Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sriyani Padmalatha Konara Mudiyanselage | + 1235 word(s) | 1235 | 2021-06-03 12:14:58 | | | |
| 2 | Ron Wang | Meta information modification | 1235 | 2021-06-08 09:31:10 | | | | |
| 3 | Ron Wang | Meta information modification | 1235 | 2021-06-08 09:39:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Konara Mudiyanselage, S.P. Herpes Zoster in Antiretroviral Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/10521 (accessed on 08 February 2026).
Konara Mudiyanselage SP. Herpes Zoster in Antiretroviral Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/10521. Accessed February 08, 2026.
Konara Mudiyanselage, Sriyani Padmalatha. "Herpes Zoster in Antiretroviral Therapy" Encyclopedia, https://encyclopedia.pub/entry/10521 (accessed February 08, 2026).
Konara Mudiyanselage, S.P. (2021, June 04). Herpes Zoster in Antiretroviral Therapy. In Encyclopedia. https://encyclopedia.pub/entry/10521
Konara Mudiyanselage, Sriyani Padmalatha. "Herpes Zoster in Antiretroviral Therapy." Encyclopedia. Web. 04 June, 2021.
Copy Citation
The incidence of herpes zoster (HZ) in patients infected with HIV is higher than that of the general population. However, the incidence of HZ in HIV patients receiving antiretroviral therapy (ART) remains unclear.
incidence
herpes zoster
HIV-infected patients
highly active antiretroviral therapy
meta-analysis
1. Introduction
Herpes zoster (HZ), also known as shingles, is caused by the reactivation of varicella zoster virus (VZV), which remains latent in the sensory ganglia following varicella infection [1]. HZ is a human immunodeficiency virus (HIV)-associated opportunistic infection. HIV infected populations have a 3- to 20-fold higher risk of contracting HZ than HIV seronegative individuals [2][3][4][5][6]. A systematic review and meta-analysis of the HZ incidence in ART-naive patients was 9.4%. During the first year of ART, the incidence was 2.3%, but this reduction was not statistically significant [7].
Even though the introduction of ART has improved the survival of those living with HIV, the incidence of HZ among HIV cohorts remains higher than that of the general population [4][8]. The incidence of HZ in the HIV cohort was 9.3–141 per 1000 patient years (PY)s [8][9][10]; but the incidence of HZ in the general population is only 3–5 per 1000 PYs [11]. The general population lifetime risk for HZ is between 23.8% and 30% and affects approximately one in four people in Europe; however, for those aged >85 years, the risk increases to one in two people [12].
ART was previously associated with reduced risk of herpes zoster and other opportunistic infections and could substantially improve the quality of life among HIV infected patients [7]. The long-term effects of ART on HZ incidence among HIV infected cohorts have been inconsistent across previous studies. A study in 2005 noted that the HZ incidence has not significantly decreased since the advent of ART [9]. Conversely, other prior studies found a reduction in the risk of HZ after the initiation of ART in North America, Europe, Taiwan and Africa [4][8][10][13][14][15][16][17]. Several studies have shown an increase in the HZ incidence during the first to fourth months following ART therapy, which suggests that HZ may be a feature of immune reconstitution inflammatory syndrome (IRIS) [9][18][19].
A better understanding of the risk factors for HZ for the HIV infected population may provide valuable information for health care professionals to identify adult patients at a heightened risk and could help formulate appropriate policies for vaccination strategies for the HIV-infected population [20]. Therefore, the objective of meta-analysis aimed to systematically review studies estimating the incidence and risk factors for HZ in the post-ART era among people living with HIV (PLWH) and discuss implications based on the updated evidence.
2. Results
2.1. Study Characteristics
During the period from 1 January 2000 to 28 February 2021, we identified 2111 records: 221 from PubMed, 1793 from Embase, 23 from Cochrane Library and 74 from CINAHL. After removing duplications and the initial screening, 45 articles were eligible for full-text review (Figure 1).
Eleven studies met the inclusion criteria for the meta-analysis. The 11 studies received a quality score of ≥8, with nine studies receiving a score of >10 out of 12 (Table 1). The 11 studies included 10 cohort studies and 1 clinical observational study. Overall, the study quality was acceptable. These 11 studies included 195,190 total HIV infected participants. The incidence was reported for populations in America (four studies), Europe (four studies), Asia (one study) and Africa (two studies).
The pooled incidence rate of HZ in the 11 studies was 2.30 (95% CI: 1.56–3.05) per 100 person years (PYs) (Figure 2a). Due to high heterogeneity, subgroup analyses were conducted and stratified by gender, income, AIDS history, study observational years, HIV risk factors and CD4 cell count (Table 2). Among the 10 studies, patients with high income (pooled RR, 2.64; 95% CI, 1.62–3.65) had the pooled HZ incidence greater than those with low income (pooled RR, 1.33; 95% CI, −0.56–3.22). Among the 4 studies, patients without AIDS history (pooled RR, 0.60; 95% CI, 0.46–0.72) had the pooled HZ incidence greater than those who had AIDS history (pooled RR, 0.40; 95% CI, 0.28–0.54). Among the 4 studies, patients being heterosexual had the pooled HZ incidence (pooled RR, 0.41; 95% CI, 0.31–0.52) greater than IDUs and MSM. Of the 4 studies, patients with a CD4 count < 200 cells/mm3 (pooled RR, 0.78; 95% CI, 0.55–0.91) were at increased risk of HZ compared with those with a CD4 count > 200 cells/mm3 (pooled RR, 0.21; 95% CI, 0.06–0.51).
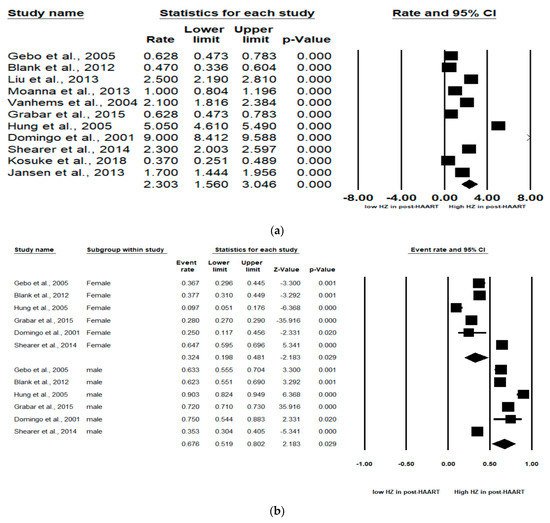
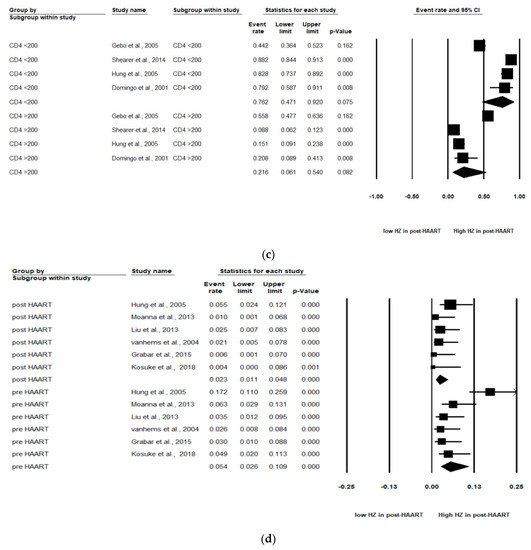
Figure 2. Meta-analyses of (a) HZ incidence of HIV-infected patients among ART-era therapy, (b) subgroup segmented by sex, (c) subgroup segmented by CD4 count level and (d) subgroup segmented by ART use. (Note: in the graph, the square represents the effect size of each study. The bigger the square, the more participants in the study. A horizontal line represents the 95% confidence intervals of the study result, with each end of the line representing the boundaries of the confidence interval. The diamond represents the combined effect. Pre-ART = not receiving ART. Post-ART = receiving ART).
Table 2. Subgroup analysis of HZ incidence in different categories.
| Subgroup Category | No. of Studies | RR (95% Confidence Interval) | I2 (%) | p Value |
|---|---|---|---|---|
| Overall HZ | 11 | 2.30 (1.56–3.05) | 99.3 | 0.001 |
| Sex | ||||
| Male | 6 | 0.68 (0.52–0.80) | 97.5 | 0.001 |
| Female | 6 | 0.32 (0.20–0.48) | 97.5 | 0.001 |
| Income | ||||
| High income | 8 | 2.64 (1.62–3.65) | 99.5 | 0.001 |
| Low income | 2 | 1.33 (−0.56–3.22) | 99.3 | 0.001 |
| AIDS history | ||||
| Yes | 4 | 0.40 (0.28–0.54) | 98.3 | 0.001 |
| No | 4 | 0.60 (0.46–0.72) | 98.3 | 0.001 |
| Observation years | ||||
| >7 years | 5 | 2.50 (1.29–3.71) | 99.6 | 0.001 |
| ≤7 years | 5 | 2.24 (1.09–3.40) | 99.1 | 0.001 |
| HIV risk factor | ||||
| Heterosexual | 4 | 0.41 (0.31–0.52) | 76.5 | 0.005 |
| IDUs | 4 | 0.35 (0.20–0.54) | 88.7 | 0.001 |
| MSM | 4 | 0.32 (0.19–0.49) | 90.2 | 0.001 |
| CD4 count | ||||
| CD4 < 200 | 4 | 0.78 (0.55–0.91) | 95.6 | 0.001 |
| CD4 > 200 | 4 | 0.21 (0.06–0.51) | 97.0 | 0.001 |
| ART use | ||||
| Pre-ART | 6 | 6.22 (3.59–8.85) | 99.6 | 0.001 |
| Post-ART | 6 | 2.00 (1.04–2.95) | 99.2 | 0.001 |
2.2. Meta-Regression Analyses of Overall HZ Moderators
Performed several meta-regression analyses, by which samples were segmented by sex, CD4 count level and ART use.
2.3. Subgroup Analyses by Sex
In six studies, males and females were analyzed. The subgroup analyses revealed that males were at significantly higher HZ risk than females (Pooled RR = 0.32 (female) and 0.68 (male); 95% CI: 0.20–0.48 (female) and 0.52–0.80 (male)). Larger effect sizes were observed for the HZ incidence for males than for females in the HIV-infected population (Figure 2b).
2.4. Subgroup Analyses by CD4 Count Level
As stated, two levels of CD4 count (CD4 count < 200 and CD4 count > 200 cells/mm3) were analyzed in these studies. Of the four studies, patients with a CD4 count < 200 cells/mm3 (pooled RR, 0.76; 95% CI, 0.47–0.92, p < 0.001) were at increased risk of HZ compared with patients with a CD4 count > 200 cells/mm3 (pooled RR, 0.22; 95% CI, 0.06–0.54, p < 0.001). A significant effect was noted in that CD4 count < 200 cells/mm3 among HIV-infected population that would increase the risk of HZ (Figure 2c).
2.5. Subgroup Segmented by ART Use
Among the six studies that enrolled patients in the pre-ART (not receiving ART) and post-ART (receiving ART) era, the subgroup analyses showed that those in the pre-ART era (pooled RR, 0.05 PYs; 95% CI, 0.03–0.11, p < 0.001) were at significantly higher HZ risk than those in the post-ART era (pooled RR, 0.02 PYs; 95% CI, 0.01–0.05, p < 0.001). The HIV-infected population who was not receiving ART was at higher HZ risk than the HIV-infected population receiving ART (Figure 2d).
2.6. Meta-Regression Analysis
We found a high level of heterogeneity in the HZ incidence across the 11 studies (I2 = 99.3%) (Figure 2a). Publication bias was analyzed by generating a funnel plot (Figure 3) and using Egger’s test.
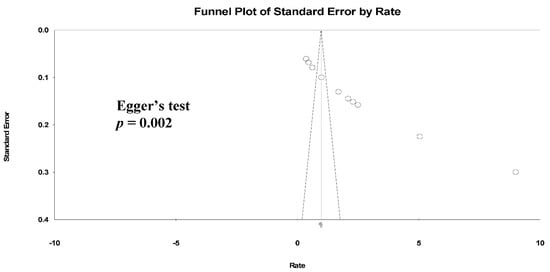
Figure 3. Funnel plots of HZ incidence. (Note: in the graph, the observed studies are shown as open circles and the observed point estimate in log units us shown as a diamond).
Furthermore, significant publication bias existed based on the Egger’s regression analysis (p = 0.002). A univariate analysis was conducted to identify significant independent covariates as potential sources of heterogeneity to be included in the meta-regression model. We found that risk factors for HZ incidence included male sex (AOR: 4.35 (95% CI: 0.54–2.41), p = 0.002), CD4 count < 200 cells/μL (AOR: 11.59 (95% CI: 0.53–4.38), p = 0.013) and not receiving ART (AOR: 2.89 (95% CI: −0.44–2.56), p = 0.16) (Table 3).
Table 3. Meta-regression analysis of HZ risk factors affecting heterogeneity.
| Variable | Coefficient | Standard Error | OR (95% CI) | p Value |
|---|---|---|---|---|
| Sex | ||||
| Female | Reference | |||
| Male | 1.47 | 0.48 | 0.54–2.41 | 0.002 * |
| HIV risk factor | ||||
| Heterosexual | Reference | |||
| IDU | −0.77 | 0.51 | −1.76–0.22 | 0.13 |
| MSM | 0.19 | 0.48 | −0.76–1.13 | 0.70 |
| CD4 count | ||||
| CD4 > 200 | Reference | |||
| CD4 < 200 | 2.45 | 0.98 | 0.53–4.38 | 0.013 * |
| AIDS history | ||||
| No | Reference | |||
| Yes | −0.57 | 0.42 | −1.39–0.25 | 0.17 |
| ART use | ||||
| With ART | Reference | |||
| Absence of ART | 1.06 | 0.77 | −0.44–2.56 | 0.16 |
* p < 0.05.
References
- Hope-Simpson, R.E. The nature of herpes zoster: A long-term study and a new hypothesis. Proc. R. Soc. Med. 1965, 58, 9–20.
- Buchbinder, S.P.; Katz, M.H.; Hessol, N.A.; Liu, J.Y.; O’Malley, P.M.; Underwood, R.; Holmberg, S.D. Herpes zoster and human immunodeficiency virus infection. J. Infect. Dis. 1992, 166, 1153–1156.
- Glesby, M.J.; Hoover, D.R.; Tan, T.; Shi, Q.; Gao, W.; French, A.L.; Toby, M.; Mary, Y.; Jack, D.; Jenny, R.; et al. Herpes zoster in women with and at risk for HIV: Data from the Women’s Interagency HIV Study. J. Acquir. Immune Defic. Syndr. 2004, 37, 1604–1609.
- Grabar, S.; Tattevin, P.; Selinger-Leneman, H.; De La Blanchardiere, A.; De Truchis, P.; Rabaud, C.; Rey, D.; Daneluzzi, V.; Ferret, S.; Lascaux, A.-S.; et al. Incidence of Herpes Zoster in HIV-Infected Adults in the Combined Antiretroviral Therapy Era: Results From the FHDH-ANRS CO4 Cohort. Clin. Infect. Dis. 2015, 60, 1269–1277.
- Morgan, D.; Mahe, C.; Malamba, S.; Okongo, M.; Mayanja, B.; Whitworth, J. Herpes zoster and HIV-1 infection in a rural Ugandan cohort. AIDS 2001, 15, 223–229.
- Veenstra, J.; Krol, A.; Van Praag, R.M.; Frissen, P.M.J.; Schellekens, P.T.; Lange, J.M.; Coutinho, R.A.; Van Der Meer, J.T. Herpes zoster, immunological deterioration and disease progression in HIV-1 infection. AIDS 1995, 9, 1153–1158.
- Low, A.; Gavriilidis, G.; Larke, N.; B-Lajoie, M.R.; Drouin, O.; Stover, J.; Muhe, L.; Easterbrook, P. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: A systematic review and meta-analysis. Clin. Infect. Dis. Off. Publ. Infect Dis. Soc. Am. 2016, 62, 1595–1603.
- Blank, L.J.; Polydefkis, M.J.; Moore, R.D.; Gebo, K.A. Herpes Zoster among Persons Living With HIV in the Current Antiretroviral Therapy Era. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 61, 203–207.
- Gebo, A.K.; Kalyani, R.; Moore, R.D.; Polydefkis, M.J. The Incidence of, Risk Factors for, and Sequelae of Herpes Zoster among HIV Patients in the Highly Active Antiretroviral Therapy Era. JAIDS J. Acquir. Immune Defic. Syndr. 2005, 40, 169–174.
- Moanna, A.; Rimland, D. Decreasing Incidence of Herpes Zoster in the Highly Active Antiretroviral Therapy Era. Clin. Infect. Dis. 2013, 57, 122–125.
- Kawai, K.; Yawn, B.P. Risk Factors for Herpes Zoster: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2017, 92, 1806–1821.
- Pinchinat, S.; Cebrián-Cuenca, A.M.; Bricout, H.; Johnson, R.W. Similar herpes zoster incidence across Europe: Results from a systematic literature review. BMC Infect. Dis. 2013, 13, 170.
- Hung, C.-C.; Hsiao, C.-F.; Wang, J.-L.; Chen, M.-Y.; Hsieh, S.-M.; Sheng, W.-H.; Chang, S.-C. Herpes zoster in HIV-1-infected patients in the era of highly active antiretroviral therapy: A prospective observational study. Int. J. STD AIDS 2005, 16, 673–676.
- Jansen, K.; Haastert, B.; Michalik, C.; Guignard, A.; Esser, S.; Dupke, S.; Plettenberg, A.; Skaletz-Rorowski, A.; Brockmeyer, N.H. Incidence and risk factors of herpes zoster among hiv-positive patients in the german competence network for HIV/AIDS (KompNet): A cohort study analysis. BMC Infect. Dis. 2013, 13, 372.
- Kawai, K.; Hawkins, C.A.; Hertzmark, E.; Francis, J.M.; Sando, D.; Muya, A.N.; Ulenga, N.; Fawzi, W.W. Impact of Antiretroviral Therapy on the Risk of Herpes Zoster among Human Immunodeficiency Virus-Infected Individuals in Tanzania. Am. J. Trop. Med. Hyg. 2018, 98, 396–401.
- Liu, C.; Wang, C.; Glesby, M.J.; D’Souza, G.; French, A.; Minkoff, H.; Maurer, T.; Karim, R.; Young, M. Effects of highly active antiretroviral therapy and its adherence on herpes zoster incidence: A longitudinal cohort study. AIDS Res. Ther. 2013, 10, 34.
- Vanhems, P.; Voisin, L.; Gayet-Ageron, A.; Trepo, C.; Cotte, L.; Peyramond, D.; Chidiac, C.; Touraine, J.-L.; Livrozet, J.-M.; Fabry, J.; et al. The Incidence of Herpes Zoster Is Less Likely Than Other Opportunistic Infections to Be Reduced by Highly Active Antiretroviral Therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2005, 38, 111–113.
- Domingo, P.; Torres, O.H.; Ris, J.; Vazquez, G. Herpes zoster as an immune reconstitution disease after initiation of combination antiretroviral therapy in patients with human immunodeficiency virus type-1 infection. Am. J. Med. 2001, 110, 605–609.
- Jevtovic, D.; Salemović, D.; Ranin, J.; Pešić, I.; Žerjav, S.; Djurkovic-Djakovic, O. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2005, 6, 140–143.
- A Benson, C.; Andersen, J.W.; Macatangay, B.J.C.; Mailliard, R.B.; Rinaldo, C.R.; Read, S.; Bozzolo, D.R.; Purdue, L.; Jennings, C.; Keefer, M.C.; et al. Safety and Immunogenicity of Zoster Vaccine Live in Human Immunodeficiency Virus–Infected Adults With CD4+ Cell Counts > 200 Cells/mL Virologically Suppressed on Antiretroviral Therapy. Clin. Infect. Dis. 2018, 67, 1712–1719.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
916
Revisions:
3 times
(View History)
Update Date:
08 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




