
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Timo Gaber | + 1793 word(s) | 1793 | 2021-04-15 05:46:48 | | | |
| 2 | Peter Tang | Meta information modification | 1793 | 2021-06-09 10:57:30 | | |
Video Upload Options
Oxygen availability varies throughout the human body in health and disease. Under physiological conditions, oxygen availability drops from the lungs over the blood stream towards the different tissues into the cells and the mitochondrial cavities leading to physiological low oxygen conditions or physiological hypoxia in all organs including primary lymphoid organs. Moreover, immune cells travel throughout the body searching for damaged cells and foreign antigens facing a variety of oxygen levels.
1. Introduction
Immune cells and proper immune response require focal sites of immune cell development, maturation, activation, tolerance, and longevity also defined as immunological niches bearing a certain microenvironment to maintain immune homeostasis [1]. These organs and tissues include the bone marrow, placenta, intestinal mucosa, renal medulla, secondary lymphoid organs, and the thymus [2][3]. In tissue pathology, sites of high immunological activity lead to inflammation and as a result tissue dysfunction bearing certain pathological microenvironment features. These pathological sites include infected, inflamed, and ischemic tissues and tumors [4][5][6][7]. Of note, sites of immune activity with distinct microenvironmental entities can broadly range between a state of immune homeostasis and a state of immune pathology. Under certain conditions of severe and disorganized immune activity, inflammation can perpetuate as a result of immune dysfunction leading to autoimmunity or culminates into inflammation-driven tumor development [8]. Microenvironmental conditions at sites of physiological and pathological immune activity play a key role in the development of effective immune response and pathological immune dysfunction by modulation of immune cell function. Understanding the impact of the microenvironment in sites of immune activity and adaptation mechanisms in immune cell reprogramming may yield into new therapeutic treatment strategies against a dysfunctional immune response as found in autoimmunity and cancer. At sites of immune activity under physiological and pathophysiological circumstances, immune cells become highly metabolically active and activate bystander cells and surrounding tissue. As a result, microenvironmental features rapidly change by increasing the amount and number of humoral factors, metabolites and a decrease in oxygen leading to a state of hypoxia—a condition where cellular oxygen demand exceeds the oxygen supply [9][10]. Constant supply of oxygen is a prerequisite for the energy homeostasis of respiring cells. Oxygen plays a vital role in all eukaryotes, being the terminal electron acceptor of the mitochondrial electron transport chain, which finally feeds the proton gradient for the generation of ATP via oxidative phosphorylation. If the constant supply with oxygen does not anymore meet the requirements of cells, hypoxic conditions will be established and, if sustained, these conditions will ultimately result in cell death. Hypoxia arises in a variety of immunological situations under physiological and pathophysiological immune activity [10][11].
2. Physiological Hypoxia Influences Immunity
Fundamental principle of the vasculature is to supply all organs, tissues and cells with oxygen and nutrients according to their needs and to dispose of refuse (carbon dioxide and metabolic products) establishing a balance between supply and consumption which is unique for the respective organ, tissue and cell. With regard to oxygen, its availability to the cells in the human body depends on various factors, such as (i) oxygen uptake, (ii) the transport capacity of the blood, (iii) the transport of the oxygen carrier, i.e., vascularization, and finally (iv) cell respiration itself.
Even under physiological conditions oxygen partial pressure (pO2) varies throughout the human body (Figure 1) [12][13][14][15]. Arterial blood owns an average oxygen partial pressure of ~80–100 mmHg which corresponds to an oxygen air-content (O2 air-content) at sea level of 10–12.5%. The extreme values are 100 mmHg in the pulmonary veins and 40 mmHg in the pulmonary arteries. The tissue oxygen partial pressure varies depending on the tissue anatomy and function in the range of 30–50 mmHg (~3–6% O2 air-content) dropping to a cellular range of 9.9–19 mmHg (~1–2% O2 air-content) and further to a mitochondrial pO2 of <9.9 mmHg (~1% O2 air-content) [13]. Consequently, current standardized cell culture conditions are oriented towards of atmospheric pO2 with oxygen concentrations 2–5 times higher than physiologically relevant, which are ignoring in vivo situation [12][13].
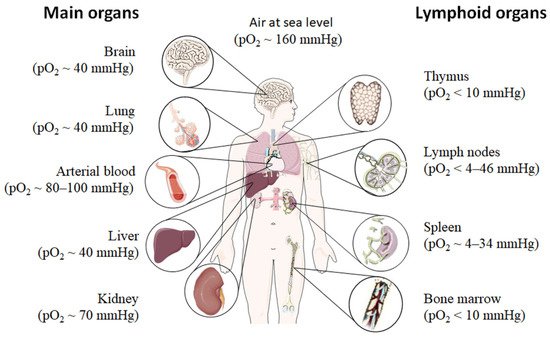
Low pO2 have been detected in various compartments of healthy and inflamed tissues as well as in tumors, often as a characteristic of tissue architecture, vascularization and microenvironment [1]. Tissues and cells vary in their (i) circulatory distance from lung oxygenation (ii) density, functionality and relative proximity of/to their capillary network (iii) oxygen consuming microenvironmental, (iv) the rate of oxygen consumption within the cells and thus in pO2 leading to distinct thresholds and susceptibilities to hypoxia [12][14][15]. However, at a cellular level, hypoxia and hypoxic responses generally occur at a pO2 ~7–10 mmHg (~1% O2 air-content) [17].
Although most tissues of the body are provided with a level of oxygen that exceeds the basal metabolic requirements, in some tissues, the pO2 is comparatively low, which results in regions of “physiological hypoxia” [1]. Such regions can occur in the intestinal outermost mucosal surface where a controlled oxygen gradient establishes as a result of anatomical features such as juxtapositioning of the mucosal surface to the anoxic gut lumen and the functional countercurrent oxygen exchange system in the intestinal villi [18]. In kidney, oxygen gradients are necessary for organ function which is to maximize the concentration of urine by counter-current exchange of oxygen in the renal medulla [13][19]. Moreover, oxygen gradients are important for the synthesis of erythropoietin (EPO) in kidney and liver [20]. In the developmental process of the placenta and the fetus, physiological hypoxia can be observed in several regions due to constant outgrowing of the existing local blood supply [21]. If blood supply is limited due to the lack of vasculature such as found in the eye’s retina but also in the outer layer of the skin, the epidermis, ‘physiological hypoxia’ has been demonstrated to be well established [21][22]. Moreover, the major organs of the immune system, including bone marrow (pO2 < 10 mmHg) [23][24], thymus (pO2 < 10 mmHg) [24][25][26], spleen (pO2 ~ 4–34 mmHg) [26][27], and lymph nodes (pO2 < 4–46 mmHg) [28], exhibit regions of immune activity with locally significantly lower pO2 than surrounding tissues and even lower than inhaled air. These hypoxic regions are of functional importance because they impact immunity by providing a niche for hematopoietic stem cells (HSCs) in the bone marrow, where hypoxia maintains the self-renewal capacity of HSCs favors a slow turnover of HSCs and sustains survival by promoting their quiescence [29][30][31][32] or an environment for the antigen challenging of B cells in germinal centers (GCs), where hypoxia increase glycolytic metabolism supporting the generation and expansion of antigen-specific GC B cells and the production of high-affinity immunoglobulin G (IgG) antibodies [33][34].
3. Pathophysiological Hypoxia Shapes Immune Response
Hypoxia also exists in pathophysiological states, which are more severe and confused as compared to physiological hypoxia [35] (Figure 2). Solid tumor microenvironment is one of the well-known typical pathophysiological immunological hypoxia caused by an imbalance between oxygen supply and oxygen demand [36]. The rapidly proliferating tumor cells are outgrowing from the vascular network, which limits the diffusion of oxygen into the intratumor microenvironment leading to hypoxia. In the hypoxic microenvironment of the tumor induces proangiogenic factors, such as vascular endothelial growth factor (VEGF), and promotes tumor vascularization and growth. However, the tumor’s blood vessels are usually irregularly structured and poorly functional, and also tend to form clots and local edema, aggravating local hypoxia. Moreover, the tumor induced neovasculature has gaps between endothelial cells resulting in that tumor cells leak into bloodstream and disseminate [37][38][39]. The hypoxic milieu recruits myeloid-derived suppressor cells (MDSCs) to the primary tumor site by activating the transcription of chemokine ligand in cancer cells [40] and promoting ectonucleoside triphosphate diphosphohydrolase 2 (ENTPD2/CD39L1) [41]. MDSCs play a key role in tumor immunosuppression by inhibition of anti-tumor T cell effector function. Usually MDSCs inhibit antigen-specific CD8+ T cells in lymphoid organs thereby reducing collateral damage and controlling effector function, but MDSCs at the tumor site preferentially differentiate into tumor associated macrophages (TAMs) facilitated by the hypoxic tumor microenvironment inhibiting not only antigen-specific but also nonspecific T cell activity [42]. Furthermore, the hypoxic microenvironment increases the expression programmed death ligand 1 (PD-L1) on MDSCs, which is when blocked resulting in enhanced MDSC-mediated T cell activation [43] evidencing hypoxia-mediated suppression of anti-tumor T cell effector function supporting tumor development [44][45][46].

Apart from the hypoxic tumor sites, hypoxic areas may appear as a consequence of infection with pathogenic bacteria, viruses, fungi, and protozoa [47][48][49][50]. Many factors contribute to the establishment of an hypoxic environment including an increased oxygen consumption by inflamed resident cells, infiltrating immune cells and pathogens as well as a decreased oxygen supply caused by the combination of vascular pathology and microthrombosis [51]. In this scenario, the hypoxic microenvironment protects the host by decreasing host cell death and reducing pathogenicity of invaders, while deleterious effects such as increases in antibiotic resistance and bacterial invasion make hypoxia a double-edged sword [52][53][54].
However, sites of inflammation undergo significant shifts in metabolic activity leading to O2 deficiency, which is defined as “inflammatory hypoxia” [35][55]. The reasons for this kind of hypoxia include the increase in oxygen consumption by infiltration and transmigration of immune cells such as monocytes and polymorphonuclear neutrophils (PMN), by local T and B cell proliferation, by activation of oxygenase, such as oxidases, monooxygenases and dioxygenases, and the immunometabolic switch in effector cells itself. The influence of inflammatory hypoxia on the severity of inflammation is particularly tissue-specific and depends on the composition and distribution of the involved cell types, the local microenvironment, the duration and severity of hypoxia [56].The induction of hypoxia by tissue infiltrating PMNs for instance in the intestinal epithelia ameliorates colitis [56], while in the lung enhances the severity of lung injury [57].
Blocking the blood supplying vessels by thrombus, embolus, or other blockages and followed by the subsequent restoration of perfusion and concomitant reoxygenation leads to ischemia and reperfusion injuring the demanding tissue [58]. Ischemia and reperfusion often occur in small capillaries of cerebral, coronary, or peripheral arteries [59] and is one of the leading causes of morbidity and mortality. Imbalance of oxygen supply and demand during ischemia and reperfusion leads to tissue hypoxia and immune cell attraction, which trigger inflammation and result in the tissue damage in ischemic disease [60][61].
References
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774.
- Beerman, I.; Luis, T.C.; Singbrant, S.; Lo Celso, C.; Mendez-Ferrer, S. The evolving view of the hematopoietic stem cell niche. Exp. Hematol. 2017, 50, 22–26.
- Shah, D.K.; Zuniga-Pflucker, J.C. An overview of the intrathymic intricacies of T cell development. J. Immunol. 2014, 192, 4017–4023.
- Lin, E.W.; Karakasheva, T.A.; Hicks, P.D.; Bass, A.J.; Rustgi, A.K. The tumor microenvironment in esophageal cancer. Oncogene 2016, 35, 5337–5349.
- Maru, Y. The lung metastatic niche. J. Mol. Med. (Berl) 2015, 93, 1185–1192.
- Biswas, S.; Davis, H.; Irshad, S.; Sandberg, T.; Worthley, D.; Leedham, S. Microenvironmental control of stem cell fate in intestinal homeostasis and disease. J. Pathol. 2015, 237, 135–145.
- Hallenbeck, J.M.; Hansson, G.K.; Becker, K.J. Immunology of ischemic vascular disease: Plaque to attack. Trends Immunol. 2005, 26, 550–556.
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98.
- Lin, N.; Simon, M.C. Hypoxia-inducible factors: Key regulators of myeloid cells during inflammation. J. Clin. Investig. 2016, 126, 3661–3671.
- Taylor, C.T.; Doherty, G.; Fallon, P.G.; Cummins, E.P. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J. Clin. Investig. 2016, 126, 3716–3724.
- Scholz, C.C.; Taylor, C.T. Targeting the HIF pathway in inflammation and immunity. Curr. Opin. Pharmacol. 2013, 13, 646–653.
- McNamee, E.N.; Korns Johnson, D.; Homann, D.; Clambey, E.T. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res. 2013, 55, 58–70.
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 2011, 15, 1239–1253.
- Boveris, D.L.; Boveris, A. Oxygen delivery to the tissues and mitochondrial respiration. Front. Biosci. 2007, 12, 1014–1023.
- Leach, R.M.; Treacher, D.F. Oxygen transport-2. Tissue hypoxia. BMJ 1998, 317, 1370–1373.
- Available online: (accessed on 1 March 2021).
- Jiang, B.H.; Semenza, G.L.; Bauer, C.; Marti, H.H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 1996, 271, C1172–C1180.
- Glover, L.E.; Lee, J.S.; Colgan, S.P. Oxygen metabolism and barrier regulation in the intestinal mucosa. J. Clin. Investig. 2016, 126, 3680–3688.
- Maxwell, P.H.; Ferguson, D.J.; Nicholls, L.G.; Iredale, J.P.; Pugh, C.W.; Johnson, M.H.; Ratcliffe, P.J. Sites of erythropoietin production. Kidney Int. 1997, 51, 393–401.
- Haase, V.H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013, 27, 41–53.
- Macklin, P.S.; McAuliffe, J.; Pugh, C.W.; Yamamoto, A. Hypoxia and HIF pathway in cancer and the placenta. Placenta 2017, 56, 8–13.
- Wang, W.; Winlove, C.P.; Michel, C.C. Oxygen partial pressure in outer layers of skin of human finger nail folds. J. Physiol. 2003, 549, 855–863.
- Takubo, K.; Goda, N.; Yamada, W.; Iriuchishima, H.; Ikeda, E.; Kubota, Y.; Shima, H.; Johnson, R.S.; Hirao, A.; Suematsu, M.; et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010, 7, 391–402.
- Parmar, K.; Mauch, P.; Vergilio, J.A.; Sackstein, R.; Down, J.D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. USA 2007, 104, 5431–5436.
- Hale, L.P.; Braun, R.D.; Gwinn, W.M.; Greer, P.K.; Dewhirst, M.W. Hypoxia in the thymus: Role of oxygen tension in thymocyte survival. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1467–H1477.
- Braun, R.D.; Lanzen, J.L.; Snyder, S.A.; Dewhirst, M.W. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2533–H2544.
- Caldwell, C.C.; Kojima, H.; Lukashev, D.; Armstrong, J.; Farber, M.; Apasov, S.G.; Sitkovsky, M.V. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 2001, 167, 6140–6149.
- Huang, J.H.; Cardenas-Navia, L.I.; Caldwell, C.C.; Plumb, T.J.; Radu, C.G.; Rocha, P.N.; Wilder, T.; Bromberg, J.S.; Cronstein, B.N.; Sitkovsky, M.; et al. Requirements for T lymphocyte migration in explanted lymph nodes. J. Immunol. 2007, 178, 7747–7755.
- Eliasson, P.; Rehn, M.; Hammar, P.; Larsson, P.; Sirenko, O.; Flippin, L.A.; Cammenga, J.; Jonsson, J.I. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture. Exp. Hematol. 2010, 38, 301.e302–310.e302.
- Hermitte, F.; Brunet de la Grange, P.; Belloc, F.; Praloran, V.; Ivanovic, Z. Very low O2 concentration (0.1%) favors G0 return of dividing CD34+ cells. Stem Cells 2006, 24, 65–73.
- Ivanovic, Z.; Hermitte, F.; Brunet de la Grange, P.; Dazey, B.; Belloc, F.; Lacombe, F.; Vezon, G.; Praloran, V. Simultaneous maintenance of human cord blood SCID-repopulating cells and expansion of committed progenitors at low O2 concentration (3%). Stem Cells 2004, 22, 716–724.
- Cipolleschi, M.G.; Dello Sbarba, P.; Olivotto, M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 1993, 82, 2031–2037.
- Cho, S.H.; Raybuck, A.L.; Stengel, K.; Wei, M.; Beck, T.C.; Volanakis, E.; Thomas, J.W.; Hiebert, S.; Haase, V.H.; Boothby, M.R. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature 2016, 537, 234–238.
- Abbott, R.K.; Thayer, M.; Labuda, J.; Silva, M.; Philbrook, P.; Cain, D.W.; Kojima, H.; Hatfield, S.; Sethumadhavan, S.; Ohta, A.; et al. Germinal Center Hypoxia Potentiates Immunoglobulin Class Switch Recombination. J. Immunol. 2016, 197, 4014–4020.
- Colgan, S.P.; Campbell, E.L.; Kominsky, D.J. Hypoxia and Mucosal Inflammation. Annu. Rev. Pathol. 2016, 11, 77–100.
- Labiano, S.; Palazon, A.; Melero, I. Immune response regulation in the tumor microenvironment by hypoxia. Semin. Oncol. 2015, 42, 378–386.
- Unwith, S.; Zhao, H.; Hennah, L.; Ma, D. The potential role of HIF on tumour progression and dissemination. Int. J. Cancer 2015, 136, 2491–2503.
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283.
- Krock, B.L.; Skuli, N.; Simon, M.C. Hypoxia-induced angiogenesis: Good and evil. Genes Cancer 2011, 2, 1117–1133.
- Chiu, D.K.; Xu, I.M.; Lai, R.K.; Tse, A.P.; Wei, L.L.; Koh, H.Y.; Li, L.L.; Lee, D.; Lo, R.C.; Wong, C.M.; et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology 2016, 64, 797–813.
- Chiu, D.K.; Tse, A.P.; Xu, I.M.; Di Cui, J.; Lai, R.K.; Li, L.L.; Koh, H.Y.; Tsang, F.H.; Wei, L.L.; Wong, C.M.; et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun 2017, 8, 517.
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453.
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790.
- Doedens, A.L.; Stockmann, C.; Rubinstein, M.P.; Liao, D.; Zhang, N.; DeNardo, D.G.; Coussens, L.M.; Karin, M.; Goldrath, A.W.; Johnson, R.S. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010, 70, 7465–7475.
- Loeffler, D.A.; Keng, P.C.; Baggs, R.B.; Lord, E.M. Lymphocytic infiltration and cytotoxicity under hypoxic conditions in the EMT6 mouse mammary tumor. Int J. Cancer 1990, 45, 462–467.
- Mazurek, J. Diagnostic and therapeutic role of a psychiatrist in a diagnostic and therapeutic team in a de-habituation ward. Psychiatr. Pol. 1975, 9, 349–350.
- Yousaf, I.; Kaeppler, J.; Frost, S.; Seymour, L.W.; Jacobus, E.J. Attenuation of the Hypoxia Inducible Factor Pathway after Oncolytic Adenovirus Infection Coincides with Decreased Vessel Perfusion. Cancers 2020, 12, 851.
- Devraj, G.; Beerlage, C.; Brune, B.; Kempf, V.A. Hypoxia and HIF-1 activation in bacterial infections. Microbes Infect. 2017, 19, 144–156.
- Friedrich, D.; Fecher, R.A.; Rupp, J.; Deepe, G.S., Jr. Impact of HIF-1alpha and hypoxia on fungal growth characteristics and fungal immunity. Microbes Infect. 2017, 19, 204–209.
- Werth, N.; Beerlage, C.; Rosenberger, C.; Yazdi, A.S.; Edelmann, M.; Amr, A.; Bernhardt, W.; von Eiff, C.; Becker, K.; Schafer, A.; et al. Activation of hypoxia inducible factor 1 is a general phenomenon in infections with human pathogens. PLoS ONE 2010, 5, e11576.
- Schaffer, K.; Taylor, C.T. The impact of hypoxia on bacterial infection. FEBS J. 2015, 282, 2260–2266.
- Schaible, B.; McClean, S.; Selfridge, A.; Broquet, A.; Asehnoune, K.; Taylor, C.T.; Schaffer, K. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE 2013, 8, e56491.
- Zeitouni, N.E.; Chotikatum, S.; von Kockritz-Blickwede, M.; Naim, H.Y. The impact of hypoxia on intestinal epithelial cell functions: Consequences for invasion by bacterial pathogens. Mol. Cell. Pediatr. 2016, 3, 14.
- Schaible, B.; Taylor, C.T.; Schaffer, K. Hypoxia increases antibiotic resistance in Pseudomonas aeruginosa through altering the composition of multidrug efflux pumps. Antimicrob. Agents Chemother. 2012, 56, 2114–2118.
- Karhausen, J.; Haase, V.H.; Colgan, S.P. Inflammatory hypoxia: Role of hypoxia-inducible factor. Cell Cycle 2005, 4, 256–258.
- Campbell, E.L.; Bruyninckx, W.J.; Kelly, C.J.; Glover, L.E.; McNamee, E.N.; Bowers, B.E.; Bayless, A.J.; Scully, M.; Saeedi, B.J.; Golden-Mason, L.; et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 2014, 40, 66–77.
- Liu, Z.; Bone, N.; Jiang, S.; Park, D.W.; Tadie, J.M.; Deshane, J.; Rodriguez, C.A.; Pittet, J.F.; Abraham, E.; Zmijewski, J.W. AMP-Activated Protein Kinase and Glycogen Synthase Kinase 3beta Modulate the Severity of Sepsis-Induced Lung Injury. Mol. Med. 2016, 21, 937–950.
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401.
- Harten, S.K.; Ashcroft, M.; Maxwell, P.H. Prolyl hydroxylase domain inhibitors: A route to HIF activation and neuroprotection. Antioxid. Redox Signal. 2010, 12, 459–480.
- Jian, Z.; Liu, R.; Zhu, X.; Smerin, D.; Zhong, Y.; Gu, L.; Fang, W.; Xiong, X. The Involvement and Therapy Target of Immune Cells After Ischemic Stroke. Front. Immunol. 2019, 10, 2167.
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665.





