Osteoporosis is a systemic disease with progressive bone loss. The bone loss is associated with an imbalance between bone resorption via osteoclasts and bone formation via osteoblasts. Other cells including T cells, B cells, macrophages, and osteocytes are also involved in the pathogenesis of osteoporosis. Different cytokines from activated macrophages can regulate or stimulate the development of osteoclastogenesis-associated bone loss. The fusion of macrophages can form multinucleated osteoclasts and, thus, cause bone resorption via the expression of IL-4 and IL-13. Different cytokines, endocrines, and chemokines are also expressed that may affect the presentation of macrophages in osteoporosis.
- osteoporosis
- macrophage
- cytokine
- chemokine
- estrogen
1. Introduction
2. Osteoblast, Osteoclast and Osteocyte in Bone Formation and Homeostasis
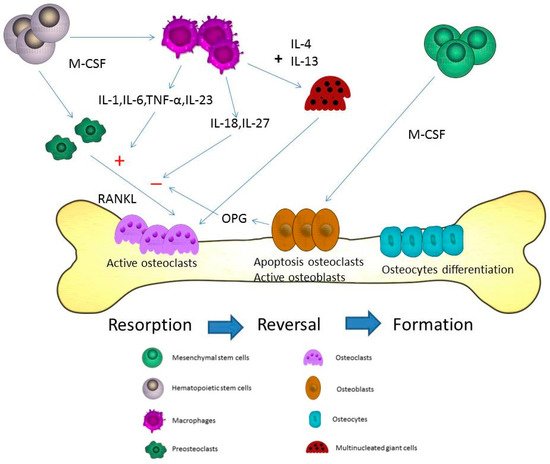
3. Macrophages in the Pathogenesis of Osteoporosis
4. The Cytokines from Macrophages Contribute to the Process of Osteoporosis
5. The Fusion of Macrophages/Monocytes to Form Multinucleated Cells—Osteoclasts
6. Different Cytokines for the Pathogenesis of Osteoporosis
This entry is adapted from the peer-reviewed paper 10.3390/ijms20092093
References
- Hadji, P.; Klein, S.; Gothe, H.; Haussler, B.; Kless, T.; Schmidt, T.; Steinle, T.; Verheyen, F.; Linder, R. The epidemiology of osteoporosis—bone evaluation study (best): An analysis of routine health insurance data. Dtsch. Arztebl. Int. 2013, 110, 52–57.
- Cunha-Henriques, S.; Costa-Paiva, L.; Pinto-Neto, A.M.; Fonsechi-Carvesan, G.; Nanni, L.; Morais, S.S. Postmenopausal women with osteoporosis and musculoskeletal status: A comparative cross-sectional study. J. Clin. Med. Res. 2011, 3, 168–176.
- Johnell, O.; Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005, 16, S3–S7.
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287.
- Kassem, M.; Kveiborg, M.; Eriksen, E.F. Production and action of transforming growth factor-beta in human osteoblast cultures: Dependence on cell differentiation and modulation by calcitriol. Eur. J. Clin. Investig. 2000, 30, 429–437.
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Parfitt, A.M.; Manolagas, S.C. Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. J. Bone Miner. Res. 1998, 13, 793–802.
- Lucas, P.A. Chemotactic response of osteoblast-like cells to transforming growth factor beta. Bone 1989, 10, 459–463.
- Kasagi, S.; Chen, W. Tgf-beta1 on osteoimmunology and the bone component cells. Cell Biosci. 2013, 3, 4.
- Zupan, J.; Jeras, M.; Marc, J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem. Med. (Zagreb) 2013, 23, 43–63.
- Baron, R.; Rawadi, G. Targeting the wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007, 148, 2635–2643.
- Canalis, E.; Giustina, A.; Bilezikian, J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007, 357, 905–916.
- Poole, K.E.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Lowik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844.
- Long, C.L.; Humphrey, M.B. Osteoimmunology: The expanding role of immunoreceptors in osteoclasts and bone remodeling. Bonekey Rep. 2012, 1.
- Kylmaoja, E.; Nakamura, M.; Tuukkanen, J. Osteoclasts and remodeling based bone formation. Curr. Stem. Cell Res. Ther. 2016, 11, 626–633.
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107.
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem Biophys 2008, 473, 201–209.
- Horwood, N.J. Macrophage polarization and bone formation: A review. Clin. Rev. Allergy Immunol. 2016, 51, 79–86.
- Dou, C.; Ding, N.; Zhao, C.; Hou, T.; Kang, F.; Cao, Z.; Liu, C.; Bai, Y.; Dai, Q.; Ma, Q.; et al. Estrogen deficiency-mediated m2 macrophage osteoclastogenesis contributes to m1/m2 ratio alteration in ovariectomized osteoporotic mice. J. Bone Miner. Res. 2018, 33, 899–908.
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131.
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566.
- Dey, A.; Allen, J.; Hankey-Giblin, P.A. Ontogeny and polarization of macrophages in inflammation: Blood monocytes versus tissue macrophages. Front. Immunol. 2014, 5, 683.
- Silva, M.T. When two is better than one: Macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 2010, 87, 93–106.
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of osteoporosis-role of t cells. Front. Immunol. 2018, 9, 657.
- Yun, T.J.; Chaudhary, P.M.; Shu, G.L.; Frazer, J.K.; Ewings, M.K.; Schwartz, S.M.; Pascual, V.; Hood, L.E.; Clark, E.A. Opg/fdcr-1, a tnf receptor family member, is expressed in lymphoid cells and is up-regulated by ligating cd40. J. Immunol. 1998, 161, 6113–6121.
- Jung, Y.K.; Kang, Y.M.; Han, S. Osteoclasts in the inflammatory arthritis: Implications for pathologic osteolysis. Immune Netw. 2019, 19, e2.
- Sims, N.A.; Green, J.R.; Glatt, M.; Schlict, S.; Martin, T.J.; Gillespie, M.T.; Romas, E. Targeting osteoclasts with zoledronic acid prevents bone destruction in collagen-induced arthritis. Arthritis Rheum. 2004, 50, 2338–2346.
- Mansoori, M.N.; Shukla, P.; Kakaji, M.; Tyagi, A.M.; Srivastava, K.; Shukla, M.; Dixit, M.; Kureel, J.; Gupta, S.; Singh, D. Il-18bp is decreased in osteoporotic women: Prevents inflammasome mediated il-18 activation and reduces th17 differentiation. Sci. Rep. 2016, 6, 33680.
- Maugeri, D.; Mamazza, C.; Lo Giudice, F.; Puglisi, N.; Muscoso, E.G.; Rizzotto, M.; Testai, M.; Bennati, E.; Lentini, A.; Panebianco, P. Interleukin-18 (il-18) and matrix metalloproteinase-9 (mmp-9) in post-menopausal osteoporosis. Arch. Gerontol. Geriatr. 2005, 40, 299–305.
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-gamma-mediated osteoimmunology. Front. Immunol 2018, 9, 1508.
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper t cell subset that links t cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682.
- Shukla, P.; Mansoori, M.N.; Singh, D. Efficacy of anti-il-23 monotherapy versus combination therapy with anti-il-17 in estrogen deficiency induced bone loss conditions. Bone 2018, 110, 84–95.
- Khera, A.; Kanta, P.; Kalra, J.; Dumir, D.; M, T. Resveratrol restores the level of key inflammatory cytokines and rankl/opg ratio in the femur of rat osteoporosis model. J. Women Aging 2018, 1–13.
- Woodward, J. Regulation of haematopoietic progenitor cell proliferation and survival: The involvement of the osteoblast. Cell Adh. Migr. 2010, 4, 4–6.
- Shukla, P.; Mansoori, M.N.; Kakaji, M.; Shukla, M.; Gupta, S.K.; Singh, D. Interleukin 27 (il-27) alleviates bone loss in estrogen-deficient conditions by induction of early growth response-2 gene. J. Biol. Chem. 2017, 292, 4686–4699.
- Boyce, B.F.; Xing, L. Functions of rankl/rank/opg in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146.
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264.
- Kao, W.J.; McNally, A.K.; Hiltner, A.; Anderson, J.M. Role for interleukin-4 in foreign-body giant cell formation on a poly(etherurethane urea) in vivo. J. Biomed. Mater. Res. 1995, 29, 1267–1275.
- DeFife, K.M.; Jenney, C.R.; McNally, A.K.; Colton, E.; Anderson, J.M. Interleukin-13 induces human monocyte/macrophage fusion and macrophage mannose receptor expression. J. Immunol. 1997, 158, 3385–3390.
- DeFife, K.M.; Jenney, C.R.; Colton, E.; Anderson, J.M. Cytoskeletal and adhesive structural polarizations accompany il-13-induced human macrophage fusion. J. Histochem. Cytochem. 1999, 47, 65–74.
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. Opgl is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323.
- Khosla, S. Minireview: The opg/rankl/rank system. Endocrinology 2001, 142, 5050–5055.
- Garnero, P.; Darte, C.; Delmas, P.D. A model to monitor the efficacy of alendronate treatment in women with osteoporosis using a biochemical marker of bone turnover. Bone 1999, 24, 603–609.
- Greenblatt, M.B.; Shim, J.H. Osteoimmunology: A brief introduction. Immune Netw. 2013, 13, 111–115.
- Mann, G.N.; Jacobs, T.W.; Buchinsky, F.J.; Armstrong, E.C.; Li, M.; Ke, H.Z.; Ma, Y.F.; Jee, W.S.; Epstein, S. Interferon-gamma causes loss of bone volume in vivo and fails to ameliorate cyclosporin a-induced osteopenia. Endocrinology 1994, 135, 1077–1083.
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8.
- Kong, Y.Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated t cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999, 402, 304–309.
- Geusens, P.; Lems, W.F. Osteoimmunology and osteoporosis. Arthritis Res. Ther. 2011, 13, 242.
- Manabe, N.; Kawaguchi, H.; Chikuda, H.; Miyaura, C.; Inada, M.; Nagai, R.; Nabeshima, Y.; Nakamura, K.; Sinclair, A.M.; Scheuermann, R.H.; et al. Connection between b lymphocyte and osteoclast differentiation pathways. J. Immunol. 2001, 167, 2625–2631.
- Nemeth, K.; Schoppet, M.; Al-Fakhri, N.; Helas, S.; Jessberger, R.; Hofbauer, L.C.; Goettsch, C. The role of osteoclast-associated receptor in osteoimmunology. J. Immunol. 2011, 186, 13–18.
