Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leslie Chavez-Galan | + 3288 word(s) | 3288 | 2021-05-25 05:12:18 | | | |
| 2 | Bruce Ren | -21 word(s) | 3267 | 2021-06-03 03:43:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chavez-Galan, L. Transmembrane TNF in Mycobacterial Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/10434 (accessed on 07 February 2026).
Chavez-Galan L. Transmembrane TNF in Mycobacterial Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/10434. Accessed February 07, 2026.
Chavez-Galan, Leslie. "Transmembrane TNF in Mycobacterial Infections" Encyclopedia, https://encyclopedia.pub/entry/10434 (accessed February 07, 2026).
Chavez-Galan, L. (2021, June 02). Transmembrane TNF in Mycobacterial Infections. In Encyclopedia. https://encyclopedia.pub/entry/10434
Chavez-Galan, Leslie. "Transmembrane TNF in Mycobacterial Infections." Encyclopedia. Web. 02 June, 2021.
Copy Citation
Tumor necrosis factor (TNF) is one of the main cytokines regulating a pro-inflammatory environment. It has been related to several cell functions, for instance, phagocytosis, apoptosis, proliferation, mitochondrial dynamic. Moreover, during mycobacterial infections, TNF plays an essential role to maintain granuloma formation. Several effector mechanisms have been implicated according to the interactions of the two active forms, soluble TNF (solTNF) and transmembrane TNF (tmTNF), with their receptors TNFR1 and TNFR2.
tumor necrosis factor
TNF receptors
mycobacterial infections
tumor necrosis factor-α converting enzyme (TACE)
1. Tumor Necrosis Factor (TNF) and Tumor Necrosis Factor-α Converting Enzyme (TACE)
TNF was described for the first time in the middle of 1970; it is a polypeptide considered a potent pro-inflammatory cytokine and encoded in the major histocompatibility complex in human and mice, and it is produced by immune system cells both myeloid and lymphoid origin [1][2][3].
TNF is synthesized as a monomeric protein and stored in vesicles, which use the route of rough endoplasmic reticulum (ER) to cross the cytoplasm. TNF monomers form a compact trimer through non-covalent interactions; the TNF-trimeric has high thermodynamic stability, the molecular mass of the human TNF is 50.4-kDa and murine TNF 50-kDa [4][5]. Active TNF is also expressed as a trimeric transmembrane form on the cell surface (hereafter tmTNF); after an activation stimulus, tmTNF is proteolytically processed, and a soluble form is released (hereafter solTNF); both tmTNF and solTNF display physiological functions [6].
TACE or also called A Disintegrin and Metalloproteinase (ADAM) domain 17 (ADAM17), cleaves tmTNF between residues Ala76 and Val77 to obtain solTNF [7]. TACE is the only, or at least the major sheddase of TNF in vivo, other ADAM family members such as ADAM10, ADAM9, and ADAM19 have been shown to shed TNF only in vitro and the cleavage site does not match with the physiologically relevant site [8]. TACE is not a TNF specific protease; several other proteins such as transforming growth factor-beta (TGF-β), beta-amyloid precursor protein, and TNF receptors are released by the TACE action [8][9]. It has been well documented that TACE also mediates the cleavage of the receptor Angiotensin-converting enzyme-2, a receptor used by viruses such as SARS-CoV and SARS-CoV2 to infect the cells [10][11][12].
It is currently well established that TACE is necessary to deliver solTNF; however, how its catalytic activity is activated or regulated is still limited. Probably, the presence of a functional TACE is dependent on the action of multiple molecules. For instance, in TGF-β-mediated TACE activation, when TGF-β binds to its receptors, the sarcoma kinase (Src) molecule is phosphorylated, mediating NOX 1 activation and producing reactive oxygen species (ROS), which is finally activating TACE [13]. The TNF-dependent pathway activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling, whereas the Uev1A-Ubc13 complex catalyzes the ubiquitination of RHBDF2, which is a crucial factor to promote TACE maturation. Consequently, Uev1A-Ubc13 inhibition interferes with RHBDF2-promoted TACE maturation [14].
Three mechanisms proposed to explain how the TACE’ catalytic activity is regulated are discussed below.
(1) Nardilysin (NRD)-dependent pathway: NRD is a metalloendopeptidase of the M16 family. NRD binds to TACE (at cytosol or extracellular level) and potentiates its catalytic activity, a process promoted by phorbol esters. The NRD gene’s overexpression increases TNF and induces ADAM10 activation, suggesting that the high TNF shedding could be due to the function of both TACE and ADAM10 [15][16] (Figure 1, dotted green line). Although the TNF shedding by ADAM10 has been observed only as an in-vitro process, using cultures of TACE-deficient fibroblasts, solTNF was detected, suggesting that ADAM10 is the TNF shedder when TACE is not present [17].
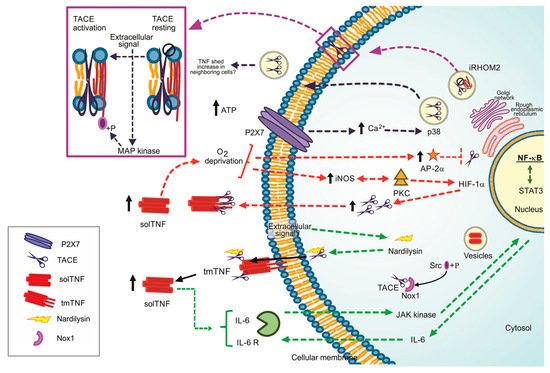
Figure 1. Regulation pathways to the TNF/TACE axis, ways to lead the TACE activation. Dotted green line: Nardilysin-dependent pathways. Dotted red line: iNOS/AP-2a-dependent pathways. +P Phosphorylate form. Dotted blue black line: P2X7 dependent pathway, Dotted purple line: iRHOM2 as the TACE maturation promoter. Black arrow up: increased level.
TNF can promote cancer cells’ proliferation [18]. NRD increases TNF shedding; consequently, NF-κB signalling pathway and pro-inflammatory microenvironment, characterized by the presence of interleukin (IL)-1β, IL-6 and prostaglandin E2 are activated. In turn, STAT3 is phosphorylated by the autocrine function of IL-6, and growth-related genes are upregulated [19] (Figure 1, dotted green line). Several questions remain open, for instance, if a specific extracellular signal is necessary or not to induce the complex NRD/TACE or if ADAM10 can or not cleave TNF in vivo.
(2) Oxygen deprivation-dependent pathway. Reports suggested that oxygen deprivation induces increased TACE expression and, consequently, high solTNF levels [20]. By this pathway, the protein kinase C (PKC) and inducible nitric oxide synthase (iNOS) induce a nuclear accumulation of NF-κB and hypoxic-inducible factor-1 subunit α (HIF-1α) to stimulate TACE promotor activity and solTNF increase by an autocrine function, thus the shedding of TNF could be perpetuated [21][22] (Figure 1, dotted red line).
Reports also suggested that solTNF upregulates the transcription factor AP-2α. However, the TACE promoter contains an AP-2α binding sequence, and it can bind in a TNF-dependent manner, indicating that solTNF downregulates TACE expression and function because AP-2α is enhanced [23] (Figure 1, dotted red line). The role played by AP-2α in the synthesis and function of TACE is controversial. Evidence showed that TNF induces caspase-6 activation, which in turn cleaves AP-2α, suggesting that TNF downregulates AP-2α by a caspase 6-dependent pathway [24]. It is possible that the concentration of solTNF and tmTNF, or maybe one of the receptors, could be responsible for determining the up-or down-regulation of AP-2α and its consequent role.
(3) P2 and iRHOM2 pathways. The P2 purinergic receptors have bi-functional effects on TNF release. On the one hand, P2X receptor activation attenuates TNF release and simultaneously, on the other hand, P2Y induces TNF release [25]. ATP induces intracellular Ca2+ rise by P2X7-dependent pathway, activating kinase p38 and finally promoting TACE’s release into exosomes. It could be a mechanism to shed membrane proteins to neighboring cells, thus propagating inflammation [26] (Figure 1, dotted black line).
iRHOM2 (rhomboid 5 homolog 2) or RHBDF2 is a member of the rhomboid protein family found in the ER. iRHOM2 is considered an essential regulator for the crosstalk TNF/TACE as it helps TACE to get the plasma membrane [27]. Data indicate that Ubiquitin (Ub)-conjugating enzyme variant 1A (Uev1A) polyubiquitinates iRHOM2 promoting TACE maturation (Figure 1, dotted purple line). However, the role of iRHOM2 is not limited to induce TACE maturation. The phosphorylation of the iRHOM2 cytoplasmic tail by MAP kinases (p38, JNK and ERK1/2) is a crucial step to expose TACE proteolytic site activating its sheddase function [14][28] (Figure 1, purple box).
2. Tumor Necrosis Factor Receptors
Two receptors have been identified to mediate interactions with TNF, tumor necrosis factor receptor 1 (TNFR1), also called CD120a and p55 (its molecular weight is 55 kDa), and tumor necrosis factor receptor 2 (TNFR2), also called CD120b and p75 (its molecular weight is 75 kDa) [29]. TNFR1 and TNFR2 are not specific to TNF, they interact also with lymphotoxin alpha (LTα, previously known as TNFβ). LTα is a cytokine closely related to TNF, activated by similar stimuli than those activating TNF, produced mainly by lymphoid cells in a soluble form and can combine with LTβ interacting with another different receptor, LTβR [30].
TNFR1 and TNFR2 are on the cellular membrane or in a soluble form following TACE activation; their cytoplasmic domains are unrelated, and intracellular signalling pathways are independent. TNFR1 is involved in cytotoxicity, whereas TNFR2 plays a role in cytotoxicity and proliferation [31][32]. As described in the previous section, the exact mechanism involving TACE in the shedding of TNFR1 and TNFR2 is still unclear.
2.1. Tumor Necrosis Factor Receptor 1
TNFR1 contains a cytoplasmic region designed death domain (DD), which initiates a signal of cytotoxicity with homology to the intracellular domain of Fas antigen [33]. TNFR1 activates different signalling pathways, including neutrophil migration, complement pathway, regulation of other cytokines and chemokines, adhesion molecules and their receptors, generally promoting inflammatory responses [34]. Several molecules were described as essential for NF-κB activation through a TNFR1-dependent pathway, for example, TRADD (TNFR1-associated death domain), RIPK1 (receptor-interacting protein kinase 1, also known as RIP1), TRAF2 (TNFR-associated factor 2) and FADD (Fas-associated death domain) [35]. The TNF/tmTNFR1 complex induces activation signalling pathways leading to opposite effects such as cell survival and cell death. Once TNF binds tmTNFR1, TRADD, RIPK1, TRAF2 and TAK1 molecules are recruited near DD and complex I. This complex I mediates the activation of MAPK and NF-κB, promoting cell survival. To activate this pathway, the phosphorylation of Jak-(Janus kinase)1 and Jak2, STAT-(signal transducer and activator of transcription)3 and STAT5 are required [36][37]. RIPK1 (RIPK1U) is ubiquitinated to recruit Ikappa B kinase (IKK), and then there is a binding between RIPK1U/NEMO (regulatory subunit IKKγ) to finally activate NF-κB [38] (Figure 2, left). However, if TRADD, RIPK1 and TRAF2 are dissociated from DD, they can interact with FADD, assembling the complex II that activates Caspase-8, inducing cell death. It has been suggested that to induce a full caspase 8-activation, ROS generation is required upstream and downstream of complex II [37][39] (Figure 2, left).
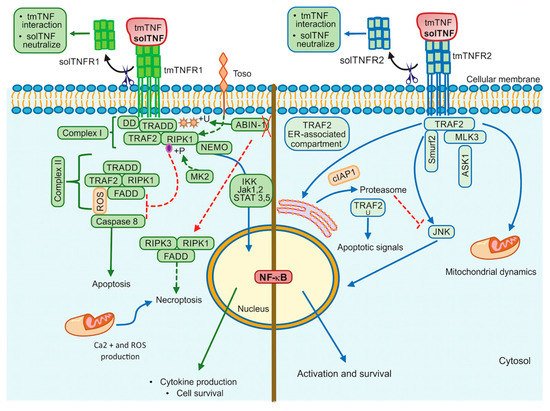
Figure 2. TNFR1 and TNFR2 activation pathways. TNFR1 signalling is involved in cell death events and inflammatory processes by classical NF-kB, whereas TNRF2 triggers signalling that may activate classical or non-classical NF-kB pathway. Additionally, TNFR2 impacts mitochondrial dynamics. ABIN-1: Ubiquitin binding protein, DD: Death domain, FADD: Fas-associated death domain, IKK: IkappaB kinase, Jak: Janus kinase protein, NEMO: The NF-κB essential modulator (or IKKγ), RIPK1: Receptor interacting protein kinase 1, RIPK3: Receptor interacting protein kinase 3, ROS: Reactive oxygen species, STAT: Signal transducer and activator of transcription proteins, Toso: Human Fcμ receptor (hFCMR) or FAIM3, TRADD: TNFR1-associated death domain, TRAF2: TNFR-associated factor 2, +P: Phosphorylation, +U: Ubiquitination. Continuous lines green and blue: activation signalling. Dotted lines: inhibition signalling. A red cross: absence.
The interactions of solTNF or tmTNF with tmTNFR1 are also crucial to trigger apoptotic signals. It has been recently shown that tmTNF induces the binding of STAT1 to a region spanning amino acids 319–337 of tmTNFR1. STAT1-phosphorylation (at the serine residue in position 727) favors its binding to TRADD and FADD, promoting apoptosis but not NF-κB activation [40]. Thus, the regulatory balance between survival versus death by the TNFR1 pathway is crucial to maintain homeostasis, and several authors suggested that TNFR1-mediated-signal transduction includes a checkpoint resulting in cell death when the signal activating NF-κB fails. Although it is not yet clarified how this checkpoint is controlled, experimental evidence suggested that RIPK1 is a crucial target to regulate this balance. It was reported that IKKα/IKKβ mediates direct phosphorylation of RIPK1 as the last step regulating cell death [41].
Toso, also known as FAIM3 (Fas apoptosis inhibitory molecule 3) or FcμR, is a transmembrane protein with a negative regulatory function, which promotes the ubiquitination of RIP1 inducing NF-κB activation and consequently cell survival [42], affecting caspase 8 activation [43]. Another molecule that has been involved in cell death is MAPKAP kinase-2 (MK2) [44]. If the effector MK2 induces phosphorylation of the kinase RIPK1, the binding with FADD/Caspase 8 is inhibited, thus complex-II-dependent cell death is blocked [44][45]. TNFR1-mediated cell survival may depend on ubiquitination and phosphorylation of RIPK1 (Figure 2, left).
Additionally, TNF/tmTNFR1 pathway can induce cell death called necroptosis as the assembly of FADD, RIPK1, and RIPK3 form the complex called necrosome [46]. Roca and collaborators [47], have shown in zebrafish or in human macrophages (infected with Mycobacterium marinum or Mycobacterium tuberculosis) that an excess of TNF triggers necrosis through TNF-RIPK1-RIPK3 interactions increasing the production of ROS, cyclophilin D (mitochondrial matrix protein), BID and BAX (pro-apoptotic proteins) [48]. The same group has recently reported that TNF triggers the production of ROS activating cyclophilin D (mitochondrial matrix protein) and leading to BID and BAX (pro-apoptotic proteins) activation, which results in mitochondrial Ca2+ overload through ER ryanodine receptor and necrosis [47].
The Ubiquitin-binding protein ABIN-1 is critical for the activation of RIPK1 [49]. This protein is recruited into the complex I and plays a critical role in the control of ubiquitylation and deubiquitylation. It has been proposed that ABIN-1 deficiency reduces the recruitment of A20 molecule (negative regulator of NFκB), and consequently, there are ubiquitylation and activation of RIPK1 to mediate necroptosis [49] (Figure 2, left).
2.2. Tumor Necrosis Factor Receptor 2
Although the tmTNFR2 signalling pathway has been mainly implicated in cell proliferation, activation and survival, data have also reported to transduce apoptotic signal under specific models and potentiate TNFR1-induced cell death [50][51].
Two molecules with the ability to interact with the TNFR2 cytoplasmic domain and to induce signalling were reported and called TNF receptor-associated factor 1 (TRAF1) and TRAF2 [52]. TNFR2 signalling was first simplified as a pathway where TRAF2 induces c-Jun-N-terminal kinase (JNK) activation, using the apoptosis signal-regulating kinase 1 (ASK1) as a mediator to activate NF-κB and facilitating anti-apoptotic signals [53].
JNK plays a fundamental role to determine the outcome of the TNFR2 pathway. It has been shown that after TNF/tmTNFR2 engagement, TRAF2 and the inhibitor of apoptosis molecules called cIAP1 (molecule to mediate the TRAF2 ubiquitination) are recruited to the TNFR2 cytoplasmic domain, and later it is translocated to an ER-associated compartment, where TRAF2 ubiquitination occurs [54][55] (Figure 2, right). The ER is sensitive to homeostasis alterations favoring the accumulation of misfolded proteins, which trigger ER stress and promote apoptotic cell death [56]. Thus, after ER-stress, TRAF2 forms a complex with the ER-stress sensor called IRE1 and interacts with procaspase-12 promoting its activation [57]. It has also been proposed that ER-stress induces expression of TNF in an IRE1- and NF-κB-dependent manner, and TRAF2 is decreased; these together inhibit the JNK activation and makes cells susceptible to cell death [58]. Those data support evidence for a relevant role of the ER in an additional apoptotic control point of the TNFR2 pathway.
It has also been proposed that following tmTNFR2 activation, the E3 ubiquitin ligase Smurf2 forms a ternary complex with tmTNFR2 and TRAF2, inducing relocalization of TNFR2 to the insoluble membrane/cytoskeletal fraction promoting the JNK activation [59]. Mixed lineage kinase 3 (MLK3), a mitogen-activated protein kinase kinase kinase (MAP3K) required for optimal activation of JNK signalling, has been shown to associate with TRAF2, TRAF5 and TRAF6. However, only TRAF2 induces the kinase activity of MLK3 by conjugating with polyubiquitin chains to activate JNK [60][61].
Finally, there is controversial evidence about the role of TNF on mitochondrial integrity. Reports have shown that TNF causes mitochondrial fragmentation (fission), but other authors suggested that TNF stimulates mitochondrial biogenesis [62][63]. In human airway smooth muscle (hASM) non-asthmatic cells, fission was associated with an increased level of Drp1 (Dynamin related protein (1) and the decreased level of Mfn2 (GTPase Mitofusin (2), and TNF was involved in Mfn2 reduction [64]. Recently, it was reported that hASM cells exposed to TNF showed fission and mitochondrial biogenesis, with increased organelle volume density, cell proliferation, reduction of Ca2+ influx, and decrease of O2 consumption per mitochondrion [65]. In this regard, the TNFR2-activation mediates interactions between Stat3 (signal transducer and activator of transcription (3) and Re1A (Stat-Re1A protein) as the Stat3/Re1A complex interacts with two lysine residues within Stat3 that is acetylated by p300 and OPA1 (optic atrophy 1) expression is increased, suggesting an enhanced mitochondrial fusion [66].
In summary, the tmTNFR2 pathway is tightly regulated depending on different cellular organelles, which are alternative for specific cellular systems when TNF is produced in inappropriate concentration. These mechanisms can help to develop novel therapeutic targets for treating or preventing diseases where TNF expression is dysregulated.
3. tmTNF Signalling Pathway
As previously discussed, tmTNF is a stable homotrimer that is cleaved by TACE and released as solTNF. The first publications on tmTNF suggested that tmTNF interacts primarily with TNFR2, whereas solTNF binds mainly to TNFR1 [67][68]. However, we need to consider that the tmTNF form that showed the specific interaction with TNFR2 are artificial because that tmTNF form contains mutations, which are not found in the native tmTNF molecule, and it is identical to solTNF. Also, tmTNF mutations to be retained on the cell membrane can differ, resulting in different outcome after an infection in vivo and in vitro [30][69]. At present, no data have been reported, excluding the interaction of TNFR1 with tmTNF. Many data have reported on TNFR1-tmTNF interactions in vivo and in vitro and, in particular, in the context of mycobacterial infections using tmTNF mutant mice that will be treated in this review.
tmTNF is a molecule that induces crosstalk between tmTNF-bearing cells and tmTNFR-bearing cells signalling both as a ligand and as a receptor. This means that tmTNF not only mediates the forward signal to the target cell but also mediates the reverse signalling inside the tmTNF-bearing cell [69] (Figure 3A).
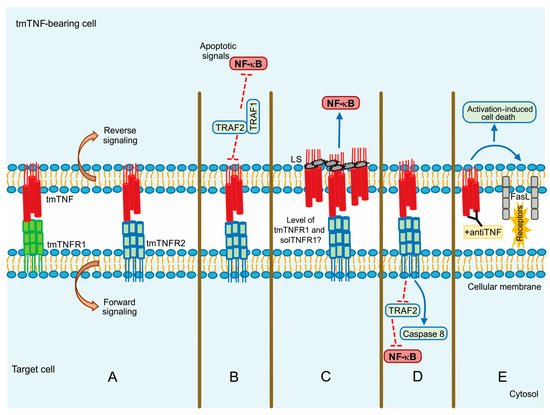
Figure 3. tmTNF signalling pathway. (A) Forward and reverse signalling; (B) Apoptotic signal by TRAF1 and TRAF2; (C) Direct NF-kB activation by LS (leader sequence) in tmTNF; (D) Actin involvement in tm-TNF transduction; (E) Activation Induced Cell Death (AICD) by upregulation of FASL (CD95L). Continuous blue lines: activation signalling. Dotted red lines: inhibition signalling.
Evidence suggests that although solTNF and tmTNF mediate cytotoxicity, tmTNF can exert apoptosis on solTNF-resistant cells. Using a model of TNF-resistant cells (the HL-60 cell line), it has been reported that tmTNF promotes the interaction with TRAF1 and TRAF2. TRAF1 plays a suppressor role in blocking the translocation of TRAF2 from the cytoplasm to the cell membrane. Consequently, NF-κB activation is inhibited resulting in cell death (Figure 3B) [70]. The same group has also proposed that forward signalling can result in opposite activities since tmTNF acting as a receptor promotes NF-κB activation, whereas acting as a ligand inhibits NF-κB activity [71].
It has been reported that MCF-7 human tumor cells with a high expression level of tmTNF are resistant to cell death associated with solTNF and constitutive NF-κB activation. tmTNF contains a leader sequence (LS) in the cytoplasmic segment (76 amino acid residues) through which tmTNF is anchored into the membrane. LS appears to affect both forward and reverse signalling, and it seems that LS induces directly the constitutive activation of NF-κB [72][73] (Figure 3C).
Using a pleural cell model, we have previously shown that tmTNF but not solTNF controls the expression of TNFR2 on myeloid cells expressing tmTNF, TNFR1 also contributes but in a minor way. Besides, the inflammatory process of BCG-induced pleurisy was downregulated mainly by tmTNF and TNFR2 [74]. Recent studies have shown that tmTNF efficiently activates both TNFR1 and TNFR2, but solTNF interacts and activates TNFR1 [75][76]. In several liver injury models, it has been shown that only solTNF causes liver toxicity but not tmTNF which can protect the host against mycobacterial infections [77][78]. Recently, we reported that TNFR1 is necessary to recruit myeloid cells, while TNFR2 is implicated in cell activation after BCG-induced pleural infection [78][79].
The actin cytoskeleton plays an essential role in different functions of the cell, for instance, intracellular trafficking and cellular contractility, but also it has been recognized that the actin’s dynamic structure is involved in apoptosis and necrosis [80]. The actin’s dynamic is another molecular mechanism by which the signalling activated can differ between both forms of TNF. Data have shown that solTNF induces actin depolymerization and morphological changes through ERK activation and p38 MAPK inducing cell death [81]. In contrast, tmTNF does not affect the state of actin microfilaments; apparently, actin is involved in tmTNF-mediated signal transduction by uncoupling TRAF2 and cFLIP from TNFR2 and consequently activating caspase-8 to induce apoptosis and inhibiting NF-κB activation (Figure 3D) [82].
Activation-induced cell death (AICD) plays a role in regulating peripheral immune tolerance by deleting overactivated or autoreactive T cells. It has been described that NF-κB is required to mediate the expression of the pro-apoptotic molecule called Fas ligand (FasL or CD95L) inducing AICD [83]. It was recently reported that when tmTNF functions as a receptor, using an anti-TNF polyclonal antibody to trigger reverse signalling, tmTNF can upregulate FasL expression and, consequently, increase AICD. Moreover, tmTNF-dependent reverse signalling also significantly increases several ligands, including TNFRs and Fas (Figure 3E) [84].
References
- Müller, U.; Jongeneel, C.V.; Nedospasov, S.A.; Lindahl, K.F.; Steinmetz, M. Tumour Necrosis Factor and Lymphotoxin Genes Map Close to H-2D in the Mouse Major Histocompatibility Complex. Nature 1987, 325, 265–267.
- Spies, T.; Morton, C.C.; Nedospasov, S.A.; Fiers, W.; Pious, D.; Strominger, J.L. Genes for the Tumor Necrosis Factors Alpha and Beta Are Linked to the Human Major Histocompatibility Complex. Proc. Natl. Acad. Sci. USA 1986, 83, 8699–8702.
- Brehm, M.A.; Daniels, K.A.; Welsh, R.M. Rapid Production of TNF-Alpha Following TCR Engagement of Naive CD8 T Cells. J. Immunol. 2005, 175, 5043–5049.
- Wingfield, P.; Pain, R.H.; Craig, S. Tumour Necrosis Factor Is a Compact Trimer. FEBS Lett. 1987, 211, 179–184.
- Chensue, S.W.; Remick, D.G.; Shmyr-Forsch, C.; Beals, T.F.; Kunkel, S.L. Immunohistochemical Demonstration of Cytoplasmic and Membrane-Associated Tumor Necrosis Factor in Murine Macrophages. Am. J. Pathol. 1988, 133, 564–572.
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S.D. A Novel Form of TNF/Cachectin Is a Cell Surface Cytotoxic Transmembrane Protein: Ramifications for the Complex Physiology of TNF. Cell 1988, 53, 45–53.
- Schlöndorff, J.; Becherer, J.D.; Blobel, C.P. Intracellular Maturation and Localization of the Tumour Necrosis Factor Alpha Convertase (TACE). Biochem. J. 2000, 347 Pt 1, 131–138.
- Zheng, Y.; Saftig, P.; Hartmann, D.; Blobel, C. Evaluation of the Contribution of Different ADAMs to Tumor Necrosis Factor Alpha (TNFalpha) Shedding and of the Function of the TNFalpha Ectodomain in Ensuring Selective Stimulated Shedding by the TNFalpha Convertase (TACE/ADAM17). J. Biol. Chem. 2004, 279, 42898–42906.
- Reddy, P.; Slack, J.L.; Davis, R.; Cerretti, D.P.; Kozlosky, C.J.; Blanton, R.A.; Shows, D.; Peschon, J.J.; Black, R.A. Functional Analysis of the Domain Structure of Tumor Necrosis Factor-Alpha Converting Enzyme. J. Biol. Chem. 2000, 275, 14608–14614.
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor Necrosis Factor-Alpha Convertase (ADAM17) Mediates Regulated Ectodomain Shedding of the Severe-Acute Respiratory Syndrome-Coronavirus (SARS-CoV) Receptor, Angiotensin-Converting Enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119.
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-Alpha-Converting Enzyme by the Spike Protein of SARS-CoV and ACE2 Induces TNF-Alpha Production and Facilitates Viral Entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814.
- Zipeto, D.; Palmeira, J.d.F.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745.
- Moreno-Càceres, J.; Mainez, J.; Mayoral, R.; Martín-Sanz, P.; Egea, G.; Fabregat, I. Caveolin-1-Dependent Activation of the Metalloprotease TACE/ADAM17 by TGF-β in Hepatocytes Requires Activation of Src and the NADPH Oxidase NOX1. FEBS J. 2016, 283, 1300–1310.
- Zhang, Y.; Li, Y.; Yang, X.; Wang, J.; Wang, R.; Qian, X.; Zhang, W.; Xiao, W. Uev1A-Ubc13 Catalyzes K63-Linked Ubiquitination of RHBDF2 to Promote TACE Maturation. Cell. Signal. 2018, 42, 155–164.
- Nishi, E.; Hiraoka, Y.; Yoshida, K.; Okawa, K.; Kita, T. Nardilysin Enhances Ectodomain Shedding of Heparin-Binding Epidermal Growth Factor-like Growth Factor through Activation of Tumor Necrosis Factor-Alpha-Converting Enzyme. J. Biol. Chem. 2006, 281, 31164–31172.
- Hiraoka, Y.; Yoshida, K.; Ohno, M.; Matsuoka, T.; Kita, T.; Nishi, E. Ectodomain Shedding of TNF-Alpha Is Enhanced by Nardilysin via Activation of ADAM Proteases. Biochem. Biophys. Res. Commun. 2008, 370, 154–158.
- Mezyk-Kopeć, R.; Bzowska, M.; Stalińska, K.; Chełmicki, T.; Podkalicki, M.; Jucha, J.; Kowalczyk, K.; Mak, P.; Bereta, J. Identification of ADAM10 as a Major TNF Sheddase in ADAM17-Deficient Fibroblasts. Cytokine 2009, 46, 309–315.
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The Dual Role of Tumor Necrosis Factor-alpha (TNF-α) in Breast Cancer: Molecular Insights and Therapeutic Approaches. Cell Oncol. 2020, 43, 1–18.
- Kanda, K.; Komekado, H.; Sawabu, T.; Ishizu, S.; Nakanishi, Y.; Nakatsuji, M.; Akitake-Kawano, R.; Ohno, M.; Hiraoka, Y.; Kawada, M.; et al. Nardilysin and ADAM Proteases Promote Gastric Cancer Cell Growth by Activating Intrinsic Cytokine Signalling via Enhanced Ectodomain Shedding of TNF-α. EMBO Mol. Med. 2012, 4, 396–411.
- Romera, C.; Hurtado, O.; Botella, S.H.; Lizasoain, I.; Cárdenas, A.; Fernández-Tomé, P.; Leza, J.C.; Lorenzo, P.; Moro, M.A. In Vitro Ischemic Tolerance Involves Upregulation of Glutamate Transport Partly Mediated by the TACE/ADAM17-Tumor Necrosis Factor-Alpha Pathway. J. Neurosci. 2004, 24, 1350–1357.
- Hurtado, O.; Cárdenas, A.; Lizasoain, I.; Boscá, L.; Leza, J.C.; Lorenzo, P.; Moro, M.A. Up-Regulation of TNF-Alpha Convertase (TACE/ADAM17) after Oxygen-Glucose Deprivation in Rat Forebrain Slices. Neuropharmacology 2001, 40, 1094–1102.
- Charbonneau, M.; Harper, K.; Grondin, F.; Pelmus, M.; McDonald, P.P.; Dubois, C.M. Hypoxia-Inducible Factor Mediates Hypoxic and Tumor Necrosis Factor Alpha-Induced Increases in Tumor Necrosis Factor-Alpha Converting Enzyme/ADAM17 Expression by Synovial Cells. J. Biol. Chem. 2007, 282, 33714–33724.
- Ge, L.; Vujanovic, N.L. Soluble TNF Regulates TACE via AP-2α Transcription Factor in Mouse Dendritic Cells. J. Immunol. 2017, 198, 417–427.
- Nyormoi, O.; Wang, Z.; Doan, D.; Ruiz, M.; McConkey, D.; Bar-Eli, M. Transcription Factor AP-2alpha Is Preferentially Cleaved by Caspase 6 and Degraded by Proteasome during Tumor Necrosis Factor Alpha-Induced Apoptosis in Breast Cancer Cells. Mol. Cell. Biol. 2001, 21, 4856–4867.
- Kucher, B.M.; Neary, J.T. Bi-Functional Effects of ATP/P2 Receptor Activation on Tumor Necrosis Factor-Alpha Release in Lipopolysaccharide-Stimulated Astrocytes. J. Neurochem. 2005, 92, 525–535.
- Barberà-Cremades, M.; Gómez, A.I.; Baroja-Mazo, A.; Martínez-Alarcón, L.; Martínez, C.M.; de Torre-Minguela, C.; Pelegrín, P. P2X7 Receptor Induces Tumor Necrosis Factor-α Converting Enzyme Activation and Release to Boost TNF-α Production. Front. Immunol. 2017, 8, 862.
- Adrain, C.; Zettl, M.; Christova, Y.; Taylor, N.; Freeman, M. Tumor Necrosis Factor Signaling Requires IRhom2 to Promote Trafficking and Activation of TACE. Science 2012, 335, 225–228.
- Cavadas, M.; Oikonomidi, I.; Gaspar, C.J.; Burbridge, E.; Badenes, M.; Félix, I.; Bolado, A.; Hu, T.; Bileck, A.; Gerner, C.; et al. Phosphorylation of IRhom2 Controls Stimulated Proteolytic Shedding by the Metalloprotease ADAM17/TACE. Cell Rep. 2017, 21, 745–757.
- Loetscher, H.; Schlaeger, E.J.; Lahm, H.W.; Pan, Y.C.; Lesslauer, W.; Brockhaus, M. Purification and Partial Amino Acid Sequence Analysis of Two Distinct Tumor Necrosis Factor Receptors from HL60 Cells. J. Biol. Chem. 1990, 265, 20131–20138.
- Garcia, I.; Olleros, M.L. The Roles of Tumor Necrosis Factor and Other Macrophage-Derived Cytokines in Host Defense Mechanisms During the Course of Mycobacterium tuberculosis Infection. In Current Topics on The Profiles of Host Immunological Response to Mycobacterial Infections; Research Signpost: Thiruvananthapuram, Kerala, India, 2009; pp. 1–46.
- Dembic, Z.; Loetscher, H.; Gubler, U.; Pan, Y.C.; Lahm, H.W.; Gentz, R.; Brockhaus, M.; Lesslauer, W. Two Human TNF Receptors Have Similar Extracellular, but Distinct Intracellular, Domain Sequences. Cytokine 1990, 2, 231–237.
- Jacobsen, F.W.; Rothe, M.; Rusten, L.; Goeddel, D.V.; Smeland, E.B.; Veiby, O.P.; Slørdal, L.; Jacobsen, S.E. Role of the 75-KDa Tumor Necrosis Factor Receptor: Inhibition of Early Hematopoiesis. Proc. Natl. Acad. Sci. USA 1994, 91, 10695–10699.
- Tartaglia, L.A.; Ayres, T.M.; Wong, G.H.; Goeddel, D.V. A Novel Domain within the 55 Kd TNF Receptor Signals Cell Death. Cell 1993, 74, 845–853.
- Dostert, C.; Grusdat, M.; Letellier, E.; Brenner, D. The TNF Family of Ligands and Receptors: Communication Modules in the Immune System and Beyond. Physiol. Rev. 2019, 99, 115–160.
- Idress, M.; Milne, B.F.; Thompson, G.S.; Trembleau, L.; Jaspars, M.; Houssen, W.E. Structure-Based Design, Synthesis and Bioactivity of a New Anti-TNFα Cyclopeptide. Molecules 2020, 25, 922.
- Guo, D.; Dunbar, J.D.; Yang, C.H.; Pfeffer, L.M.; Donner, D.B. Induction of Jak/STAT Signaling by Activation of the Type 1 TNF Receptor. J. Immunol. 1998, 160, 2742–2750.
- Micheau, O.; Tschopp, J. Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell 2003, 114, 181–190.
- Ea, C.-K.; Deng, L.; Xia, Z.-P.; Pineda, G.; Chen, Z.J. Activation of IKK by TNFalpha Requires Site-Specific Ubiquitination of RIP1 and Polyubiquitin Binding by NEMO. Mol. Cell 2006, 22, 245–257.
- Dondelinger, Y.; Aguileta, M.A.; Goossens, V.; Dubuisson, C.; Grootjans, S.; Dejardin, E.; Vandenabeele, P.; Bertrand, M.J.M. RIPK3 Contributes to TNFR1-Mediated RIPK1 Kinase-Dependent Apoptosis in Conditions of CIAP1/2 Depletion or TAK1 Kinase Inhibition. Cell Death Differ. 2013, 20, 1381–1392.
- Jiang, Y.; Yu, M.; Hu, X.; Han, L.; Yang, K.; Ba, H.; Zhang, Z.; Yin, B.; Yang, X.-P.; Li, Z.; et al. STAT1 Mediates Transmembrane TNF-Alpha-Induced Formation of Death-Inducing Signaling Complex and Apoptotic Signaling via TNFR1. Cell Death Differ. 2017, 24, 660–671.
- Dondelinger, Y.; Jouan-Lanhouet, S.; Divert, T.; Theatre, E.; Bertin, J.; Gough, P.J.; Giansanti, P.; Heck, A.J.R.; Dejardin, E.; Vandenabeele, P.; et al. NF-ΚB-Independent Role of IKKα/IKKβ in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Mol. Cell 2015, 60, 63–76.
- Nguyen, X.H.; Lang, P.A.; Lang, K.S.; Adam, D.; Fattakhova, G.; Föger, N.; Kamal, M.A.; Prilla, P.; Mathieu, S.; Wagner, C.; et al. Toso Regulates the Balance between Apoptotic and Nonapoptotic Death Receptor Signaling by Facilitating RIP1 Ubiquitination. Blood 2011, 118, 598–608.
- Song, Y.; Jacob, C.O. The Mouse Cell Surface Protein TOSO Regulates Fas/Fas Ligand-Induced Apoptosis Through its Binding to Fas-Associated Death Domain. J. Biol. Chem. 2005, 280, 9618–9626.
- Jaco, I.; Annibaldi, A.; Lalaoui, N.; Wilson, R.; Tenev, T.; Laurien, L.; Kim, C.; Jamal, K.; Wicky John, S.; Liccardi, G.; et al. MK2 Phosphorylates RIPK1 to Prevent TNF-Induced Cell Death. Mol. Cell 2017, 66, 698–710.e5.
- Dondelinger, Y.; Delanghe, T.; Rojas-Rivera, D.; Priem, D.; Delvaeye, T.; Bruggeman, I.; Van Herreweghe, F.; Vandenabeele, P.; Bertrand, M.J.M. MK2 Phosphorylation of RIPK1 Regulates TNF-Mediated Cell Death. Nat. Cell Biol. 2017, 19, 1237–1247.
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-Alpha. Cell 2009, 137, 1100–1111.
- Roca, F.J.; Whitworth, L.J.; Redmond, S.; Jones, A.A.; Ramakrishnan, L. TNF Induces Pathogenic Programmed Macrophage Necrosis in Tuberculosis through a Mitochondrial-Lysosomal-Endoplasmic Reticulum Circuit. Cell 2019, 178, 1344–1361.e11.
- Roca, F.J.; Ramakrishnan, L. TNF Dually Mediates Resistance and Susceptibility to Mycobacteria Through Mitochondrial Reactive Oxygen Species. Cell 2013, 153, 521–534.
- Dziedzic, S.A.; Su, Z.; Jean Barrett, V.; Najafov, A.; Mookhtiar, A.K.; Amin, P.; Pan, H.; Sun, L.; Zhu, H.; Ma, A.; et al. ABIN-1 Regulates RIPK1 Activation by Linking Met1 Ubiquitylation with Lys63 Deubiquitylation in TNF-RSC. Nat. Cell Biol. 2018, 20, 58–68.
- Tartaglia, L.A.; Weber, R.F.; Figari, I.S.; Reynolds, C.; Palladino, M.A.; Goeddel, D.V. The Two Different Receptors for Tumor Necrosis Factor Mediate Distinct Cellular Responses. Proc. Natl. Acad. Sci. USA 1991, 88, 9292–9296.
- Chan, F.K.; Lenardo, M.J. A Crucial Role for P80 TNF-R2 in Amplifying P60 TNF-R1 Apoptosis Signals in T Lymphocytes. Eur. J. Immunol. 2000, 30, 652–660.
- Rothe, M.; Wong, S.C.; Henzel, W.J.; Goeddel, D.V. A Novel Family of Putative Signal Transducers Associated with the Cytoplasmic Domain of the 75 KDa Tumor Necrosis Factor Receptor. Cell 1994, 78, 681–692.
- Nishitoh, H.; Saitoh, M.; Mochida, Y.; Takeda, K.; Nakano, H.; Rothe, M.; Miyazono, K.; Ichijo, H. ASK1 Is Essential for JNK/SAPK Activation by TRAF2. Mol. Cell 1998, 2, 389–395.
- Li, X.; Yang, Y.; Ashwell, J.D. TNF-RII and c-IAP1 Mediate Ubiquitination and Degradation of TRAF2. Nature 2002, 416, 345–347.
- Wu, C.-J.; Conze, D.B.; Li, X.; Ying, S.-X.; Hanover, J.A.; Ashwell, J.D. TNF-Alpha Induced c-IAP1/TRAF2 Complex Translocation to a Ubc6-Containing Compartment and TRAF2 Ubiquitination. EMBO J. 2005, 24, 1886–1898.
- Cao, Y.; Long, J.; Liu, L.; He, T.; Jiang, L.; Zhao, C.; Li, Z. A Review of Endoplasmic Reticulum (ER) Stress and Nanoparticle (NP) Exposure. Life Sci. 2017, 186, 33–42.
- Yoneda, T.; Imaizumi, K.; Oono, K.; Yui, D.; Gomi, F.; Katayama, T.; Tohyama, M. Activation of Caspase-12, an Endoplastic Reticulum (ER) Resident Caspase, through Tumor Necrosis Factor Receptor-Associated Factor 2-Dependent Mechanism in Response to the ER Stress. J. Biol. Chem. 2001, 276, 13935–13940.
- Hu, P.; Han, Z.; Couvillon, A.D.; Kaufman, R.J.; Exton, J.H. Autocrine Tumor Necrosis Factor Alpha Links Endoplasmic Reticulum Stress to the Membrane Death Receptor Pathway through IRE1alpha-Mediated NF-KappaB Activation and down-Regulation of TRAF2 Expression. Mol. Cell. Biol. 2006, 26, 3071–3084.
- Carpentier, I.; Coornaert, B.; Beyaert, R. Smurf2 Is a TRAF2 Binding Protein That Triggers TNF-R2 Ubiquitination and TNF-R2-Induced JNK Activation. Biochem. Biophys. Res. Commun. 2008, 374, 752–757.
- Korchnak, A.C.; Zhan, Y.; Aguilar, M.T.; Chadee, D.N. Cytokine-Induced Activation of Mixed Lineage Kinase 3 Requires TRAF2 and TRAF6. Cell. Signal. 2009, 21, 1620–1625.
- Sondarva, G.; Kundu, C.N.; Mehrotra, S.; Mishra, R.; Rangasamy, V.; Sathyanarayana, P.; Ray, R.S.; Rana, B.; Rana, A. TRAF2-MLK3 Interaction Is Essential for TNF-Alpha-Induced MLK3 Activation. Cell Res. 2010, 20, 89–98.
- Sente, T.; Van Berendoncks, A.M.; Fransen, E.; Vrints, C.J.; Hoymans, V.Y. Tumor Necrosis Factor-α Impairs Adiponectin Signalling, Mitochondrial Biogenesis, and Myogenesis in Primary Human Myotubes Cultures. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1164–H1175.
- Drabarek, B.; Dymkowska, D.; Szczepanowska, J.; Zabłocki, K. TNFα Affects Energy Metabolism and Stimulates Biogenesis of Mitochondria in EA.Hy926 Endothelial Cells. Int. J. Biochem. Cell Biol. 2012, 44, 1390–1397.
- Aravamudan, B.; Kiel, A.; Freeman, M.; Delmotte, P.; Thompson, M.; Vassallo, R.; Sieck, G.C.; Pabelick, C.M.; Prakash, Y.S. Cigarette Smoke-Induced Mitochondrial Fragmentation and Dysfunction in Human Airway Smooth Muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L840–L854.
- Delmotte, P.; Marin Mathieu, N.; Sieck, G.C. TNFα Induces Mitochondrial Fragmentation and Biogenesis in Human Airway Smooth Muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L137–L151.
- Nan, J.; Hu, H.; Sun, Y.; Zhu, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Zhang, N.; Wang, Y.; Wang, Y.; et al. TNFR2 Stimulation Promotes Mitochondrial Fusion via Stat3- and NF-KB-Dependent Activation of OPA1 Expression. Circ. Res. 2017, 121, 392–410.
- Grell, M.; Douni, E.; Wajant, H.; Löhden, M.; Clauss, M.; Maxeiner, B.; Georgopoulos, S.; Lesslauer, W.; Kollias, G.; Pfizenmaier, K.; et al. The Transmembrane Form of Tumor Necrosis Factor Is the Prime Activating Ligand of the 80 KDa Tumor Necrosis Factor Receptor. Cell 1995, 83, 793–802.
- Grell, M.; Wajant, H.; Zimmermann, G.; Scheurich, P. The Type 1 Receptor (CD120a) Is the High-Affinity Receptor for Soluble Tumor Necrosis Factor. Proc. Natl. Acad. Sci. USA 1998, 95, 570–575.
- Olleros, M.L.; Vesin, D.; Bisig, R.; Santiago-Raber, M.L.; Schuepbach-Mallepell, S.; Kollias, G.; Gaide, O.; Garcia, I. Membrane-Bound TNF Induces Protective Immune Responses to M. bovis BCG Infection: Regulation of memTNF and TNF Receptors Comparing two memTNF molecules. PLoS ONE 2012, 7, e31469.
- Shi, W.; Li, L.; Shi, X.; Zheng, F.; Zeng, J.; Jiang, X.; Gong, F.; Zhou, M.; Li, Z. Inhibition of Nuclear Factor-KappaB Activation Is Essential for Membrane-Associated TNF-Alpha-Induced Apoptosis in HL-60 Cells. Immunol. Cell Biol. 2006, 84, 366–373.
- Zhang, H.; Yan, D.; Shi, X.; Liang, H.; Pang, Y.; Qin, N.; Chen, H.; Wang, J.; Yin, B.; Jiang, X.; et al. Transmembrane TNF-Alpha Mediates “Forward” and “Reverse” Signaling, Inducing Cell Death or Survival via the NF-KappaB Pathway in Raji Burkitt Lymphoma Cells. J. Leukoc. Biol. 2008, 84, 789–797.
- Yan, D.; Qin, N.; Zhang, H.; Liu, T.; Yu, M.; Jiang, X.; Feng, W.; Wang, J.; Yin, B.; Zhang, T.; et al. Expression of TNF-Alpha Leader Sequence Renders MCF-7 Tumor Cells Resistant to the Cytotoxicity of Soluble TNF-Alpha. Breast Cancer Res. Treat. 2009, 116, 91–102.
- Zheng, F.; Liu, N.; Chen, Q.; Yang, L.; Liu, L.; Xiong, P.; Feng, W.; Jiang, X.; Gong, F.; Li, Z. Leader Sequence Is Required for Activity of Transmembrane Tumor Necrosis Factor-Alpha. Mol. Immunol. 2009, 46, 3336–3344.
- Uysal, H.; Chavez-Galan, L.; Vesin, D.; Blaser, G.; Benkhoucha, M.; Ryffel, B.; Quesniaux, V.F.J.; Garcia, I. Transmembrane TNF and Partially TNFR1 Regulate TNFR2 Expression and Control Inflammation in Mycobacterial-Induced Pleurisy. Int. J. Mol. Sci. 2018, 19, 1959.
- Chavez-Galan, L.; Vesin, D.; Segueni, N.; Prasad, P.; Buser-Llinares, R.; Blaser, G.; Pache, J.C.; Ryffel, B.; Quesniaux, V.F.; Garcia, I. Tumor Necrosis Factor and Its Receptors Are Crucial to Control Mycobacterium bovis Bacillus Calmette-Guerin Pleural Infection in a Murine Model. Am. J. Pathol. 2016, 186, 2364–2377.
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell. Dev. Biol. 2019, 7.
- Olleros, M.L.; Vesin, D.; Fotio, A.L.; Santiago-Raber, M.L.; Tauzin, S.; Szymkowski, D.E.; Garcia, I. Soluble TNF, but not membrane TNF is critical in LPS-induced hepatitis. J. Hepatol. 2010, 53, 1059–1068.
- Chavez-Galan, L.; Vesin, D.; Blaser, G.; Uysal, H.; Benmerzoug, S.; Rose, S.; Ryffel, B.; Quesniaux, V.F.J.; Garcia, I. Myeloid Cell TNFR1 Signaling Dependent Liver Injury and Inflammation upon BCG Infection. Sci. Rep. 2019, 9, 5297.
- Rodriguez-Cruz, A.; Vesin, D.; Ramon-Luing, L.; Zuñiga, J.; Quesniaux, V.; Ryffel, B.; Lascurain, R.; Garcia, I.; Chávez-Galán, L. CD3+ Macrophages Deliver Pro-Inflammatory Cytokines by a CD3- and Transmembrane TNF-Dependent Pathway and Are Increased at the BCG-Infection Site. Front. Immunol. 2019, 10, 2550.
- Cabado, A.G.; Leira, F.; Vieytes, M.R.; Vieites, J.M.; Botana, L.M. Cytoskeletal Disruption Is the Key Factor That Triggers Apoptosis in Okadaic Acid-Treated Neuroblastoma Cells. Arch. Toxicol. 2004, 78, 74–85.
- Kutsuna, H.; Suzuki, K.; Kamata, N.; Kato, T.; Hato, F.; Mizuno, K.; Kobayashi, H.; Ishii, M.; Kitagawa, S. Actin Reorganization and Morphological Changes in Human Neutrophils Stimulated by TNF, GM-CSF, and G-CSF: The Role of MAP Kinases. Am. J. Physiol. Cell Physiol. 2004, 286, C55–C64.
- Chen, H.; Xiao, L.; Zhang, H.; Liu, N.; Liu, T.; Liu, L.; Hu, X.; Yan, D.; Yang, K.; Yin, B.; et al. The Involvement of β-Actin in the Signaling of Transmembrane TNF-α-Mediated Cytotoxicity. J. Leukoc. Biol. 2011, 89, 917–926.
- Kasibhatla, S.; Genestier, L.; Green, D.R. Regulation of Fas-Ligand Expression during Activation-Induced Cell Death in T Lymphocytes via Nuclear Factor KappaB. J. Biol. Chem. 1999, 274, 987–992.
- Zhang, M.; Wang, J.; Jia, L.; Huang, J.; He, C.; Hu, F.; Yuan, L.; Wang, G.; Yu, M.; Li, Z. Transmembrane TNF-α Promotes Activation-Induced Cell Death by Forward and Reverse signaling. Oncotarget 2017, 8, 63799–63812.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
845
Revisions:
2 times
(View History)
Update Date:
03 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




