Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Deepak Ipe | + 3462 word(s) | 3462 | 2021-05-09 13:52:49 | | | |
| 2 | Lily Guo | + 1901 word(s) | 5363 | 2021-05-28 06:06:44 | | | | |
| 3 | Lily Guo | Meta information modification | 5363 | 2021-06-01 06:14:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ipe, D. DMAHDM Nanocomposite. Encyclopedia. Available online: https://encyclopedia.pub/entry/10218 (accessed on 07 February 2026).
Ipe D. DMAHDM Nanocomposite. Encyclopedia. Available at: https://encyclopedia.pub/entry/10218. Accessed February 07, 2026.
Ipe, Deepak. "DMAHDM Nanocomposite" Encyclopedia, https://encyclopedia.pub/entry/10218 (accessed February 07, 2026).
Ipe, D. (2021, May 28). DMAHDM Nanocomposite. In Encyclopedia. https://encyclopedia.pub/entry/10218
Ipe, Deepak. "DMAHDM Nanocomposite." Encyclopedia. Web. 28 May, 2021.
Copy Citation
Researchers have developed novel nanocomposites that incorporate additional biomaterials with dimethylaminohexadecyl methacrylate (DMAHDM) in order to reduce secondary caries. The aim of this review was to summarize the current literature and assess the synergistic antibacterial and remineralizing effects that may contribute to the prevention of secondary caries. An electronic search was undertaken in MEDLINE using PubMed, Embase, Scopus, Web of Science and Cochrane databases.
biomaterial
1. Introduction
Dental composite resins are widely used restorative materials due to their ability to conserve tooth structure during cavity preparation, aesthetics and direct-filling capabilities [1][2][3][4]. However, composite resin restorations still present several drawbacks including polymerization shrinkage, bulk fracture, mechanical fatigue by mastication, marginal leakage and biodegradation by acid while also being challenged by the adhesion and accumulation of cariogenic bacteria (Viz., Streptococcus mutans and Lactobacilli) when compared to other materials used for restoration [1][2][5].
Dental biofilm adjoining to the tooth-restoration margin could lead to secondary caries, the primary reason for failure of composite restorations [4], accounting for nearly 50% of failures within 10 years [6][7][8]. The replacement of failed restorations may result in further tooth structure removal, which can weaken the remaining hard tissues and affect the long-term prognosis of the tooth [9]. Consequently, efforts have been made to create a novel dental composite resins with the addition of antibacterial and remineralizing agents to combat secondary caries [1].
When copolymerized in resins, quaternary ammonium methacrylates (QAMs), which are cationic compounds, exhibit low toxicity and a broad-spectrum antimicrobial effect [2][10][11][12]. These positively charged methacrylic monomers bind and disrupt the electrical equilibrium of the negatively charged bacterial membranes thereby causing rupture and cell death [13].
The antibacterial efficacy of QAMs has been correlated with the alkyl chain length (CL) of the hydrocarbons as this causes an increase in hydrophobicity [2][14][15]. Previous studies have reported that an increase in CL from 3 to 16 greatly enhanced the efficacy of dental materials against bacteria [16], which then decreased at a CL of 18 [17][18]. A CL of 18 exhibited an increase in live bacteria and biofilm thickness and did not further decrease the metabolic activity or strengthen the bacterial inhibition effect compared to a CL of 16 [19][20]. Therefore, dimethylaminohexadecyl methacrylate (DMAHDM) monomer with a CL of 16 was found to exhibit the best antiseptic effect against oral bacterial pathogens when used as bonding agents, sealers or other dental materials [17][21].
Due to the strong antibacterial properties of DMAHDM, researchers have experimented with combining additional biomaterials with DMAHDM to explore any synergistic mechanisms of action to increase the efficacy of DMAHDM and reduce biofilm formation at the tooth-restoration interface [8][11]. DMAHDM does not possess any inherent remineralizing capabilities, so efforts have also been made to incorporate biomaterials that promote tooth remineralization as another approach for caries inhibition [1][8][22]. Furthermore, the development of this nanotechnology is quickly evolving and there has been a shift in focus to create novel composite resins that possess both antibacterial and remineralizing capabilities [23].
Despite this growing area of research, there is yet to be a review that outlines the current combinations of DMAHDM nanocomposite or any synergistic mechanisms of action that enhance the effects of DMAHDM nanocomposite. As such, this systematic review intended to summarize the literature on dental composite resins that incorporate additional biomaterials with DMAHDM, and to assess for any possible synergistic antibacterial and remineralizing effects that may aid in the prevention of secondary caries.
2. Study Selection
The initial literature search retrieved 954 results, 559 remained after removal of duplicates. After screening of titles and abstracts 25 articles remained, of which 15 were eligible for inclusion after the full-text review. Six articles were excluded as they did not use DMAHDM or had an additive biomaterial [24][25][26][27][28][29], one each used dental materials other than the composite [30] or tested materials with compromised mechanical properties [31] while two were non-English articles [32][33]. The results of the screening and search process are presented in Figure 1.
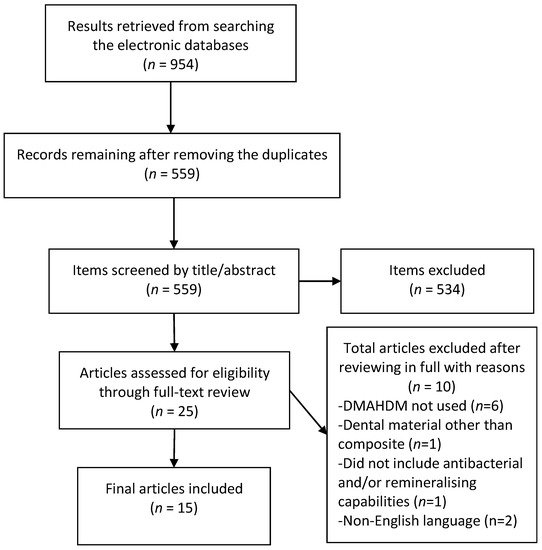
Figure 1. Preferred reporting items for systematic review and meta-analyses (PRISMA) flow chart demonstrating the selection of articles.
3. Study Characteristics
All study characteristics are reported in Supplementary Materials Table S1. There were no studies published prior to 2015. All the studies were conducted in USA and/or China, or Japan. Eight of the studies used EBPM (ethoxylated bisphenol A-dimethacrylate (EBPADMA) and pyromellitic glyceroldimethacrylate (PMGDM)) as the composite resin matrix while seven of the studies used a BisGMA-TEGDMA (bisphenol A-glycidyl methacrylate and triethylene glycol dimethacrylate) resin matrix. Nine studies used the heliomolar commercial composite as a control comparison group, two studies used the Renamel Microfill commercial composite and one study used both as a control group.
There were six different combinations reported in the literature, which incorporated additional biomaterials with DMAHDM: DMAHDM + nanoparticles of amorphous calcium phosphate (NACP); DMAHDM + 2-methacryloyloxyethyl phosphorylcholine (MPC); DMAHDM + NACP + MPC; DMAHDM + NACP + silver nanoparticles (AgNPs); DMAHDM + NACP + MPC + AgNPs and DMAHDM + NACP + MPC + AgNPs + polyamidoamine dendrimers (PAMAM).
All studies used a modified Menschutkin reaction method to synthesize DMAHDM [2]. NACP was synthesized with a spray-drying technique [34]. MPC was sourced commercially. AgNPs was chemically prepared by dissolving silver 2-ethylhexanoate in 2-(tert-butylamino)ethyl methacrylate (TBAEMA) [35]. PAMAM was used at 1 mg/mL concentration, by mixing PAMAM in distilled water [23]. Antibacterial and remineralizing outcomes used by the included studies are outlined in Table 1.
Table 1. Tests for antibacterial and remineralizing outcomes.
| Antibacterial Outcomes | Remineralizing Outcomes |
|---|---|
|
|
4. Risk of Bias
As reported in Table 2, all 15 studies demonstrated a medium risk of bias and reported an adequate control group, standardized sample production process, standardized antibacterial/remineralizing assessment and adequate statistical analysis. No studies reported that the antibacterial or remineralizing properties were evaluated by a single operator or presented a sample size calculation. No studies reported about the manufacturer’s instructions for using the materials. As these are experimental studies, most studies prepared their own composite materials.
Table 2. Risk of bias among the included studies.
| Reference | Sample Size Calculation | Adequate Control Group | Use of Materials According to Manufacturer’s Instruction | Standardized Sample Production Process | Standardized Antibacterial/ Remineralizing Assessment |
Evaluation of Antibacterial/ Remineralizing Properties by a Single Operator |
Adequate Statistical Analysis | Risk of Bias * |
|---|---|---|---|---|---|---|---|---|
| Wu et al., 2015 [34] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wu et al., 2015 [36] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhang et al., 2015 [37] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Melo et al., 2016 [38] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wang et al., 2016 [39] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wang et al., 2016 [40] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Xie et al., 2016 [41] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhang et al., 2016 [20] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Xiao et al., 2017 [23] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhang et al., 2017 [42] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Al-Dulaijan et al., 2018 [1] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wang et al., 2019 [43] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Xiao et al., 2019 [35] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Bhadila et al., 2020 [2] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhou et al., 2020 [44] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
5. Antibacterial Results
There were 14 studies that assessed the antibacterial potential of incorporating bioactive materials with DMAHDM to target specific bacterial species (Table 3). Seven studies assessed efficiency against all bacterial species, all streptococci and Streptococcus mutans. One study assessed efficiency against S. mutans, Lactobacillus acidophilus, Candida albicans and multispecies biofilms. Additionally, periodontal pathogens found in subgingival biofilms are highly associated with periodontal disease and root caries. Four studies looked at the antibacterial efficiency against specific root caries pathogens [45], including Prevotella. intermedia, Porphyromonas. gingivalis and Aggregatibacter. actinomycetemcomitans, with an objective of using this restorative material for cervical restorations. One study did not specify the microorganisms tested.
Table 3. Antibacterial results.
| Reference | Biomaterial Combinations Used and Comparison Group/s | Microorganisms Tested | Time Points Assessed | Methodology Used to Assess Antibacterial Effectiveness |
Results Summary |
|---|---|---|---|---|---|
| Wu et al., 2015 [37] | (1–5) Five variants with 10% DMAHDM + 20% NACP + 0%, 2.5%, 5%, 7.5% or 10%, respectively, of microcapsules of formaldehyde and urea Comparison group: (6) 20% NACP |
Total microorganism Total streptococci Streptococcus mutans |
2 days | Live/dead assay | Biofilm was primarily alive in (6) (1) showed mostly dead bacteria. |
| Metabolic assay (MTT) | Metabolic activity of (1) was reduced by 96% compared to (6) | ||||
| Production of Lactic Acid | (1) reduced lactic acid production by 99% compared to (6) | ||||
| CFUc counts | (1) reduced the biofilm CFU by 3–4 times compared to (6) | ||||
| Wu et al., 2015 [39] | (1–4) Four variants with 20% NACP and DMAHDM mass fraction of 0.75%, 1.5%, 2.25% and 3%, respectively Comparison group: (5) 20% NACP |
Total microorganisms Total streptococci S. mutans |
2 days | Live/dead assay | Biofilm was primarily alive in (5). Amounts of dead bacteria increased with increase in the mass fraction of DMAHDM |
| Metabolic assay (MTT) | Metabolic activity—(4) was 96% lower than (5) | ||||
| Production of Lactic Acid | Increasing DMAHDM mass fraction caused a monotonic decrease in the production of lactic acid | ||||
| CFUc counts | Antibacterial activity increased and CFU decreased with increase in the mass fraction of DMAHDM | ||||
| Zhang et al., 2015 [40] | (1) 1.5% DMAHDM (2) 3% MPC (3) 1.5% DMAHDM + 3% MPC Comparison groups: (4) 0% DMAHDM + 0% MPC (5) Heliomolar commercial composite |
Total microorganisms Total streptococci S. mutans |
2 days | Live/dead assay | (1) demonstrated lower bacterial adhesion but most bacteria were alive (1) showed high amounts of dead bacteria |
| Metabolic assay (MTT) | (3) had the least metabolic activity and higher reduction in biofilm growth compared to (1) or (2) | ||||
| Production of Lactic Acid | (3) had the least lactic acid production | ||||
| CFU counts | (3) reduced CFU counts by >3 logs compared to (4) or (5) and had much less biofilm CFU than (1) or (2) | ||||
| Protein adsorption | (3) had less protein adsorption compared to controls than (1) or (2) | ||||
| Melo et al., 2016 [41] | (1) 5% DMAHDM + 0.1% AgNPs + 30% NACP Comparison group: (2) 0% DMAHDM + 0% AgNPs + 0% NACP |
Did not specify | 7 days | Live/dead assay | Dental materials containing multiagents resulted in compromised bacteria at tooth-composite interface |
| Wang et al., 2016 [42] | (1) 3% DMAHDM + 20% NACP Comparison groups: (2) 20% NACP (3) Heliomolar commercial composite |
P. gingivalis, P. intermedia, Prevotella nigrescens, A. actinomycetemcomitans, F. nucleatum, Enterococcus faecalis |
2 days | Live/dead assay | (1) mainly had dead bacteria while (2) and (3) had primarily live bacteria |
| CFU counts | (1) CFU reduction differed between the bacterial species differently, few by <3 log while others by >3 log | ||||
| Crystal violet biofilm biomass assay | (1) had a significantly decreased biomass value compared to (2) and (3) | ||||
| Polysaccharide production | (1) had greatly reduced polysaccharide production for all six species compared to (2) and (3) | ||||
| Wang et al., 2016 [43] | (1) 3% DMAHDM (2) 3% MPC (3) 3% DMAHDM + 3% MPC b Comparison groups: (4) 0% DMAHDM + 0% MPC (5) Heliomolar commercial composite |
P. gingivalis, P. intermedia, A. actinomycetemcomitans, F. nucleatum |
2 days | Live/dead assay | (2) reduced the adhesion of bacteria, (3) demonstrated mostly dead bacteria |
| Metabolic activity (MTT) | (3) presented lower biofilm metabolic activity on all the tested periodontal pathogens compared to (4) and (5) | ||||
| CFU counts | Addition of DMAHDM or MPC independently into the composite decreased the CFU | ||||
| Protein adsorption | (1) had no effect on protein adsorption (2) substantially decreased the protein adsorption by one log compared to (4) and (5) |
||||
| Polysaccharide production | (3) had much less polysaccharide production compared to (4) and (5) | ||||
| Xie et al., 2016 [44] | (1) 30% NACP + 3% MPC (2) 30% NACP + 3% MPC + 1.5% DMAHDM (3) 30% NACP + 3% MPC + 3% DMAHDM Comparison groups: (4) 30% NACP (5) Heliomolar commercial composite |
Total microorganisms Total streptococci S. mutans |
2 days (with 2-day biofilm) and 4 days (pH required 72 h of incubation) | Live/dead assay | (4) and (5) were completely covered by live bacteria. Bacterial adhesion was reduced by MPC, DMAHDM produced an antibacterial effect. (3) had the most dead bacteria followed by (2) and (1) |
| Metabolic assay (MTT) | Metabolic activity of biofilms of (3) < (2) < (1) (3) had the lowest metabolic activity of biofilms among all |
||||
| CFU counts | (3) had the least biofilm CFU, count reduced by 3 logs compared to (4) and (5). | ||||
| Protein adsorption | (1) had protein adsorption one log less than (5); (2) and (3) had no effect on the protein adsorption | ||||
| pH of biofilm culture medium | (3) maintained a pH above 6.5. | ||||
| Zhang et al., 2016 [20] | (1–5) Five variants with 20% NACP with QAM CL of 3, 6, 12, 16 and 18, respectively Comparison groups: (6) 20% NACP (7) Renamel Microfill commercial composite |
Total microorganisms Total streptococci S. mutans |
30 days | Live/dead assay | (6) and (7) were covered by live bacteria. Dead bacteria increased progressively from CL3 up to CL16 with maximum antibacterial potency at CL16 before decreasing in potency at CL18 as indicated by some live bacteria. |
| Metabolic assay (MTT) | Metabolic activity of biofilms decreased with increase in CL from 3 to 16. CL16 had maximum reduction on metabolic activity, which remained constant with any increase in CL |
||||
| Production of Lactic Acid | The biofilms on (6) and (7) produced the most acid. Acid production capacity of biofilm increased with an increase in CL from 3 to 16 CL16 minimized lactic acid production by 10-fold compared to (6) and (7) |
||||
| CFU counts | CFU counts decreased with an increase in the CL from 3 to 16. Antibacterial activity was strongest at CL16, which lowered at C18. CL16 reduced all three CFU counts by 2 logs compared to (6) and (7) | ||||
| Zhang et al., 2017 [45] | (1) 1.5% DMAHDM (2) 3% MPC (3) 1.5% DMAHDM + 3% MPC Comparison group: (4) Heliomolar commercial composite |
Total microorganisms Total streptococci S. mutans |
185 days | Live/dead assay | (3) had high levels of dead bacteria and lower bacterial attachment. Protein-repellent and anti-biofilm activities remained same from day 1 to 180 |
| Metabolic assay (MTT) | (1) and (2) showed higher reduction of biofilm viability than (4) (3) had the least metabolic activity Antibacterial function remained the same from day 1 to 180, being unimpacted by water-aging |
||||
| Production of Lactic Acid | (3) had the least lactic acid production | ||||
| CFU counts | (1) and (2) decreased the CFU compared to (4). (3) had greater antibacterial properties compared to (1) and (2) and was nearly 3 logs lower than (4), both at 1 day and 180 days of water-aging (p < 0.05). | ||||
| Protein adsorption | MPC greatly inhibited protein adsorption with no difference between 1 day and 180 days. (3) had the same protein adsorption as (2) (p > 0.1), which was about one tenth that of (4) and (1) (p < 0.05). | ||||
| Al-Dulaijan et al., 2018 [1] | (1) 20% NACP + 3% DMAHDM (2) 20% NACP Comparison group: (3) Heliomolar commercial composite |
Total microorganisms Total streptococci S. mutans |
2 days | Live/dead assay | (1) had much less live bacteria compared to (2) and (3). |
| Metabolic assay (MTT) | (1) greatly decreased the metabolic activity of the biofilms compared to (2) and (3) (p < 0.05). (2) had similar metabolic activity to (3) indicating that NACP had little effect on biofilm viability. | ||||
| Production of Lactic Acid | (1) had the least lactic acid production. | ||||
| CFU counts | (1) decreased all three CFU counts by 3–4 logs compared to (2) and (3). | ||||
| Wang et al., 2019 [46] | (1) 3% MPC + 20% NACP (2) 3% DMAHDM + 20% NACP (3) 3% DMAHDM + 3% MPC + 20% NACP Comparison groups: (4) 20% NACP (5) Heliomolar commercial composite |
(1) Biofilm with one species: P. gingivalis (2) Biofilm with three species: P. gingivalis, S. gordonii and F. nucleatum (3) Biofilm with six species: P. gingivalis, S. gordonii, F. nucleatum, A. naeslundii, P. intermedia and A. actinomycetemcomitans (4) Nine-species biofilm: P. gingivalis, S. gordonii, F. nucleatum, A. naeslundii, P. intermedia, A. actinomycetemcomitans, P. nigrescens, Tannerella forsythia and Parvimonas micra |
4 days | Live/dead assay | (2) had large quantity of dead bacteria, (1) showed lower bacterial adhesion. (3) had large quantity of dead bacteria but lower bacterial adhesion that (4) and (5) which were largely covered by live bacteria |
| Metabolic assay (MTT) | (1) and (2) reduced the metabolic activity greatly compared to (3) Killing power of DMAHDM decreased with increase in the number of species in the biofilm (3) had stronger killing efficacy on all biofilm types |
||||
| CFU | (3) had higher reduction of CFU than (1) and (2), by >3 log on all four biofilm types | ||||
| Protein adsorption | DMAHDM demonstrated no effect on protein adsorption (1) decreased the protein adsorption by approximately 1 log, compared to (2) and (5) |
||||
| Polysaccharide production | Single species biofilms produced less polysaccharides than multi species biofilms (1) and (2) decreased the amount of polysaccharide produced by biofilms, (3) showed least production in all the biofilm types |
||||
| Xiao et al., 2019 [38] | (1) 30% NACP + 3% MPC + 3% DMAHDM (2) 30% NACP + 3% MPC + 3% DMAHDM + 0.12% AgNPs a,c Comparison groups: (3) 30% NACP (4) Renamel Microfill commercial composite |
(1) P. gingivalis (2) A. actinomycetemcomitans (3) F. nucleatum |
2 days | Live/dead assay | (1) and (2) had much less biofilms, with mostly dead bacteria compared to (3) and (4), which were mostly covered by live bacteria. |
| Metabolic assay (MTT) | Metabolic activity of (1) and (2) lower than (3) and (4) (2) showed lower biofilm metabolic activity for all three bacteria species than (1) |
||||
| Production of Lactic Acid | (1) had significantly decreased CFU counts for all three species, (2) showed the lowest CFU. (1) reduced the CFU counts by 4 logs on P. gingivalis and A. actinomycetemcomitans, while (2) reduced the CFU counts by 5 log. (1) and (2) reduced the CFU counts for F. nucleatum, by 3 and 5 logs, respectively. |
||||
| Protein adsorption | (1) and (2) decreased protein adsorption, it was e tenth of (3) and (4) | ||||
| Polysaccharide production | (1) had much less polysaccharide production than (3) and (4 The lowest production of polysaccharides from biofilms was caused by (2) |
||||
| Bhadila et al., 2020 [2] | (1) 20% NACP (2) 3% DMAHDM + 20% NACP Comparison group: (3) Heliomolar commercial composite |
S. mutans | 2 days | Live/dead assay | (2) had primarily dead bacteria compared to (1) and (3) which were primarily covered by live bacteria. |
| Production of Lactic Acid | (2) caused lowest production of lactic acid production from biofilms than (1) and (3) | ||||
| CFU counts | (2) showed a CFU reduction of 3–4 logs less than (1) and (3). | ||||
| Zhou et al., 2020 [47] | (1) 30% NACP (2) 3% DMAHDM (3) 30% NACP + 3% DMAHDM Comparison groups: (4) 0% NACP + 0% DMAHDM (5) Heliomolar commercial composite |
S. mutans L. acidophilus C. albicans Multi-species |
2 days | Live/dead assay | (2) had substantial dead bacteria for all species tested while (1), (4) and (5) were covered with live bacteria. |
| Metabolic assay (MTT) | (2) reduced the metabolic activity of biofilms significantly | ||||
| Production of Lactic Acid | (1), (4) and (5) showed higher lactic acid production from S. mutans and polymicrobial biofilms while (2) and (3) inhibited. All materials produced lower levels of lactic acid from L. acidophilus and C. albicans | ||||
| CFU counts | (2) and (3) greatly reduced S. mutans and C. albicans CFU levels by 5 and 3 logs respectively | ||||
| Polysaccharide production | (2) and (3) inhibited production of extracellular matrix from the bacterial associated with root caries |
5.1. Live/Dead Staining
All 14 articles that evaluated antibacterial potential assessed the biofilms for live/dead staining on the samples of composites. After staining, green and red fluorescence indicated live and dead bacteria respectively; this was then evaluated with an inverted epifluorescence microscope [40]. All studies reported that composites with DMAHDM had significantly reduced live bacteria than control composites. Among the ten studies that assessed the antibacterial potential of NACP alone, all reported similar results to control composites where bacteria were primarily alive, indicating NACP did not have any additional antibacterial effects with DMAHDM [1][2][34][39][43]. Composites with MPC alone showed lower levels of bacterial adhesion but the bacteria were found to be mostly alive; however, when combined with DMAHDM, there were less biofilms and the bacteria had mostly compromised cell membranes [3][35][37][40][41][42] with no significant changes in results after 180 days of water-aging [42].
5.2. CFU Counts
There were 13 articles that assessed the CFU counts to understand the antibacterial effect of DMAHDM composite with or without additional bioactive materials. All studies reported a decrease in CFU counts for all microorganisms tested against composites containing DMAHDM compared to control composites or composites without DMAHDM, confirming its antibacterial potency. One study reported increased antibacterial activity (decreasing CFU counts) with an increase in the mass fraction of DMAHDM [36]. Adding MPC to the DMAHDM composite had a synergistic antibacterial effect that decreased CFU counts much more than using either biomaterial alone as observed by three studies [37][41][42]. One study described a synergistic effect against CFU counts for Fusobacterium nucleatum, P. gingivalis and A. actinomycetemcomitans when incorporating 0.12% AgNPs into the composite [35].
5.3. MTT Metabolic Assays
Eleven articles assessed metabolic assays where the biofilms were incubated with MTT solution, which resulted in the bacteria that were metabolically active. Four studies reported that the metabolic activity of biofilms on DMAHDM composites was lower than commercial controls or NACP alone, which had little effect on biofilm viability [1][34][36][44]. Five studies showed that DMAHDM + MPC combinations resulted in the least metabolic activity of all biofilms including total microorganisms or total streptococci, and all periodontal pathogens [37][40][41][42][43]. One study explained that DMAHDM + MPC was effective in killing single or polymicrobial biofilms, compared to DMAHDM alone whose killing efficacy decreased as the number of species in the biofilm increased [43]. Xiao et al. (2019) reported that the addition of 0.12% AgNPs reduced the metabolic activity of biofilms for periodontal pathogens (F. nucleatum, A. actinomycetemcomitans and P. gingivalis) compared to composites without AgNPs [35].
5.4. Lactic Acid Production
Eight articles assessed the production of lactic acid from biofilms by adding buffered-peptone water to the composite disks and incubating them. Lactic acid production was significantly lower in the DMAHDM group than the NACP composite group [1][2][20][34][44]. NACP alone produced higher levels of lactic acid production that were comparable to commercial control composites [20][44]. It was also reported by two studies that DMAHDM + MPC had the least lactic acid production compared to using the biomaterials alone [37][42].
5.4.5. Protein Adsorption
The protein adsorption of biofilms was evaluated by six studies by using a micro bicinchoninic acid (BCA) protein assay. One study reported that DMAHDM + MPC had less protein adsorption compared to that of the biomaterials alone [37]. However, four studies reported that DMAHDM alone had no significant effects on protein adsorption, whereas MPC substantially decreased the protein adsorption [40][41][42][43], with no difference in efficacy over 180 days [42]. A synergistic effect was found when incorporating 0.12% AgNPs, which significantly reduced protein absorption compared to other concentrations of AgNPs as reported by Xiao et al. (2019) [35].
5.4.6. Polysaccharide Production
Polysaccharide production in biofilms’ extracellular polymeric substance (EPS) was assessed by five articles. Composites containing DMAHDM greatly reduced polysaccharide production for all biofilms compared to NACP composites, which had similar polysaccharide production to commercial control composites [35][39][44]. MPC alone also significantly reduced polysaccharide production, however, a combination of DMAHDM + MPC displayed a synergistic effect exhibiting the least amount of polysaccharides for all types of biofilms tested [40][43]. Furthermore, Xiao et al. (2019) also described a synergistic effect by adding 0.12% AgNPs, which had a further reduction of polysaccharides than DMAHDM + MPC [35].
5.4.7. Other Results
One study tested the pH for the media that was incubated with bacterial biofilm and composite disks with or without bioactive materials [41]. Xie et al. (2016) reported that DMAHDM + MPC composite maintained a safe pH of >6.5, in comparison to the cariogenic pH of 4.2 in the control group [41]. Wang, Melo, et al. (2016) evaluated biofilm biomass and reported that DMAHDM had a significantly decreased biofilm biomass value compared to NACP composite and commercial control composite [39].
5.5. Remineralization Results
There were five studies that assessed the remineralization potential of incorporating bioactive materials with DMAHDM (Table 4). All five studies incorporated NACP as a remineralizing agent, while one study also incorporated MPC, AgNPs and PAMAM to assess its remineralizing potential.
Table 4. Remineralization results.
| Reference | Biomaterial Combinations and Comparison Group/s | Time Points Assessed | Methodology Used to Assess Remineralization | Results Summary |
|---|---|---|---|---|
| Zhang et al., 2016 [20] | (1) 20% NACP with QAM CL16 a Comparison group: (2) 20% NACP |
1, 3, 7, 14, 21 and 28 days | Release of Ca and P ions | (1) released lower levels of Ca and P ions than (2) |
| Xiao et al., 2017 [23] | (1) 0.12% AgNPs + 3% MPC + 3% DMAHDM + 30% NACP (2) 0.12% AgNPs + 3% MPC + 3% DMAHDM + 30% NACP + PAMAM Comparison groups: (3) Demineralized root dentine specimen (4) Demineralized root dentine specimen + PAMAM |
1, 3, 5, 7, 10, 14 and 21 days | Concentration of Ca and P ions | (1) and (2) demonstrated greater concentrations of Ca and P concentrations than PAMAM and control groups |
| Acid neutralization | (1) and (2) had greater acid neutralization than PAMAM and comparison groups. | |||
| Dentine hardness | (2) had the greatest dentine hardness, remineralization and mineral growth. | |||
| SEMexamination | (2) had the greatest remineralization and mineral growth. | |||
| Al-Dulaijan et al., 2018 [1] | (1) 20% NACP + 3% DMAHDM (2) 20% NACP Comparison group: (3) Heliomolar commercial composite |
1, 3, 5, 7, 14, 21, 28, 35, 42, 49, 56, 63 and 70 days | Concentration of Ca and P ions | No differences in concentrations of Ca and P ions, their recharge and re-release between (1) and (2) groups |
| 1, 2, 3, 5, 9, 11 and 14 days | Recharge and rerelease of Ca and P | Specimens could release the ions for 42 days after one charge | ||
| Bhadila et al., 2020 [2] | (1) 20% NACP (2) 3% DMAHDM + 20% NACP Comparison group: (3) Heliomolar commercial composite |
1, 3, 5, 7, 14, 21, 28, 35, 42, 49, 56, 63 and 70 days | Release of Ca and P ions | No significant difference in Ca and P ion release between (1) and (2) |
| 1, 3, 5, 7, 9 and 14 days | Recharge and rerelease of Ca and P | Both composites showed increasing ion concentration with time, and release continued after each recharge. | ||
| Zhou et al., 2020 [47] | (1) 30% NACP (2) 3% DMAHDM (3) 30% NACP + 3% DMAHDM Comparison groups: (4) 0% NACP+ 0% DMAHDM (5) Heliomolar commercial composite |
1, 3, 7, 14, 21, 28, 35, 42, 49, 54, 63 and 70 days | Release of Ca and P ions | No difference in release of Ca and P ions between (1) and (3) Lower pH increased ion release |
| Dentine hardness | (3) caused the highest dentine hardness, which was twice |
5.5.1. Calcium and Phosphate Ion Release
Five studies evaluated and compared the levels of calcium (Ca) and phosphate (P) ion release. The total time before evaluating varied from 21 to 70 days. One early study reported a moderately lower Ca and P ion release in DMAHDM + NACP composite group than the NACP composite group [20]. This was contradicted by three later studies, which found no significant difference between the NACP and DMAHDM + NACP composites [1][2][44]. Xiao et al. (2017) reported that DMAHDM + MPC + NACP + AgNPs and DMAHDM + MPC + NACP + AgNPs + PAMAM had higher calcium and phosphate concentrations than other control groups [23]. Two articles assessed ion recharge and rerelease and Ca and P ions. Both studies reported that NACP and DMAHDM + NACP continuously released ions after being recharged with no significant differences between them [1][2].
5.5.2. Other Results
Two studies evaluated dentine hardness at the dentine-restoration interface. Xiao et al. (2017) concluded that DMAHDM + MPC + NACP + AgNPs + PAMAM had the greatest dentine hardness, remineralization and mineral growth. Zhou et al. (2020) found that dentine hardness in DMAHDM + NACP group was more than double that of the control groups [23]. Xiao et al. (2017) further analyzed acid neutralization and a scanning electron microscopic examination (Table 4). It was reported that DMAHDM + MPC + NACP + AgNPs and DMAHDM + MPC + NACP + AgNPs + PAMAM had greater acid neutralization than PAMAM alone or other control groups [23].
6. Discussion
The aim of this systematic review was to report the current combinations of the DMAHDM composite and to assess the synergistic effects on the prevention of secondary caries. The findings from the studies included indicate that incorporating additional biomaterials with DMAHDM produces a positive synergistic effect on the prevention of secondary caries through antibacterial and remineralizing capabilities.
Regardless of the type of biomaterial combination added to the composite, all studies considered in this review reported a strong antibacterial efficacy of DMAHDM alone, with one study observing increased potency as the mass fraction increased [36]. It is suggested that the mechanism of action of QAMs is through contact inhibition leading to cell death [40][41]. However, it is essential that salivary protein adsorption occurs on the composite surface for bacterial adhesion to follow, and this pellicle separating the resin surface from the biofilm could decrease QAM’s antibacterial efficacy [3][23][40].
MPC is a biocompatible polymer that was shown to have a synergistic mechanism of action when incorporated with DMAHDM compared to either agent alone [35][37][40][41][42][43]. Due to its hydrophilic surface, MPC can inhibit bacterial adhesion and decrease protein adsorption [35][40][46], thus, forming direct contact of the resin surface with the overlaying biofilms and enhancing the contact-killing mechanism of DMAHDM [35][40].
The literature reported DMAHDM + MPC having less biofilms with mostly compromised cell membranes, decreased CFU counts, the least metabolic activity, stronger killing efficacy, least lactic acid production, decreased protein adsorption and maintained a pH above 6.5 [41]. However, Zhang et al. (2017) observed live bacteria on the MPC + DMAHDM composite despite a reduction in lactic acid production from biofilm and total microorganism CFU counts [42]. This further emphasizes the need for more research into the long-term effectiveness of these novel composites on the prevention of secondary caries in orally healthy individuals.
Several articles also reported on the efficacy of novel composites against root caries pathogens [23][35][39][40][43][44]. Composites containing DMAHDM and MPC produced significantly lower levels of lactic acid from S. mutans and polymicrobial biofilms [1][2][20][34][44]. Lactic acid is a known byproduct of caries-causing bacteria, and coupled with their aciduric properties, allows them to survive in low pH conditions [44]. By reducing the amount of lactic acid, root dentine demineralization at the restoration margins may be reduced [44], which can improve the longevity of these restorations.
Combining MPC with DMAHDM was effective in inhibiting extracellular matrix synthesis of root caries pathogens by having substantially reduced polysaccharide production [44]. Polysaccharides are a key component of the extracellular polymeric substances (EPS) that surrounds biofilms and protects pathogens from antibacterial agents. Reducing polysaccharide production reduces this protection and the virulence of these pathogens, potentially reducing caries and inhibiting local periodontitis [47].
AgNPs have been associated with many dental applications such as acrylic resins for dentures [48], endodontic irrigants and intracanal medications [49][50] and other restorative materials [15][51]. The antibacterial properties of AgNPs is hypothesized to be due to the release of silver ions to the bacterial environment where the nanoparticle promotes infiltration into the bacterial cell membranes and affects intracellular processes [38]. Furthermore, the incorporation of AgNPs at 0.12% concentration with MPC and DMAHDM composite was shown to have even greater synergistic mechanisms of action, with further reductions of CFU counts, metabolic activity, protein adsorption and polysaccharide production [35]. AgNPs are particularly effective in inhibiting S. mutans and F. nucleatum, and are also capable of long-distance bacterial killing [35]. Incorporating small particles sizes of AgNPs increases the surface area, thus achieving a strong antimicrobial function with a relatively low filler level of AgNPs without compromising the mechanical properties or aesthetics of the resin [35][38].
Despite the interdisciplinary role AgNP has in modern medicine, recent reviews have discussed the possible environmental and economic impacts of AgNPs that are synthesized either naturally (green synthesis) or chemically [52][53][54][55]. Moreover, development of AgNPs using the biological method of synthesis with the use of bacteria, fungi and plant extracts [52][53] have shown to be ecofriendlier, cost effective and energy efficient [56], with reports of greater antimicrobial activity against pathogenic bacteria [52]. All three studies that experimented with AgNPs in this review synthesized the nanoparticles through chemical reduction. As such, further research is required to examine the effects of incorporating biosynthesized AgNPs on the efficacy of DMAHDM nanocomposite.
While NACP did not exhibit any antibacterial effect and showed comparable results to commercial control composites, NACP was effective on remineralization of tooth structure and pH neutralization [1][2][3][20][34][35][36][39][41][44]. The addition of NACP enables the release of Ca and P ions to increase the pH during cariogenic challenges, thereby, preventing demineralization and facilitating remineralization [35] and thus, reducing the potential for secondary caries development. Recent studies found no significant difference between the NACP and DMAHDM + NACP composites, meaning that DMAHDM did not impact the release of ions and can therefore be incorporated at no disadvantage [1][2][35]. Furthermore, the addition of NACP in the composite resin allows for repeated recharges, facilitates ion rerelease and remineralization over a longer period of time [1]. DMAHDM maintained potent antibiofilm properties after 12 cycles of recharge and did not affect ion re-release concentrations [1][2]. The findings from Bhadila et al. (2020) indicate that the concentration of Ca and P ion releases from NACP and DMAHDM combinations surpassed the required levels for tooth remineralization [57]. These results are very promising for improving the longevity and prognosis of current restorative materials.
Xiao et al. (2017) reported synergistic remineralizing effects such as greater acid neutralization, dentine hardness and mineral growth when combining NACP and third generation PAMAM. PAMAM exhibits the ability to remineralize tooth lesions through its role as an excellent nucleation template whereby Ca and P ions are rapidly absorbed leading to remineralization [23][58][59]. PAMAM has also shown lasting dentine mineral regeneration when incorporated with recharged NACP after prolonged fluid exposure [60], which indicates successful long-term therapeutic effects in reducing demineralization and aiding in the reduction of secondary caries.
Xiao et al. (2017) suggested that the incorporation of MPC, AgNPs, MPC and PAMAM with DMAHDM yielded the maximum antibacterial and remineralization capacity. The addition of MPC and AgNPs with DMAHDM produced significant synergistic antibacterial effects, while NACP and PAMAM provided continuous ion release and combined remineralization mechanisms of action [23]. Therefore, this novel bioactive composite combination shows promising results that may adjunctively reduce the rate of secondary caries and increase the longevity of these restorations.
The findings of this systematic review suggest that incorporation of antibacterial and remineralizing biomaterials have the potential to aid in the prevention of secondary caries. However, caries is a complex multifactorial disease and other factors must be considered when determining the clinical success of composite resins. These include but are not limited to, quality of the restoration such as the presence of microgaps and patient caries risk including oral hygiene habits, salivary flow and composition, consumption of dietary sugars and exposure to fluoride [61].
The limitations of this review include medium risk of bias and in vitro conditions in all studies. Additionally, there were wide variations of comparison groups between the studies. This study only focused on antibacterial and remineralization properties and did not consider mechanical qualities during water-aging. The bacterial incubation period for samples tested for antibacterial efficacy were heterogeneous across the studies, ranging from two days in some studies to 185 days in one study. The differences in incubation protocols may have depended on the manufacturer’s instructions, pH of biofilm culture medium and that different types of bacteria required different incubation times. Therefore, it is important to note that these variations were adjusted accordingly to target specific bacterial species for different studies.
References
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Wang, L.; Xu, H.H.K. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J. Dent. 2018, 72, 44–52.
- Bhadila, G.; Baras, B.H.; Weir, M.D.; Wang, H.; Melo, M.A.S.; Hack, G.D.; Bai, Y.; Xu, H.H.K. Novel antibacterial calcium phosphate nanocomposite with long-term ion recharge and re-release to inhibit caries. Dent. Mater. J. 2020, 4, 678–689.
- Wang, W.; Zhu, S.; Zhang, G.; Wu, F.; Ban, J.; Wang, L. Antibacterial and thermomechanical properties of experimental dental resins containing quaternary ammonium monomers with two or four methacrylate groups. RSC Adv. 2019, 9, 40681–40688.
- Ferracane, J.L. Resin composite--state of the art. Dent. Mater. 2011, 27, 29–38.
- Rasines Alcaraz, M.G.; Veitz-Keenan, A.; Sahrmann, P.; Schmidlin, P.R.; Davis, D.; Iheozor-Ejiofor, Z. Direct composite resin fillings versus amalgam fillings for permanent or adult posterior teeth. Cochrane Database Syst. Rev. 2014, 3.
- Cheng, L.; Zhang, K.; Melo, M.A.S.; Weir, M.D.; Zhou, X.; Xu, H.H.K. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604.
- Eltahlah, D.; Lynch, C.D.; Chadwick, B.L.; Blum, I.R.; Wilson, N.H.F. An update on the reasons for placement and replacement of direct restorations. J. Dent. 2018, 72, 1–7.
- Xie, X.; Wang, L.; Xing, D.; Zhang, K.; Weir, M.D.; Liu, H.; Bai, Y.; Xu, H.H.K. Novel dental adhesive with triple benefits of calcium phosphate recharge, protein-repellent and antibacterial functions. Dent. Mater. 2017, 33, 553–563.
- Askar, H.; Krois, J.; Göstemeyer, G.; Bottenberg, P.; Zero, D.; Banerjee, A.; Schwendicke, F. Secondary caries: What is it, and how it can be controlled, detected, and managed? Clin. Oral Investig. 2020, 24, 1869–1876.
- Chrószcz, M.; Barszczewska-Rybarek, I. Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials. Polymers 2020, 12, 2551.
- Xue, J.; Wang, J.; Feng, D.; Huang, H.; Wang, M. Application of Antimicrobial Polymers in the Development of Dental Resin Composite. Molecules 2020, 25, 4738.
- Zhang, K.; Zhang, N.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Bioactive Dental Composites and Bonding Agents Having Remineralizing and Antibacterial Characteristics. Dent. Clin. N. Am. 2017, 61, 669–687.
- Rego, G.F.; Vidal, M.L.; Viana, G.M.; Cabral, L.M.; Schneider, L.F.J.; Portela, M.B.; Cavalcante, L.M. Antibiofilm properties of model composites containing quaternary ammonium methacrylates after surface texture modification. Dent. Mater. 2017, 33, 1149–1156.
- He, J.; Söderling, E.; Österblad, M.; Vallittu, P.K.; Lassila, L.V. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules 2011, 16, 9755–9763.
- Chen, H.; Tang, Y.; Weir, M.D.; Gao, J.; Imazato, S.; Oates, T.W.; Lei, L.; Wang, S.; Hu, T.; Xu, H.H.K. Effects of S. mutans gene-modification and antibacterial monomer dimethylaminohexadecyl methacrylate on biofilm growth and acid production. Dent. Mater. 2020, 36, 296–309.
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938.
- Makvandi, P.; Esposito Corcione, C.; Paladini, F.; Gallo, A.L.; Montagna, F.; Jamaledin, R.; Pollini, M.; Maffezzoli, A. Antimicrobial modified hydroxyapatite composite dental bite by stereolithography. Polym. Adv. Technol. 2018, 29, 364–371.
- Zhou, H.; Li, F.; Weir, M.D.; Xu, H.H. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. J. Dent. 2013, 41, 1122–1131.
- Zhou, H.; Weir, M.D.; Antonucci, J.M.; Schumacher, G.E.; Zhou, X.D.; Xu, H.H.K. Evaluation of three-dimensional biofilms on antibacterial bonding agents containing novel quaternary ammonium methacrylates. Int. J. Oral Sci. 2014, 6, 77–86.
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.X.; Xu, H.H. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53.
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441.
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769.
- Xiao, S.; Liang, K.; Weir, M.D.; Cheng, L.; Liu, H.; Zhou, X.; Ding, Y.; Xu, H.H.K. Combining Bioactive Multifunctional Dental Composite with PAMAM for Root Dentin Remineralization. Materials 2017, 10, 89.
- Beigi Burujeny, S.; Atai, M.; Yeganeh, H. Assessments of antibacterial and physico-mechanical properties for dental materials with chemically anchored quaternary ammonium moieties: Thiol-ene-methacrylate vs. conventional methacrylate system. Dent. Mater. 2015, 31, 244–261.
- Zhou, C.; Weir, M.D.; Zhang, K.; Deng, D.; Cheng, L.; Xu, H.H. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dent. Mater. 2013, 29, 859–870.
- Cheng, L.; Weir, M.D.; Zhang, K.; Xu, S.M.; Chen, Q.; Zhou, X.; Xu, H.H. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. J. Dent. Res. 2012, 91, 460–466.
- Wang, S.; Wang, H.; Ren, B.; Li, X.; Wang, L.; Zhou, H.; Weir, M.D.; Zhou, X.; Masri, R.M.; Oates, T.W.; et al. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018, 8, 5509.
- Buruiana, T.; Melinte, V.; Popa, I.D.; Buruiana, E.C. New urethane oligodimethacrylates with quaternary alkylammonium for formulating dental composites. J. Mater. Sci. 2014, 25, 1183–1194.
- Cheng, L.; Zhang, K.; Zhou, C.-C.; Weir, M.D.; Zhou, X.-D.; Xu, H.H.K. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016, 8, 172–181.
- Li, Y.; Hu, X.; Ruan, J.; Arola, D.D.; Ji, C.; Weir, M.D.; Oates, T.W.; Chang, X.; Zhang, K.; Xu, H.H.K. Bonding durability, antibacterial activity and biofilm pH of novel adhesive containing antibacterial monomer and nanoparticles of amorphous calcium phosphate. J. Dent. 2019, 81, 91–101.
- Campos, K.d.P.L.; Viana, G.M.; Cabral, L.M.; Portela, M.B.; Hirata Junior, R.; Cavalcante, L.M.; Lourenço, E.J.V.; Telles, D.d.M. Self-cured resin modified by quaternary ammonium methacrylates and chlorhexidine: Cytotoxicity, antimicrobial, physical, and mechanical properties. Dent. Mater. 2020, 36, 68–75.
- Junling, W.; Qiang, Z.; Ruinan, S.; Ting, Z.; Jianhua, G.; Chuanjian, Z. Dental plaque microcosm biofilm behavior on a resin composite incorporated with nano-antibacterial inorganic filler containing long-chain alkyl quaternary ammonium salt. Hua Xi Kou Qiang Yi Xue Za Zhi. West China J. Stomatol. 2015, 33, 565–569.
- Wu, J.; Zhang, Q.; Zhu, T.; Ge, J.; Zhou, C. Development of novel self-healing and antibacterial resin composite containing microcapsules filled with polymerizable healing monomer. Zhonghua Kou Qiang Yi Xue Za Zhi. Chin. J. Stomatol 2015, 50, 469–473.
- Wu, J.; Weir, M.D.; Melo, M.A.; Xu, H.H. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J. Dent. 2015, 43, 317–326.
- Xiao, S.; Wang, H.; Liang, K.; Tay, F.; Weir, M.D.; Melo, M.A.S.; Wang, L.; Wu, Y.; Oates, T.W.; Ding, Y.; et al. Novel multifunctional nanocomposite for root caries restorations to inhibit periodontitis-related pathogens. J. Dent. 2019, 81, 17–26.
- Wu, J.; Zhou, H.; Weir, M.D.; Melo, M.A.; Levine, E.D.; Xu, H.H. Effect of dimethylaminohexadecyl methacrylate mass fraction on fracture toughness and antibacterial properties of CaP nanocomposite. J. Dent. 2015, 43, 1539–1546.
- Zhang, N.; Ma, J.; Melo, M.A.; Weir, M.D.; Bai, Y.; Xu, H.H. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234.
- Melo, M.A.; Orrego, S.; Weir, M.D.; Xu, H.H.; Arola, D.D. Designing Multiagent Dental Materials for Enhanced Resistance to Biofilm Damage at the Bonded Interface. ACS Appl. Mater. Interfaces 2016, 8, 11779–11787.
- Wang, L.; Melo, M.A.; Weir, M.D.; Xie, X.; Reynolds, M.A.; Xu, H.H. Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent. Mater. 2016, 32, e351–e361.
- Wang, L.; Xie, X.; Imazato, S.; Weir, M.D.; Reynolds, M.A.; Xu, H.H. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 702–710.
- Xie, X.; Wang, L.; Xing, D.; Arola, D.D.; Weir, M.D.; Bai, Y.; Xu, H.H. Protein-repellent and antibacterial functions of a calcium phosphate rechargeable nanocomposite. J. Dent. 2016, 52, 15–22.
- Zhang, N.; Zhang, K.; Melo, M.A.; Weir, M.D.; Xu, D.J.; Bai, Y.; Xu, H.H. Effects of Long-Term Water-Aging on Novel Anti-Biofilm and Protein-Repellent Dental Composite. Int. J. Mol. Sci. 2017, 18, 186.
- Wang, L.; Xie, X.; Qi, M.; Weir, M.D.; Reynolds, M.A.; Li, C.; Zhou, C.; Xu, H.H.K. Effects of single species versus multispecies periodontal biofilms on the antibacterial efficacy of a novel bioactive Class-V nanocomposite. Dent. Mater. 2019, 35, 847–861.
- Zhou, W.; Zhou, X.; Huang, X.; Zhu, C.; Weir, M.D.; Melo, M.A.S.; Bonavente, A.; Lynch, C.D.; Imazato, S.; Oates, T.W.; et al. Antibacterial and remineralizing nanocomposite inhibit root caries biofilms and protect root dentin hardness at the margins. J. Dent. 2020.
- Takahashi, N.; Nyvad, B. Ecological hypothesis of dentin and root caries. Caries Res. 2016, 50, 422–431.
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330.
- Wang, L.; Li, C.; Weir, M.D.; Zhang, K.; Zhou, Y.; Xu, H.H.K.; Reynolds, M.A. Novel multifunctional dental bonding agent for Class-V restorations to inhibit periodontal biofilms. RSC Adv. 2017, 7, 29004–29014.
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777.
- Lotfi, M.; Vosoughhosseini, S.; Ranjkesh, B.; Khani, S.; Saghiri, M.; Zand, V. Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis. Afr. J. Biotechnol. 2011, 10, 6799–6803.
- Samiei, M.; Aghazadeh, M.; Lotfi, M.; Shakoei, S.; Aghazadeh, Z.; Pakdel, S.M.V. Antimicrobial efficacy of mineral trioxide aggregate with and without silver nanoparticles. Iran. Endod. J. 2013, 8, 166.
- Chen, G.; Lu, J.; Lam, C.; Yu, Y. A novel green synthesis approach for polymer nanocomposites decorated with silver nanoparticles and their antibacterial activity. Analyst 2014, 139, 5793–5799.
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721.
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23.
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074.
- Bhat, R.; Deshpande, R.; Ganachari, S.V.; Huh, D.S.; Venkataraman, A. Photo-irradiated biosynthesis of silver nanoparticles using edible mushroom pleurotus Florida and their antibacterial activity studies. Bioinorg. Chem. Appl. 2011, 2011, 650979.
- Yasin, S.; Liu, L.; Yao, J. Biosynthesis of silver nanoparticles by bamboo leaves extract and their antimicrobial activity. J. Fiber Bioeng. Inf. 2013, 6, 77–84.
- Dickens, S.H.; Flaim, G.M.; Takagi, S. Mechanical properties and biochemical activity of remineralizing resin-based Ca–PO4 cements. Dent. Mater. 2003, 19, 558–566.
- Gao, Y.; Liang, K.; Li, J.; Yuan, H.; Liu, H.; Duan, X.; Li, J. Effect and stability of poly (amido amine)-induced biomineralization on dentinal tubule occlusion. Materials 2017, 10, 384.
- Liang, K.; Yuan, H.; Li, J.; Yang, J.; Zhou, X.; He, L.; Cheng, L.; Gao, Y.; Xu, X.; Zhou, X. Remineralization of Demineralized Dentin Induced by Amine-Terminated PAMAM Dendrimer. Macromol. Mater. Eng. 2015, 300, 107–117.
- Liang, K.; Wang, S.; Tao, S.; Xiao, S.; Zhou, H.; Wang, P.; Cheng, L.; Zhou, X.; Weir, M.D.; Oates, T.W.; et al. Dental remineralization via poly(amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. Oral Sci. 2019, 11, 1–12.
- Cocco, A.R.; da Rosa, W.L.d.O.; da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
01 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




