Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Deng-Ho Yang | + 1790 word(s) | 1790 | 2021-06-01 09:51:59 | | | |
| 2 | Peter Tang | Meta information modification | 1790 | 2021-06-02 04:54:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, D. Macrophage in Pathogenesis of Osteoporosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/10365 (accessed on 07 February 2026).
Yang D. Macrophage in Pathogenesis of Osteoporosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/10365. Accessed February 07, 2026.
Yang, Deng-Ho. "Macrophage in Pathogenesis of Osteoporosis" Encyclopedia, https://encyclopedia.pub/entry/10365 (accessed February 07, 2026).
Yang, D. (2021, June 01). Macrophage in Pathogenesis of Osteoporosis. In Encyclopedia. https://encyclopedia.pub/entry/10365
Yang, Deng-Ho. "Macrophage in Pathogenesis of Osteoporosis." Encyclopedia. Web. 01 June, 2021.
Copy Citation
Osteoporosis is a systemic disease with progressive bone loss. The bone loss is associated with an imbalance between bone resorption via osteoclasts and bone formation via osteoblasts. Other cells including T cells, B cells, macrophages, and osteocytes are also involved in the pathogenesis of osteoporosis. Different cytokines from activated macrophages can regulate or stimulate the development of osteoclastogenesis-associated bone loss. The fusion of macrophages can form multinucleated osteoclasts and, thus, cause bone resorption via the expression of IL-4 and IL-13. Different cytokines, endocrines, and chemokines are also expressed that may affect the presentation of macrophages in osteoporosis.
osteoporosis
macrophage
cytokine
chemokine
estrogen
1. Introduction
Osteoporosis is a systemic skeletal disorder characterized by a generalized increase in bone fragility that results in fractures of the hip, spine, or wrist. There are many risk factors associated with the progression of osteoporosis, including advanced age, low body-mass index, long-term glucocorticoid therapy, history of smoking, family history of hip fracture, excessive alcohol intake and previous fragility fracture. Osteoporosis occurs in all populations with a greater prevalence in postmenopausal women [1]. Women with osteoporosis have poorer musculoskeletal status than women without osteoporosis [2]. Osteoporosis is associated with limitations of daily activities, an increase in the occurrence of falls, and a consequent increased risk of fracture. The etiology of osteoporotic fractures is complex and the incidence of osteoporotic fractures is high among people aged 50 to 54 years [3]. A higher mortality is observed in these patients after osteoporotic fractures occur [4]. Osteoporotic fracture is associated with significant morbidity, mortality, poor quality of life, and increasing health care costs. Therefore, the pathogenesis of bone loss or fracture is an important issue for the prevention of osteoporosis.
2. Osteoblast, Osteoclast and Osteocyte in Bone Formation and Homeostasis
Osteoblasts originate from the mesenchymal stem cells and are important for the progression of bone formation. Co-expression of bone-specific alkaline phosphatase, type I collagen, and non-collagenous matrix proteins is observed in mature osteoblasts. Transforming growth factor-β (TGF-β) has an important role in bone formation by enhancing osteoblast proliferation [5]. TGF-β can also block osteoblast apoptosis and recruit osteoblastic precursors to the bone surface [6][7][8]. interleukin (IL)-4, IL-10, IL-13, and IL-18 may induce elevation of osteoprotegerin (OPG) and reduction of receptor activator of NF-κB ligand (RANKL) [9]. Final differentiation of osteoblasts can form osteocytes, which become embedded in the mineralized matrix. The processes of bone formation by osteoblasts are enhanced by administration of vitamin D and parathyroid hormone. The Wnt/β-catenin is important for osteoblastic differentiation in skeletal biology and disease [10]. Dickkopf-1 and sclerostin are Wnt inhibitors and can regulate the expression of Wnt/β-catenin in osteoblasts [11]. More than 90% of bone cells are osteocytes, which release chemicals to the bone surface that attract osteoclasts. Osteocytes may secrete sclerostin to limit further bone formation by osteoblasts, and play a major role in matrix mineralization [12]. Bone remodeling involves coupling and regulation of osteoclasts and osteoblasts. Besides these resorptive and formative cells, T-cells, B-cells and macrophages may also influence the immune system and bone loss [13]. The bone remodeling cycle is shown in Figure 1.
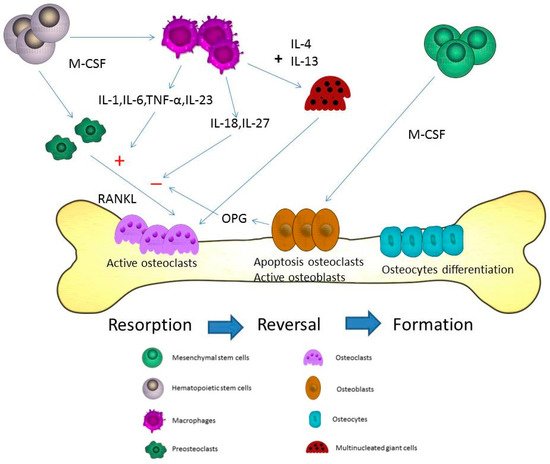
Figure 1. Microdamages in the bone-remodeling units of cancellous or cortical bone induced by osteoclasts. Osteoclasts may be activated by different cytokines including receptor activator of NF-κB ligand (RANKL), interleukin (IL)-1, IL-6, and macrophage-colony stimulating factor (M-CSF) in the resorption state. After the resorption process, the reversal state progresses. In the reversal state, apoptosis of osteoclasts may be induced to stop the bone resorption. The replacement of osteoblasts is observed at the same time. The activated osteoblasts refill the resorption pits and tunnels on the bone surface. In the formation state, osteoblasts directly adhere to the bone surface and progressively form into osteocytes. The proliferation of osteocytes can use these lacuna-canalicular networks to connect within the bone matrix. The cytokines from macrophages including IL-6, tumor necrosis factor-α (TNF-α), IL-23, IL-18, and IL-27 can induce and inhibit osteoclastogenesis through RANKL in bone remodeling. M-CSF is the most important cytokine in the initial stage of macrophage differentiation from hematopoietic stem cells. Different phases are observed during the formation of the multinucleation. RANKL is the major cytokine that stimulates osteoclasts into mature multinucleated osteoclasts. The cytokines, IL-4 and IL-13, may induce macrophages to form multinucleated giant cells during the course of bone resorption.
3. Macrophages in the Pathogenesis of Osteoporosis
In the process of bone remodeling, different states of resorption, reversal, and formation are found. Activated monocytes or bone marrow macrophage precursors adhere to the bone surface to form multinucleated osteoclasts [14]. The bone remodeling cycle is regulated by local and systemic factors. Osteoclasts and osteoblasts are both important for the pathogenesis and progression of osteoporosis. Osteoclasts induce bone resorption and osteoblasts are associated with bone formation. Normal bone quality involves a neutral balance between resorption and formation. A multinucleated osteoclast is differentiated from the mononuclear osteoclast precursor of hemopoietic stem cell. RANKL and macrophage-colony stimulating factor (M-CSF) can induce the proliferation and activation of osteoclasts via the receptor, RANK [15][16]. Therefore, the balance between resorption and formation determines the progression of osteoporosis. M1 macrophages are associated with exacerbation of inflammation and express proinflammatory cytokines. M2 macrophages are associated with anti-inflammatory reactions through the expression of anti-inflammatory cytokines [17]. When macrophages are exposed the stimulation of RNAKL, macrophages may induce osteolcastogenesis and lead to increased M1/M2 ratio in ovariectomized mice. Estrogen can protect M2 macrophage from RANKL stimulation through estrogen receptor αand the downstream blockage of NF-κB p65 nuclear translocation [18]. The blocking of estrogen deficiency-mediated M2 macrophage osteoclastogenesis by reducing the M1/M2 ratio may be a potential therapeutic target in treating postmenopausal osteoporosis. Macrophages play a major role in the activation and formation of osteoclasts and are differentiated from monocytes via M-CSF [19]. The different presentations of macrophages in different organs are shown in Figure 2. Besides the differentiation of osteoclasts from the macrophage lineage, macrophage precursors also differentiate into monocytes, macrophages, and dendritic cells. The activation of macrophages may induce the elevation of interferon-γ (IFN-γ), IL-1, tumor necrosis factor-α (TNF-α), complement proteins, and prostaglandins levels [20]. Macrophages are important for the pathogenesis of osteoporosis [19].
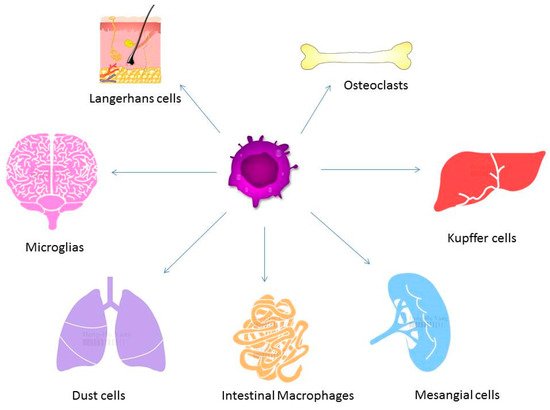
Figure 2. Macrophages exist in different tissues including lung, liver, and brain and have different functions. Different forms of macrophages include Kupffer cells in the liver, alveolar macrophages in the lung, osteoclasts in the bone, and microglia in the brain.
4. The Cytokines from Macrophages Contribute to the Process of Osteoporosis
Macrophages play a major role in the innate and adaptive immune system. Differentiation of macrophages can be found in various tissues including liver, lung, brain, and bone marrow. Macrophages are divided into M1, which express proinflammatory mediators, and M2 that are involved in anti-inflammatory reactions [21]. In the innate immune system, macrophages can execute phagocytosis and opsonization [22]. Different cell receptors of CD14, Fcγ, and CD25 are found in macrophages. Major histocompatibility complex class II molecules and CD23 are also found. These receptors are important for the progression of phagocytosis. IL-4 and IFN-γ can regulate the different functions of macrophages. Macrophages, in turn, may be activated to induce IL-6, TNF-α, IFN-γ, complement protein, and prostaglandins in the immune system. The cytokines expressed by macrophages associated with stimulation or inhibition of osteoclastogenesis include IL-6, IL-18, IL-23, IL-27, and TNF-α [23]. IL-6, a proinflammatory cytokine, can activate osteoclastogenesis [24]. During the inflammation state, proinflammatory cytokines including TNFα, IL-1β, and IL-6, may promote the differentiation and activation of osteoclasts [25]. IL-18 secreted via macrophages may regulate the Th1 differentiation and the IFN-γ production, and is an inhibitor of the TNF-α mediated osteoclastogenesis [26]. Among osteoporotic women, decreased levels of serum IL-18 binding protein and elevated levels of serum IL-18 are observed [27][28]. IFN-γ has a dual role in osteoclasts including the promotion of osteoblast differentiation and inhibition of bone marrow adipocyte formation in different stages [29]. IFN-γ can activate macrophages, but macrophages can secrete IL-18 to regulate the IFN-γ production. IL-23 has been shown to activate osteoclasts [30]. Adding IL-23 to bone marrow stromal cells led to an increased differentiation towards the osteoblast lineage [31]. In the femur of a rat osteoporosis model, IL-23 is reduced after adequate estrogen therapy for improvement of bone mineral density [32]. IL-27 may suppress the expression of RANKL in Th17 cells and CD4+ T cells [33]. IL-27 also inhibited osteoblast apoptosis through increased Egr-2 expression [34]. TNF-α from macrophages may induce indirect osteoclastic activation through RANKL in bone remodeling [35]. Therefore, inflammatory arthritis, such as rheumatoid arthritis, can induce progressive bone loss when the disease is poorly controlled. The different cytokines of macrophages associated with osteoporosis are shown in Figure 1.
5. The Fusion of Macrophages/Monocytes to Form Multinucleated Cells—Osteoclasts
Macrophages have the ability to fuse and develop into multinucleated cells during an acute infection and inflammation state. The formation of granuloma induced by tuberculosis infection and vasculitis may result in these multinucleated giant cells. In systemic inflammation, macrophages can release reactive oxygen and reactive nitrogen species to induce the formation of multinucleated giant cells. M-CSF is the most important cytokine in the initial stage of macrophages differentiation from hematopoietic stem cells. Different phases are observed during the formation of multinucleated cells. RANKL is the major cytokine responsible for the stimulation of osteoclasts into mature multinucleated osteoclasts [36]. The cytokines, IL-4 and IL-13, may induce macrophages to form multinucleated giant cells during the course of bone resorption [37][38][39]. The proliferation and differentiation of macrophages may be stimulated by M-CSF. After the stimulation via M-CSF, RANKL may activate the proliferation of osteoclasts. Fusion-competent osteoclasts may be induced by RANKL. Multinucleated giant cells originate from this fusion of cells, which develops to form multinucleated osteoclasts or giant cells [19]. The fusion of macrophages to form multinucleated osteoclasts is shown in Figure 1.
6. Different Cytokines for the Pathogenesis of Osteoporosis
RANKL can be expressed by different cells including T cells, B cells, bone-marrow stromal cells, and bone-forming osteoblasts. Mice with depletion of RANKL gene show severe osteopetrosis and lack mature circulating osteoclasts [40]. The differentiation of osteoclasts may be inhibited by the decoy receptor OPG, which is produced by osteoblasts [41]. Proinflammatory cytokines including IL-1 and TNF-α can stimulate osteoclastogenesis in vitro [42]. Other osteoclastogenic cytokines include IL-6, IL-8, IL-15, IL-17, and IFN-γ [9][43]. High dosage of IFN-γ may promote the differentiation of osteoclasts, and the effect of bone loss is enhanced in situations of estrogen deficiency [44][45]. The immune response in osteoclastogenesis via IFN-γ include activation of RANKL/RANK pathway and promotion of fused mononucleated osteoclasts [29]. In patients with rheumatoid arthritis (RA), activated T cells can directly trigger osteoclastogenesis through RANKL/RANK/OPG pathway [46][47]. Therefore, juxta-articular osteopenia of both hands and osteoporotic fracture are usually found during the disease course of RA. The role of T cells in regulating osteoclastogenesis is associated with the formation of osteoclasts. B cells may participate in osteoclastogenesis by expression of RANKL for osteoclast differentiation and serve as osteoclast progenitors [48]. Osteoclast-associated receptor may be expressed by macrophages or monocytes in order to modulate the innate and adaptive immune response [49].
References
- Hadji, P.; Klein, S.; Gothe, H.; Haussler, B.; Kless, T.; Schmidt, T.; Steinle, T.; Verheyen, F.; Linder, R. The epidemiology of osteoporosis—bone evaluation study (best): An analysis of routine health insurance data. Dtsch. Arztebl. Int. 2013, 110, 52–57.
- Cunha-Henriques, S.; Costa-Paiva, L.; Pinto-Neto, A.M.; Fonsechi-Carvesan, G.; Nanni, L.; Morais, S.S. Postmenopausal women with osteoporosis and musculoskeletal status: A comparative cross-sectional study. J. Clin. Med. Res. 2011, 3, 168–176.
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16, S3–S7.
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287.
- Kassem, M.; Kveiborg, M.; Eriksen, E.F. Production and action of transforming growth factor-beta in human osteoblast cultures: Dependence on cell differentiation and modulation by calcitriol. Eur. J. Clin. Investig. 2000, 30, 429–437.
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Parfitt, A.M.; Manolagas, S.C. Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. J. Bone Miner. Res. 1998, 13, 793–802.
- Lucas, P.A. Chemotactic response of osteoblast-like cells to transforming growth factor beta. Bone 1989, 10, 459–463.
- Kasagi, S.; Chen, W. Tgf-beta1 on osteoimmunology and the bone component cells. Cell Biosci. 2013, 3, 4.
- Zupan, J.; Jeras, M.; Marc, J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem. Med. (Zagreb) 2013, 23, 43–63.
- Baron, R.; Rawadi, G. Targeting the wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007, 148, 2635–2643.
- Canalis, E.; Giustina, A.; Bilezikian, J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007, 357, 905–916.
- Poole, K.E.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Lowik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844.
- Long, C.L.; Humphrey, M.B. Osteoimmunology: The expanding role of immunoreceptors in osteoclasts and bone remodeling. Bonekey Rep. 2012, 1.
- Kylmaoja, E.; Nakamura, M.; Tuukkanen, J. Osteoclasts and remodeling based bone formation. Curr. Stem. Cell Res. Ther. 2016, 11, 626–633.
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107.
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem Biophys 2008, 473, 201–209.
- Horwood, N.J. Macrophage polarization and bone formation: A review. Clin. Rev. Allergy Immunol. 2016, 51, 79–86.
- Dou, C.; Ding, N.; Zhao, C.; Hou, T.; Kang, F.; Cao, Z.; Liu, C.; Bai, Y.; Dai, Q.; Ma, Q.; et al. Estrogen deficiency-mediated m2 macrophage osteoclastogenesis contributes to m1/m2 ratio alteration in ovariectomized osteoporotic mice. J. Bone Miner. Res. 2018, 33, 899–908.
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131.
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566.
- Dey, A.; Allen, J.; Hankey-Giblin, P.A. Ontogeny and polarization of macrophages in inflammation: Blood monocytes versus tissue macrophages. Front. Immunol. 2014, 5, 683.
- Silva, M.T. When two is better than one: Macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 2010, 87, 93–106.
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of osteoporosis-role of t cells. Front. Immunol. 2018, 9, 657.
- Yun, T.J.; Chaudhary, P.M.; Shu, G.L.; Frazer, J.K.; Ewings, M.K.; Schwartz, S.M.; Pascual, V.; Hood, L.E.; Clark, E.A. Opg/fdcr-1, a tnf receptor family member, is expressed in lymphoid cells and is up-regulated by ligating cd40. J. Immunol. 1998, 161, 6113–6121.
- Jung, Y.K.; Kang, Y.M.; Han, S. Osteoclasts in the inflammatory arthritis: Implications for pathologic osteolysis. Immune Netw. 2019, 19, e2.
- Sims, N.A.; Green, J.R.; Glatt, M.; Schlict, S.; Martin, T.J.; Gillespie, M.T.; Romas, E. Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis. Arthritis Rheum. 2004, 50, 2338–2346.
- Mansoori, M.N.; Shukla, P.; Kakaji, M.; Tyagi, A.M.; Srivastava, K.; Shukla, M.; Dixit, M.; Kureel, J.; Gupta, S.; Singh, D. Il-18bp is decreased in osteoporotic women: Prevents inflammasome mediated il-18 activation and reduces th17 differentiation. Sci. Rep. 2016, 6, 33680.
- Maugeri, D.; Mamazza, C.; Lo Giudice, F.; Puglisi, N.; Muscoso, E.G.; Rizzotto, M.; Testai, M.; Bennati, E.; Lentini, A.; Panebianco, P. Interleukin-18 (il-18) and matrix metalloproteinase-9 (mmp-9) in post-menopausal osteoporosis. Arch. Gerontol. Geriatr. 2005, 40, 299–305.
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-gamma-mediated osteoimmunology. Front. Immunol 2018, 9, 1508.
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper t cell subset that links t cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682.
- Shukla, P.; Mansoori, M.N.; Singh, D. Efficacy of anti-il-23 monotherapy versus combination therapy with anti-il-17 in estrogen deficiency induced bone loss conditions. Bone 2018, 110, 84–95.
- Khera, A.; Kanta, P.; Kalra, J.; Dumir, D.; M, T. Resveratrol restores the level of key inflammatory cytokines and rankl/opg ratio in the femur of rat osteoporosis model. J. Women Aging 2018, 1–13.
- Woodward, J. Regulation of haematopoietic progenitor cell proliferation and survival: The involvement of the osteoblast. Cell Adh. Migr. 2010, 4, 4–6.
- Shukla, P.; Mansoori, M.N.; Kakaji, M.; Shukla, M.; Gupta, S.K.; Singh, D. Interleukin 27 (il-27) alleviates bone loss in estrogen-deficient conditions by induction of early growth response-2 gene. J. Biol. Chem. 2017, 292, 4686–4699.
- Boyce, B.F.; Xing, L. Functions of rankl/rank/opg in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146.
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264.
- Kao, W.J.; McNally, A.K.; Hiltner, A.; Anderson, J.M. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J. Biomed. Mater. Res. 1995, 29, 1267–1275.
- DeFife, K.M.; Jenney, C.R.; McNally, A.K.; Colton, E.; Anderson, J.M. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J. Immunol. 1997, 158, 3385–3390.
- DeFife, K.M.; Jenney, C.R.; Colton, E.; Anderson, J.M. Cytoskeletal and adhesive structural polarizations accompany il-13-induced human macrophage fusion. J. Histochem. Cytochem. 1999, 47, 65–74.
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. Opgl is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323.
- Khosla, S. Minireview: The opg/rankl/rank system. Endocrinology 2001, 142, 5050–5055.
- Garnero, P.; Darte, C.; Delmas, P.D. A model to monitor the efficacy of alendronate treatment in women with osteoporosis using a biochemical marker of bone turnover. Bone 1999, 24, 603–609.
- Greenblatt, M.B.; Shim, J.H. Osteoimmunology: A brief introduction. Immune Netw. 2013, 13, 111–115.
- Mann, G.N.; Jacobs, T.W.; Buchinsky, F.J.; Armstrong, E.C.; Li, M.; Ke, H.Z.; Ma, Y.F.; Jee, W.S.; Epstein, S. Interferon-gamma causes loss of bone volume in vivo and fails to ameliorate cyclosporin a-induced osteopenia. Endocrinology 1994, 135, 1077–1083.
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8.
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated t cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402, 304–309.
- Geusens, P.; Lems, W.F. Osteoimmunology and osteoporosis. Arthritis Res. Ther. 2011, 13, 242.
- Manabe, N.; Kawaguchi, H.; Chikuda, H.; Miyaura, C.; Inada, M.; Nagai, R.; Nabeshima, Y.; Nakamura, K.; Sinclair, A.M.; Scheuermann, R.H.; et al. Connection between b lymphocyte and osteoclast differentiation pathways. J. Immunol. 2001, 167, 2625–2631.
- Nemeth, K.; Schoppet, M.; Al-Fakhri, N.; Helas, S.; Jessberger, R.; Hofbauer, L.C.; Goettsch, C. The role of osteoclast-associated receptor in osteoimmunology. J. Immunol. 2011, 186, 13–18.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




