 1995, 6, 869–872.
- Abdallah, C.G.; De Feyter, H.M.; Averill, L.A.; Jiang, L.; Averill, C.L.; Chowdhury, G.M.I.; Purohit, P.; de Graaf, R.A.; Esterlis, I.; Juchem, C.; et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 2018, 43, 2154–2160.
- Basar, E.; Schurmann, M.; Basar-Eroglu, C.; Karakas, S. Alpha oscillations in brain functioning: An integrative theory. Int. J. Psychophysiol. 1997, 26, 5–29.
- Basar-Eroglu, C.; Basar, E.; Demiralp, T.; Schurmann, M. P300-response: Possible psychophysiological correlates in delta and theta frequency channels. A review. Int. J. Psychophysiol. 1992, 13, 161–179.
- Miller, R. Cortico-Hippocampal Interplay and the Representation of Contexts in the Brain; Springer: Berlin, Germany, 1991.
- Klimesch, W.; Schimke, H.; Schwaiger, J. Episodic and semantic memory: An analysis in the EEG theta and alpha band. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 428–441.
- Basar, E.; Basar-Eroglu, C.; Karakas, S.; Schurmann, M. Are cognitive processes manifested in eventrelated gamma, alpha, theta and delta oscillations in the EEG? Neurosci. Lett. 1999, 259, 165–168.
- Catts, V.S.; Lai, Y.L.; Weickert, C.S.; Weickert, T.W.; Catts, S.V. A quantitative review of the postmortem evidence for decreased cortical N-methyl-D-aspartate receptor expression levels in schizophrenia: How can we link molecular abnormalities to mismatch negativity deficits? Biol. Psychology 2016, 116, 57–67.
- Sokolov, B.P. Expression of NMDAR1, GluR1, GluR7, and KA1 glutamate receptor mRNAs is decreased in frontal cortex of “neuroleptic-free” schizophrenics: Evidence on reversible up-regulation by typical neuroleptics. J. Neurochem. 1998, 71, 2454–2464.
- Catts, V.S.; Derminio, D.S.; Hahn, C.G.; Weickert, C.S. Postsynaptic density levels of the NMDA receptor NR1 subunit and PSD-95 protein in prefrontal cortex from people with schizophrenia. NPJ Schizophr. 2015, 1, 15037.
- Gao, X.M.; Sakai, K.; Roberts, R.C.; Conley, R.R.; Dean, B.; Tamminga, C.A. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: Effects of schizophrenia. Am. J. Psychiatry 2000, 157, 1141–1149.
- Law, A.J.; Deakin, J.F. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport 2001, 12, 2971–2974.
- Akbarian, S.; Sucher, N.J.; Bradley, D.; Tafazzoli, A.; Trinh, D.; Hetrick, W.P.; Potkin, S.G.; Sandman, C.A.; Bunney, W.E., Jr.; Jones, E.G. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J. Neurosci. 1996, 16, 19–30.
- Weickert, C.S.; Fung, S.J.; Catts, V.S.; Schofield, P.R.; Allen, K.M.; Moore, L.T.; Newell, K.A.; Pellen, D.; Huang, X.F.; Catts, S.V.; et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatry 2013, 18, 1185–1192.
- Banerjee, A.; Wang, H.Y.; Borgmann-Winter, K.E.; MacDonald, M.L.; Kaprielian, H.; Stucky, A.; Kvasic, J.; Egbujo, C.; Ray, R.; Talbot, K.; et al. Src kinase as a mediator of convergent molecular abnormalities leading to NMDAR hypoactivity in schizophrenia. Mol. Psychiatry 2015, 20, 1091–1100.
- Seeman, P.; Lee, T. Antipsychotic drugs: Direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 1975, 188, 1217–1219.
- Creese, I.; Burt, D.R.; Snyder, S.H. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 1976, 192, 481–483.
- Abi-Dargham, A.; Rodenhiser, J.; Printz, D.; Zea-Ponce, Y.; Gil, R.; Kegeles, L.S.; Weiss, R.; Cooper, T.B.; Mann, J.J.; Van Heertum, R.L.; et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. USA 2000, 97, 8104–8109.
- Vollenweider, F.X.; Vontobel, P.; Oye, I.; Hell, D.; Leenders, K.L. Effects of (S)-ketamine on striatal dopamine: A [11C] raclopride PET study of a model psychosis in humans. J. Psychiatr. Res. 2000, 34, 35–43.
- Kristiansen, L.V.; Huerta, I.; Beneyto, M.; Meador-Woodruff, J.H. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 2007, 7, 48–55.
- Moghaddam, B.; Javitt, D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012, 37, 4–15.
- Okabe, S.; Miwa, A.; Okado, H. Alternative splicing of the C-terminal domain regulates cell surface expression of the NMDA receptor NR1 subunit. J. Neurosci. 1999, 19, 7781–7792.
- Pauly, T.; Schlicksupp, A.; Neugebauer, R.; Kuhse, J. Synaptic targeting of N-methyl-D-aspartate receptor splice variants is regulated differentially by receptor activity. Neuroscience 2005, 131, 99–111.
- Bauer, D.; Gupta, D.; Harotunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr. Res. 2008, 104, 108–120.
- Kalandadze, A.; Wu, Y.; Fournier, K.; Robinson, M.B. Identification of motifs involved in endoplasmic reticulum retention-forward trafficking of the GLT-1 subtype of glutamate transporter. J. Neurosci. 2004, 24, 5183–5192.
- Spangaro, M.; Bosia, M.; Zanoletti, A.; Bechi, M.; Mariachiara, B.; Pirovano, A.; Lorenzi, C.; Bramanti, P.; Smeraldi, E.; Cavallaro, R. Exploring effects of EAAT polymorphisms on cognitive functions in schizophrenia. Pharmacogenomics 2014, 15, 925–932.
- Dietz, A.G.; Goldman, S.A.; Nedergaard, M. Glial cells in schizophrenia: A unified hypothesis. Lancet Psychiatry 2020, 7, 272–281.
- Homayoun, H.; Stefani, M.R.; Adams, B.W.; Tamagan, G.D.; Moghaddam, B. Functional interaction between NMDA and mGlu5 receptors: Effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 2004, 29, 1259–1269.
- Lipska, B.K.; Weinberger, D.R. Animal models of schizophrenia. In Schizophrenia; Hirsch, S.R., Weinberger, D.R., Eds.; Blackwell Science: Malden, UK, 2003; pp. 388–402.
- Sams-Dodd, F. Effect of novel antipsychotic drugs on phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behav. Pharmacol. 1997, 8, 196–215.
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612.
- Jentsch, J.D.; Tran, A.; Taylor, J.R.; Roth, R.H. Prefrontal cortical involvement in phencyclidine-induced activation of the mesolimbic dopamine system: Behavioral and neurochemical evidence. Psychopharmacology (Berlin) 1998, 138, 89–95.
- Bakshi, V.P.; Tricklebank, M.; Neijt, H.C.; Lehmann-Masten, V.; Geyer, M.A. Disruption of prepulse inhibition and increases in locomotor activity by competitive N-methyl-D-aspartate receptor antagonists in rats. J. Pharmacol. Exp. Ther. 1999, 288, 643–652.
- Broberg, B.V.; Oranje, B.; Glenthøj, B.Y.; Fejgin, K.; Plath, N.; Bastlund, J.F. Assessment of auditory sensory processing in a neurodevelopmental animal model of schizophrenia--gating of auditory-evoked potentials and prepulse inhibition. Behav. Brain Res. 2010, 213, 142–147.
- Suzuki, Y.; Jodo, E.; Takeuchi, S.; Niwa, S.; Kayama, Y. Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience 2002, 114, 769–779.
- Jackson, M.E.; Homayoun, H.; Moghaddam, B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 8467–8472.
- Kargieman, L.; Santana, N.; Mengod, G.; Celada, P.; Artigas, F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc. Natl. Acad. Sci. USA 2007, 104, 14843–14848.
- López-Gil, X.; Jiménez-Sánchez, L.; Romón, T.; Campa, L.; Artigas, F.; Adell, A. Importance of inter-hemispheric prefrontal connection in the effects of non-competitive NMDA receptor antagonists. Int. J. Neuropsychopharmacol. 2012, 15, 945–956.
- Lladó-Pelfort, L.; Celada, P.; Riga, M.S.; Troyano-Rodríguez, E.; Santana, N.; Artigas, F. Effects of hallucinogens on neuronal activity. Curr. Top. Behav. Neurosci. 2018, 36, 75–105.
- Moghaddam, B.; Adams, B.; Verma, A.; Daly, D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997, 17, 2921–2927.
- Adams, B.W.; Moghaddam, B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol. Psychiatry 2001, 50, 750–757.
- Lorrain, D.S.; Baccei, C.S.; Bristow, L.J.; Anderson, J.J.; Varney, M.A. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: Modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 2003, 117, 697–706.
- Moghaddam, B.; Adams, B.W. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 1998, 281, 1349–1352.
- Mathé, J.M.; Nomikos, G.G.; Blakeman, K.H.; Svensson, T.H. Differential actions of dizocilpine (MK-801) on the mesolimbic and mesocortical dopamine systems: Role of neuronal activity. Neuropharmacology 1999, 38, 121–128.
- Schmidt, C.J.; Fadayel, G.M. Regional effects of MK-801 on dopamine release: Effects of competitive NMDA or 5-HT2A receptor blockade. J. Pharmacol. Exp. Ther. 1996, 277, 1541–1549.
- Martin, P.; Carlsson, M.L.; Hjorth, S. Systemic PCP treatment elevates brain extracellular 5-HT: A microdialysis study in awake rats. Neuroreport 1998, 9, 2985–2988.
- Millan, M.J.; Brocco, M.; Gobert, A.; Joly, F.; Bervoets, K.; Rivet, J.; Newman-Tancredi, A.; Audinot, V.; Maurel, S. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: Importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur. J. Neurosci. 1999, 11, 4419–4432.
- Amargós-Bosch, M.; López-Gil, X.; Artigas, F.; Adell, A. Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int. J. Neuropsychopharmacol. 2006, 9, 565–573.
- Schiffer, W.K.; Gerasimov, M.; Hofmann, L.; Marsteller, D.; Ashby, C.R.; Brodie, J.D.; Alexoff, D.L.; Dewey, S.L. Gamma vinyl-GABA differentially modulates NMDA antagonist-induced increases in mesocortical versus mesolimbic DA transmission. Neuropsychopharmacology 2001, 25, 704–712.
- Nelson, C.L.; Burk, J.A.; Bruno, J.P.; Sarter, M. Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology (Berl) 2002, 161, 168–179.
- Jiménez-Sánchez, L.; Castañé, A.; Pérez-Caballero, L.; Grifoll-Escoda, M.; López-Gil, X.; Campa, L.; Galofré, M.; Berrocoso, E.; Adell, A. Activation of AMPA receptors mediates the antidepressant action of deep brain stimulation of the infralimbic prefrontal cortex. Cereb. Cortex 2016, 26, 2778–2789.
- López-Gil, X.; Jiménez-Sánchez, L.; Campa, L.; Castro, E.; Frago, C.; Adell, A. Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chem. Neurosci. 2019, 10, 3318–3326.
- Krystal, J.H.; D’Souza, D.C.; Mathalon, D.; Perry, E.; Belger, A.; Hoffman, R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: Toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003, 169, 215–233.
- Nyíri, G.; Stephenson, F.A.; Freund, T.F.; Somogyi, P. Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience 2003, 119, 347–363.
- Freund, T.F.; Katona, I. Perisomatic inhibition. Neuron 2007, 56, 33–42.
- Razoux, F.; Garcia, R.; Lena, I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology 2007, 32, 719–727.
- Kim, S.H.; Price, M.T.; Olney, J.W.; Farber, N.B. Excessive cerebrocortical release of acetylcholine induced by NMDA antagonists is reduced by GABAergic and alpha2-adrenergic agonists. Mol. Psychiatry 1999, 4, 344–352.
- Hutson, P.H.; Hogg, J.E. Effects of and interactions between antagonists for different sites on the NMDA receptor complex on hippocampal and striatal acetylcholine efflux in vivo. Eur. J. Pharmacol. 1996, 295, 45–52.
- Yan, Q.S.; Reith, M.E.; Jobe, P.C.; Dailey, J.W. Dizocilpine (MK-801) increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Res. 1997, 765, 149–158.
- Lorrain, D.S.; Schaffhauser, H.; Campbell, U.C.; Baccei, C.S.; Correa, L.D.; Rowe, B.; Rodriguez, D.E.; Anderson, J.J.; Varney, M.A.; Pinkerton, A.B.; et al. Group II mGlu receptor activation suppresses norepinephrine release in the ventral hippocampus and locomotor responses to acute ketamine challenge. Neuropsychopharmacology 2003, 28, 1622–1632.
- Swanson, C.J.; Schoepp, D.D. A role for noradrenergic transmission in the actions of phencyclidine and the antipsychotic and antistress effects of mGlu2/3 receptor agonists. Ann. N. Y. Acad. Sci. 2003, 1003, 309–317.
- Whitton, P.S.; Maione, S.; Biggs, C.S.; Fowler, L.J. N-methyl-d-aspartate receptors modulate extracellular dopamine concentration and metabolism in rat hippocampus and striatum in vivo. Brain Res. 1994, 635, 312–316.
- Kretschmer, B.D. NMDA receptor antagonist-induced dopamine release in the ventral pallidum does not correlate with motor activation. Brain Res. 2000, 859, 147–156.
- Greenslade, R.G.; Mitchell, S.N. Selective action of (-)-2-oxa-4-aminobicyclo [3.1.0] hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell. Neuropharmacology 2004, 47, 1–8.
- Fitzgerald, P.J.; Watson, B.O. In vivo electrophysiological recordings of the effects of antidepressant drugs. Exp. Brain Res. 2019, 237, 1593–1614.
- Pitkänen, M.; Sirviö, J.; Ylinen, A.; Koivisto, E.; Riekkinen, P. Effects of NMDA receptor modulation on hippocampal type 2 theta activity in rats. Gen. Pharmacol. 1995, 26, 1065–1070.
- Lazarewicz, M.T.; Ehrlichman, R.S.; Maxwell, C.R.; Gandal, M.J.; Finkel, L.H.; Siegel, S.J. Ketamine modulates theta and gamma oscillations. J. Cogn. Neurosci. 2010, 22, 1452–1464.
- Troyano-Rodriguez, E.; Lladó-Pelfort, L.; Santana, N.; Teruel-Martí, V.; Celada, P.; Artigas, F. Phencyclidine inhibits the activity of thalamic reticular gamma-aminobutyric acidergic neurons in rat brain. Biol. Psychiatry 2014, 76, 937–945.
- Adell, A.; Jiménez-Sánchez, L.; López-Gil, X.; Romón, T. Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr. Bull. 2012, 38, 9–14.
- Egerton, A.; Reid, L.; McGregor, S.; Cochran, S.M.; Morris, B.J.; Pratt, J.A. Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology (Berl) 2008, 198, 37–49.
- Jentsch, J.D.; Roth, R.H. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999, 20, 201–225.
- Neill, J.C.; Harte, M.K.; Haddad, P.M.; Lydall, E.S.; Dwyer, D.M. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: A translational link to humans. Eur. Neuropsychopharmacol. 2014, 24, 822–823.
- Spielewoy, C.; Markou, A. Withdrawal from chronic phencyclidine treatment induces long-lasting depression in brain reward function. Neuropsychopharmacology 2003, 28, 1106–1116.
- Thomson, D.M.; McVie, A.; Morris, B.J.; Pratt, J.A. Dissociation of acute and chronic intermittent phencyclidine-induced performance deficits in the 5-choice serial reaction time task: Influence of clozapine. Psychopharmacology (Berl) 2011, 213, 681–695.
- Mouri, A.; Koseki, T.; Narusawa, S.; Niwa, M.; Mamiya, T.; Kano, S.; Sawa, A.; Nabeshima, T. Mouse strain differences in phencyclidine-induced behavioural changes. Int. J. Neuropsychopharmacol. 2012, 15, 767–779.
- Xu, X.; Domino, E.F. Genetic differences in the locomotor response to single and daily doses of phencyclidine in inbred mouse strains. Behav. Pharmacol. 1994, 5, 623–629.
- Castañé, A.; Santana, N.; Artigas, F. PCP-based mice models of schizophrenia: Differential behavioral, neurochemical and cellular effects of acute and subchronic treatments. Psychopharmacology (Berl) 2015, 232, 4085–4097.
- Kondziella, D.; Brenner, E.; Eyjolfsson, E.M.; Markinhuhta, K.R.; Carlsson, M.L.; Sonnewald, U. Glial-neuronal interactions are impaired in the schizophrenia model of repeated MK801 exposure. Neuropsychopharmacology 2006, 31, 1880–1887.
- Karlsson, R.M.; Tanaka, K.; Heilig, M.; Holmes, A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: Rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol. Psychiatry 2008, 64, 810–814.
- Zhang, C.; Li, Z.; Wu, Z.; Chen, J.; Wang, Z.; Peng, D.; Hong, W.; Yuan, C.; Wang, Z.; Yu, S.; et al. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology (Berl) 2014, 231, 685–693.
- Niciu, M.J.; Ionescu, D.F.; Richards, E.M.; Zarate, C.A.J. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J. Neural Transm. 2014, 121, 907–924.
- Kaut, O.; Schmitt, I.; Hofmann, A.; Hoffmann, P.; Schlaepfer, T.E.; Wüllner, U.; Hurlemann, R. Aberrant NMDA receptor DNA methylation detected by epigenome-wide analysis of hippocampus and prefrontal cortex in major depression. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 331–341.
- Marsden, W.N. Stressor-induced NMDAR dysfunction as a unifying hypothesis of the aetiology, pathogenesis and comorbidity of clinical depression. Med. Hypotheses 2011, 77, 508–528.
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354.
- Zarate, C.A.J.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864.
- Jaso, B.A.; Niciu, M.J.; Iadarola, N.D.; Lally, N.; Richards, E.M.; Park, M.; Ballard, E.D.; Nugent, A.C.; Machado-Vieira, R.; Zarate, C.A.J. Therapeutic modulation of glutamate receptors in major depressive disorder. Curr. Neuropharmacol. 2017, 15, 57–70.
- Paul, I.A.; Nowak, G.; Layer, R.T.; Popik, P.; Skolnick, P. Adaptation of the NMDA receptor complex following chronic antidepressant treatments. J. Pharmacol. Ther. 1994, 269, 95–102.
- Nowak, G.; Li, Y.; Paul, I.A. Adaptation of cortical but not hippocampal NMDA receptors after chronic citalopram treatment. Eur. J. Pharmacol. 1996, 295, 75–85.
- Skolnick, P.; Popik, P.; Trullas, R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009, 30, 563–569.
- Mathews, D.C.; Henter, I.D.; Zarate, C.A. Targeting the glutamatergic system to treat major depressive disorder: Rationale and progress to date. Drugs 2012, 72, 1313–1333.
- Ghasemi, M.; Phillips, C.; Trillo, L.; De Miguel, Z.; Das, D.; Salehi, A. The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci. Biobehav. Rev. 2014, 47, 336–358.
- Naughton, M.; Clarke, G.; O’Leary, O.F.; Cryan, J.F.; Dinan, T.G. A review of ketamine in affective disorders: Current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J. Affect. Disord. 2014, 156, 24–35.
- Deutschenbaur, L.; Beck, J.; Kiyhankhadiv, A.; Mühlhauser, M.; Borgwardt, S.; Walter, M.; Hasler, G.; Sollberger, D.; Lang, U.E. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 325–333.
- Feyissa, A.M.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 70–75.
- Beneyto, M.; Kristiansen, L.V.; Oni-Orisan, A.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 2007, 32, 1888–1902.
- Karolewicz, B.; Szebeni, K.; Gilmore, T.; Maciag, D.; Stockmeier, C.A.; Ordway, G.A. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int. J. Neuropsychopharmacol. 2009, 12, 143–153.
- Rodríguez-Muñoz, M.; Sánchez-Blázquez, P.; Callado, L.F.; Meana, J.J.; Garzón-Niño, J. Schizophrenia and depression, two poles of endocannabinoid system deregulation. Transl. Psychiatry 2017, 7, 1291.
- Karolewicz, B.; Stockmeier, C.; Ordway, G.A. Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology 2005, 30, 1557–1567.
- Underwood, M.D.; Bakalian, M.J.; Johnson, V.L.; Kassir, S.A.; Ellis, S.P.; Mann, J.J.; Arango, V. Less NMDA receptor binding in dorsolateral prefrontal cortex and anterior cingulate cortex associated with reported early life adversity but not suicide. Int. J. Neuropsychopharmacol. 2020.
- McCarthy, D.J.; Alexander, R.; Smith, M.A.; Pathak, S.; Kanes, S.; Lee, C.M.; Sanacora, G. Glutamate-based depression GBD. Med. Hypotheses 2012, 78, 675–681.
- Chandley, M.J.; Szebeni, A.; Szebeni, K.; Crawford, J.D.; Stockmeier, C.A.; Turecki, G.; Kostrzewa, R.M.; Ordway, G.A. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int. J. Neuropsychopharmacol. 2014, 17, 1569–1578.
- Gray, A.L.; Hyde, T.M.; Deep-Soboslay, A.; Kleinman, J.E.; Sodhi, M.S. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol. Psychiatry 2015, 20, 1057–1068.
- Lin, E.; Kuo, P.H.; Liu, Y.L.; Yu, Y.W.; Yang, A.C.; Tsai, S.J. A deep learning approach for predicting antidepressant response in major depression using clinical and genetic biomarkers. Front. Psychiatry 2018, 9, 290.
- Weder, N.; Zhang, H.; Jensen, K.; Yang, B.Z.; Simen, A.; Jackowski, A.; Lipschitz, D.; Douglas-Palumberi, H.; Ge, M.; Perepletchikova, F.; et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 417–424.
- Calabrese, F.; Guidotti, G.; Molteni, R.; Racagni, G.; Mancini, M.; Riva, M.A. Stress-induced changes of hippocampal NMDA receptors: Modulation by duloxetine treatment. PLoS ONE 2012, 7, e37916.
- Wang, Q.; Jie, W.; Liu, J.H.; Yang, J.M.; Gao, T.M. An astroglial basis of major depressive disorder? An overview. Glia 2017, 65, 1227–1250.
- Czéh, B.; Nagy, S.A. Clinical findings documenting cellular and molecular abnormalities of glia in depressive disorders. Front. Mol. Neurosci. 2018, 11, 56.
- Ongür, D.; Drevets, W.C.; Price, J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. USA 1998, 95, 13290–13295.
- Cotter, D.; Mackay, D.; Chana, G.; Beasley, C.; Landau, S.; Everall, I.P. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex 2002, 12, 386–394.
- Rajkowska, G.; Stockmeier, C.A. Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr. Drug Targets 2013, 14, 1225–1236.
- Cobb, J.A.; Simpson, J.; Mahajan, G.J.; Overholser, J.C.; Jurjus, G.J.; Dieter, L.; Herbst, N.; May, W.; Rajkowska, G.; Stockmeier, C.A. Hippocampal volume and total cell numbers in major depressive disorder. J. Psychiatr. Res. 2013, 47, 299–306.
- Miguel-Hidalgo, J.J.; Baucom, C.; Dilley, G.; Overholser, J.C.; Meltzer, H.Y.; Stockmeier, C.A.; Rajkowska, G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol. Psychiatry 2000, 48, 861–873.
- Moghaddam, B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: Comparison to hippocampus and basal ganglia. J. Neurochem. 1993, 60, 1650–1657.
- Bartanusz, V. Stress-induced changes in messenger RNA levels of N-methyl-d-aspartate and AMPA receptor subunits in selected regions of the rat hippocampus and hypothalamus. Neuroscience 1995, 66, 247–252.
- Fitzgerald, L.W.; Ortiz, J.; Hamedani, A.G.; Nestler, E.J. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: Common adaptations among cross-sensitizing agents. J. Neurosci. 1996, 16, 274–282.
- Shepard, R.; Page, C.E.; Coutellier, L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience 2016, 332, 1–12.
- Page, C.E.; Shepard, R.; Heslin, K.; Coutellier, L. Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Sci. Rep. 2019, 9, 19772.
- Ji, M.H.; Zhang, L.; Mao, M.J.; Zhang, H.; Yang, J.J.; Qiu, L.L. Overinhibition mediated by parvalbumin interneurons might contribute to depression-like behavior and working memory impairment induced by lipopolysaccharide challenge. Behav. Brain Res. 2020, 383, 112509.
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell. Neurosci. 2019, 13, 87.
- Masrour, F.F.; Peeri, M.; Azarbayjani, M.A.; Hosseini, M.J. Voluntary exercise during adolescence mitigated negative the effects of maternal separation stress on the depressive-like behaviors of adult male rats: Role of NMDA receptors. Neurochem. Res. 2018, 43, 1067–1074.
- Sathyanesan, M.; Haiar, J.M.; Watt, M.J.; Newton, S.S. Restraint stress differentially regulates inflammation and glutamate receptor gene expression in the hippocampus of C57BL/6 and BALB/c mice. Stress 2017, 20, 197–204.
- Pacheco, A.; Aguayo, F.I.; Aliaga, E.; Muñoz, M.; García-Rojo, G.; Olave, F.A.; Parra-Fiedler, N.A.; García-Pérez, A.; Tejos-Bravo, M.; Rojas, P.S.; et al. Chronic stress triggers expression of immediate early genes and differentially affects the expression of AMPA and NMDA subunits in dorsal and ventral hippocampus of rats. Front. Mol. Neurosci. 2017, 10, 244.
- Weiland, N.G.; Orchinik, M.; Tanapat, P. Chronic corticosterone treatment induces parallel changes in N-methyl-D-aspartate receptor subunit messenger RNA levels and antagonist binding sites in the hippocampus. Neuroscience 1997, 78, 653–662.
- Webster, H.H.; Flores, G.; Marcotte, E.R.; Cecyre, D.; Quirion, R.; Srivastava, L.K. Olfactory bulbectomy alters NMDA receptor levels in the rat prefrontal cortex. Synapse 2000, 37, 159–162.
- Ho, Y.J.; Liu, T.M.; Tai, M.Y.; Wen, Z.H.; Chow, R.S.S.; Tsai, Y.F.; Wong, C.S. Effects of olfactory bulbectomy on NMDA receptor density in the rat brain: [3H] MK-801 binding assay. Brain Res. 2001, 900, 214–218.
- Dong, B.E.; Chen, H.; Sakata, K. BDNF deficiency and enriched environment treatment affect neurotransmitter gene expression differently across ages. J. Neurochem. 2020.
- Boyce-Rustay, J.M.; Holmes, A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology 2006, 31, 2405–2414.
- Salimando, G.J.; Hyun, M.; Boyt, K.M.; Winder, D.G. BNST GluN2D-containing NMDA receptors influence anxiety- and depressive-like behaviors and modulate cell-specific excitatory/inhibitory synaptic balance. J. Neurosci. 2020, 40, 3949–3968.
- Forrest, D.; Yuzaki, M.; Soares, H.D.; Ng, L.; Luk, D.C.; Sheng, M.; Stewart, C.L.; Morgan, J.I.; Connor, J.A.; Curran, T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron 1994, 13, 325–338.
- Kutsuwada, T.; Sakimura, K.; Manabe, T.; Takayama, C.; Katakura, N.; Kushiya, E.; Natsume, R.; Watanabe, M.; Inoue, Y.; Yagi, T.; et al. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron 1996, 16, 333–344.
- Montalvo-Ortiz, J.L.; Bordner, K.A.; Carlyle, B.C.; Gelernter, J.; Simen, A.A.; Kaufman, J. The role of genes involved in stress, neural plasticity, and brain circuitry in depressive phenotypes: Convergent findings in a mouse model of neglect. Behav. Brain Res. 2016, 315, 71–74.
- Tordera, R.M.; Garcia-García, A.L.; Elizalde, N.; Segura, V.; Aso, E.; Venzala, E.; Ramírez, M.J.; Del Rio, J. Chronic stress and impaired glutamate function elicit a depressive-like phenotype and common changes in gene expression in the mouse frontal cortex. Eur. Neuropsychopharmacol. 2011, 21, 23–32.
- Sanacora, G.; Banasr, M. From pathophysiology to novel antidepressant drugs: Glial contributions to the pathology and treatment of mood disorders. Biol. Psychiatry 2013, 73, 1172–1179.
- Banasr, M.; Chowdhury, G.M.; Terwilliger, R.; Newton, S.S.; Duman, R.S.; Behar, K.L.; Sanacora, G. Glial pathology in an animal model of depression: Reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol. Psychiatry 2010, 15, 501–511.
- Gong, Y.; Sun, X.L.; Wu, F.F.; Su, C.J.; Ding, J.H.; Hu, G. Female early adult depression results in detrimental impacts on the behavioral performance and brain development in offspring. CNS Neurosci. Ther. 2012, 18, 461–470.
- Banasr, M.; Duman, R.S. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatry 2008, 64, 863–870.
- Fullana, M.N.; Ruiz-Bronchal, E.; Ferrés-Coy, A.; Juárez-Escoto, E.; Artigas, F.; Bortolozzi, A. Regionally selective knockdown of astroglial glutamate transporters in infralimbic cortex induces a depressive phenotype in mice. Glia 2019, 67, 1122–1137.




