Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Researchers have developed novel nanocomposites that incorporate additional biomaterials with dimethylaminohexadecyl methacrylate (DMAHDM) in order to reduce secondary caries. The aim of this review was to summarize the current literature and assess the synergistic antibacterial and remineralizing effects that may contribute to the prevention of secondary caries. An electronic search was undertaken in MEDLINE using PubMed, Embase, Scopus, Web of Science and Cochrane databases.
- biomaterial
1. Introduction
Dental composite resins are widely used restorative materials due to their ability to conserve tooth structure during cavity preparation, aesthetics and direct-filling capabilities [1,2,3,4]. However, composite resin restorations still present several drawbacks including polymerization shrinkage, bulk fracture, mechanical fatigue by mastication, marginal leakage and biodegradation by acid while also being challenged by the adhesion and accumulation of cariogenic bacteria (Viz., Streptococcus mutans and Lactobacilli) when compared to other materials used for restoration [1,2,5].
Dental biofilm adjoining to the tooth-restoration margin could lead to secondary caries, the primary reason for failure of composite restorations [4], accounting for nearly 50% of failures within 10 years [6,7,8]. The replacement of failed restorations may result in further tooth structure removal, which can weaken the remaining hard tissues and affect the long-term prognosis of the tooth [9]. Consequently, efforts have been made to create a novel dental composite resins with the addition of antibacterial and remineralizing agents to combat secondary caries [1].
When copolymerized in resins, quaternary ammonium methacrylates (QAMs), which are cationic compounds, exhibit low toxicity and a broad-spectrum antimicrobial effect [2,10,11,12]. These positively charged methacrylic monomers bind and disrupt the electrical equilibrium of the negatively charged bacterial membranes thereby causing rupture and cell death [13].
The antibacterial efficacy of QAMs has been correlated with the alkyl chain length (CL) of the hydrocarbons as this causes an increase in hydrophobicity [2,14,15]. Previous studies have reported that an increase in CL from 3 to 16 greatly enhanced the efficacy of dental materials against bacteria [16], which then decreased at a CL of 18 [17,18]. A CL of 18 exhibited an increase in live bacteria and biofilm thickness and did not further decrease the metabolic activity or strengthen the bacterial inhibition effect compared to a CL of 16 [19,20]. Therefore, dimethylaminohexadecyl methacrylate (DMAHDM) monomer with a CL of 16 was found to exhibit the best antiseptic effect against oral bacterial pathogens when used as bonding agents, sealers or other dental materials [17,21].
Due to the strong antibacterial properties of DMAHDM, researchers have experimented with combining additional biomaterials with DMAHDM to explore any synergistic mechanisms of action to increase the efficacy of DMAHDM and reduce biofilm formation at the tooth-restoration interface [8,11]. DMAHDM does not possess any inherent remineralizing capabilities, so efforts have also been made to incorporate biomaterials that promote tooth remineralization as another approach for caries inhibition [1,8,22]. Furthermore, the development of this nanotechnology is quickly evolving and there has been a shift in focus to create novel composite resins that possess both antibacterial and remineralizing capabilities [23].
Despite this growing area of research, there is yet to be a review that outlines the current combinations of DMAHDM nanocomposite or any synergistic mechanisms of action that enhance the effects of DMAHDM nanocomposite. As such, this systematic review intended to summarize the literature on dental composite resins that incorporate additional biomaterials with DMAHDM, and to assess for any possible synergistic antibacterial and remineralizing effects that may aid in the prevention of secondary caries.
2. Study Selection
The initial literature search retrieved 954 results, 559 remained after removal of duplicates. After screening of titles and abstracts 25 articles remained, of which 15 were eligible for inclusion after the full-text review. Six articles were excluded as they did not use DMAHDM or had an additive biomaterial [27,28,29,30,31,32], one each used dental materials other than the composite [33] or tested materials with compromised mechanical properties [34] while two were non-English articles [35,36]. The results of the screening and search process are presented in Figure 1.
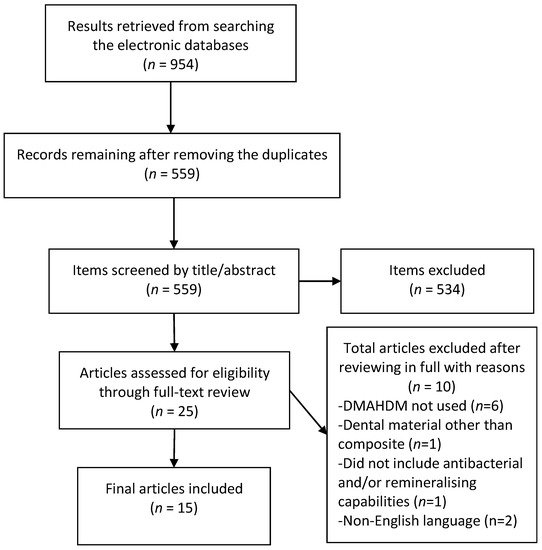
Figure 1. Preferred reporting items for systematic review and meta-analyses (PRISMA) flow chart demonstrating the selection of articles.
3. Study Characteristics
All study characteristics are reported in Supplementary Materials Table S1. There were no studies published prior to 2015. All the studies were conducted in USA and/or China, or Japan. Eight of the studies used EBPM (ethoxylated bisphenol A-dimethacrylate (EBPADMA) and pyromellitic glyceroldimethacrylate (PMGDM)) as the composite resin matrix while seven of the studies used a BisGMA-TEGDMA (bisphenol A-glycidyl methacrylate and triethylene glycol dimethacrylate) resin matrix. Nine studies used the heliomolar commercial composite as a control comparison group, two studies used the Renamel Microfill commercial composite and one study used both as a control group.
There were six different combinations reported in the literature, which incorporated additional biomaterials with DMAHDM: DMAHDM + nanoparticles of amorphous calcium phosphate (NACP); DMAHDM + 2-methacryloyloxyethyl phosphorylcholine (MPC); DMAHDM + NACP + MPC; DMAHDM + NACP + silver nanoparticles (AgNPs); DMAHDM + NACP + MPC + AgNPs and DMAHDM + NACP + MPC + AgNPs + polyamidoamine dendrimers (PAMAM).
All studies used a modified Menschutkin reaction method to synthesize DMAHDM [2]. NACP was synthesized with a spray-drying technique [37]. MPC was sourced commercially. AgNPs was chemically prepared by dissolving silver 2-ethylhexanoate in 2-(tert-butylamino)ethyl methacrylate (TBAEMA) [38]. PAMAM was used at 1 mg/mL concentration, by mixing PAMAM in distilled water [23]. Antibacterial and remineralizing outcomes used by the included studies are outlined in Table 2.
Table 2. Tests for antibacterial and remineralizing outcomes.
4. Risk of Bias
As reported in Table 3, all 15 studies demonstrated a medium risk of bias and reported an adequate control group, standardized sample production process, standardized antibacterial/remineralizing assessment and adequate statistical analysis. No studies reported that the antibacterial or remineralizing properties were evaluated by a single operator or presented a sample size calculation. No studies reported about the manufacturer’s instructions for using the materials. As these are experimental studies, most studies prepared their own composite materials.
Table 3. Risk of bias among the included studies.
| Reference | Sample Size Calculation | Adequate Control Group | Use of Materials According to Manufacturer’s Instruction | Standardized Sample Production Process | Standardized Antibacterial/ Remineralizing Assessment |
Evaluation of Antibacterial/ Remineralizing Properties by a Single Operator |
Adequate Statistical Analysis | Risk of Bias * |
|---|---|---|---|---|---|---|---|---|
| Wu et al., 2015 [37] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wu et al., 2015 [39] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhang et al., 2015 [40] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Melo et al., 2016 [41] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wang et al., 2016 [42] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wang et al., 2016 [43] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Xie et al., 2016 [44] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhang et al., 2016 [20] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Xiao et al., 2017 [23] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhang et al., 2017 [45] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Al-Dulaijan et al., 2018 [1] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Wang et al., 2019 [46] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Xiao et al., 2019 [38] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Bhadila et al., 2020 [2] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
| Zhou et al., 2020 [47] | No | Yes | No | Yes | Yes | No | Yes | Medium risk |
5. Antibacterial Results
There were 14 studies that assessed the antibacterial potential of incorporating bioactive materials with DMAHDM to target specific bacterial species (Table 4). Seven studies assessed efficiency against all bacterial species, all streptococci and Streptococcus mutans. One study assessed efficiency against S. mutans, Lactobacillus acidophilus, Candida albicans and multispecies biofilms. Additionally, periodontal pathogens found in subgingival biofilms are highly associated with periodontal disease and root caries. Four studies looked at the antibacterial efficiency against specific root caries pathogens [48], including Prevotella. intermedia, Porphyromonas. gingivalis and Aggregatibacter. actinomycetemcomitans, with an objective of using this restorative material for cervical restorations. One study did not specify the microorganisms tested.
Table 4. Antibacterial results.
5.1. Live/Dead Staining
All 14 articles that evaluated antibacterial potential assessed the biofilms for live/dead staining on the samples of composites. After staining, green and red fluorescence indicated live and dead bacteria respectively; this was then evaluated with an inverted epifluorescence microscope [43]. All studies reported that composites with DMAHDM had significantly reduced live bacteria than control composites. Among the ten studies that assessed the antibacterial potential of NACP alone, all reported similar results to control composites where bacteria were primarily alive, indicating NACP did not have any additional antibacterial effects with DMAHDM [1,2,37,42,46]. Composites with MPC alone showed lower levels of bacterial adhesion but the bacteria were found to be mostly alive; however, when combined with DMAHDM, there were less biofilms and the bacteria had mostly compromised cell membranes [3,38,40,43,44,45] with no significant changes in results after 180 days of water-aging [45].
5.2. CFU Counts
There were 13 articles that assessed the CFU counts to understand the antibacterial effect of DMAHDM composite with or without additional bioactive materials. All studies reported a decrease in CFU counts for all microorganisms tested against composites containing DMAHDM compared to control composites or composites without DMAHDM, confirming its antibacterial potency. One study reported increased antibacterial activity (decreasing CFU counts) with an increase in the mass fraction of DMAHDM [39]. Adding MPC to the DMAHDM composite had a synergistic antibacterial effect that decreased CFU counts much more than using either biomaterial alone as observed by three studies [40,44,45]. One study described a synergistic effect against CFU counts for Fusobacterium nucleatum, P. gingivalis and A. actinomycetemcomitans when incorporating 0.12% AgNPs into the composite [38].
5.3. MTT Metabolic Assays
Eleven articles assessed metabolic assays where the biofilms were incubated with MTT solution, which resulted in the bacteria that were metabolically active. Four studies reported that the metabolic activity of biofilms on DMAHDM composites was lower than commercial controls or NACP alone, which had little effect on biofilm viability [1,37,39,47]. Five studies showed that DMAHDM + MPC combinations resulted in the least metabolic activity of all biofilms including total microorganisms or total streptococci, and all periodontal pathogens [40,43,44,45,46]. One study explained that DMAHDM + MPC was effective in killing single or polymicrobial biofilms, compared to DMAHDM alone whose killing efficacy decreased as the number of species in the biofilm increased [46]. Xiao et al. (2019) reported that the addition of 0.12% AgNPs reduced the metabolic activity of biofilms for periodontal pathogens (F. nucleatum, A. actinomycetemcomitans and P. gingivalis) compared to composites without AgNPs [38].
5.4. Lactic Acid Production
Eight articles assessed the production of lactic acid from biofilms by adding buffered-peptone water to the composite disks and incubating them. Lactic acid production was significantly lower in the DMAHDM group than the NACP composite group [1,2,20,37,47]. NACP alone produced higher levels of lactic acid production that were comparable to commercial control composites [20,47]. It was also reported by two studies that DMAHDM + MPC had the least lactic acid production compared to using the biomaterials alone [40,45].
3.4.5. Protein Adsorption
The protein adsorption of biofilms was evaluated by six studies by using a micro bicinchoninic acid (BCA) protein assay. One study reported that DMAHDM + MPC had less protein adsorption compared to that of the biomaterials alone [40]. However, four studies reported that DMAHDM alone had no significant effects on protein adsorption, whereas MPC substantially decreased the protein adsorption [43,44,45,46], with no difference in efficacy over 180 days [45]. A synergistic effect was found when incorporating 0.12% AgNPs, which significantly reduced protein absorption compared to other concentrations of AgNPs as reported by Xiao et al. (2019) [38].
3.4.6. Polysaccharide Production
Polysaccharide production in biofilms’ extracellular polymeric substance (EPS) was assessed by five articles. Composites containing DMAHDM greatly reduced polysaccharide production for all biofilms compared to NACP composites, which had similar polysaccharide production to commercial control composites [38,42,47]. MPC alone also significantly reduced polysaccharide production, however, a combination of DMAHDM + MPC displayed a synergistic effect exhibiting the least amount of polysaccharides for all types of biofilms tested [43,46]. Furthermore, Xiao et al. (2019) also described a synergistic effect by adding 0.12% AgNPs, which had a further reduction of polysaccharides than DMAHDM + MPC [38].
3.4.7. Other Results
One study tested the pH for the media that was incubated with bacterial biofilm and composite disks with or without bioactive materials [44]. Xie et al. (2016) reported that DMAHDM + MPC composite maintained a safe pH of >6.5, in comparison to the cariogenic pH of 4.2 in the control group [44]. Wang, Melo, et al. (2016) evaluated biofilm biomass and reported that DMAHDM had a significantly decreased biofilm biomass value compared to NACP composite and commercial control composite [42].
3.5. Remineralization Results
There were five studies that assessed the remineralization potential of incorporating bioactive materials with DMAHDM (Table 5). All five studies incorporated NACP as a remineralizing agent, while one study also incorporated MPC, AgNPs and PAMAM to assess its remineralizing potential.
Table 5. Remineralization results.
3.5.1. Calcium and Phosphate Ion Release
Five studies evaluated and compared the levels of calcium (Ca) and phosphate (P) ion release. The total time before evaluating varied from 21 to 70 days. One early study reported a moderately lower Ca and P ion release in DMAHDM + NACP composite group than the NACP composite group [20]. This was contradicted by three later studies, which found no significant difference between the NACP and DMAHDM + NACP composites [1,2,47]. Xiao et al. (2017) reported that DMAHDM + MPC + NACP + AgNPs and DMAHDM + MPC + NACP + AgNPs + PAMAM had higher calcium and phosphate concentrations than other control groups [23]. Two articles assessed ion recharge and rerelease and Ca and P ions. Both studies reported that NACP and DMAHDM + NACP continuously released ions after being recharged with no significant differences between them [1,2].
3.5.2. Other Results
Two studies evaluated dentine hardness at the dentine-restoration interface. Xiao et al. (2017) concluded that DMAHDM + MPC + NACP + AgNPs + PAMAM had the greatest dentine hardness, remineralization and mineral growth. Zhou et al. (2020) found that dentine hardness in DMAHDM + NACP group was more than double that of the control groups [23]. Xiao et al. (2017) further analyzed acid neutralization and a scanning electron microscopic examination (Table 5). It was reported that DMAHDM + MPC + NACP + AgNPs and DMAHDM + MPC + NACP + AgNPs + PAMAM had greater acid neutralization than PAMAM alone or other control groups [23].
4. Discussion
The aim of this systematic review was to report the current combinations of the DMAHDM composite and to assess the synergistic effects on the prevention of secondary caries. The findings from the studies included indicate that incorporating additional biomaterials with DMAHDM produces a positive synergistic effect on the prevention of secondary caries through antibacterial and remineralizing capabilities.
Regardless of the type of biomaterial combination added to the composite, all studies considered in this review reported a strong antibacterial efficacy of DMAHDM alone, with one study observing increased potency as the mass fraction increased [39]. It is suggested that the mechanism of action of QAMs is through contact inhibition leading to cell death [43,44]. However, it is essential that salivary protein adsorption occurs on the composite surface for bacterial adhesion to follow, and this pellicle separating the resin surface from the biofilm could decrease QAM’s antibacterial efficacy [3,23,43].
MPC is a biocompatible polymer that was shown to have a synergistic mechanism of action when incorporated with DMAHDM compared to either agent alone [38,40,43,44,45,46]. Due to its hydrophilic surface, MPC can inhibit bacterial adhesion and decrease protein adsorption [38,43,49], thus, forming direct contact of the resin surface with the overlaying biofilms and enhancing the contact-killing mechanism of DMAHDM [38,43].
The literature reported DMAHDM + MPC having less biofilms with mostly compromised cell membranes, decreased CFU counts, the least metabolic activity, stronger killing efficacy, least lactic acid production, decreased protein adsorption and maintained a pH above 6.5 [44]. However, Zhang et al. (2017) observed live bacteria on the MPC + DMAHDM composite despite a reduction in lactic acid production from biofilm and total microorganism CFU counts [45]. This further emphasizes the need for more research into the long-term effectiveness of these novel composites on the prevention of secondary caries in orally healthy individuals.
Several articles also reported on the efficacy of novel composites against root caries pathogens [23,38,42,43,46,47]. Composites containing DMAHDM and MPC produced significantly lower levels of lactic acid from S. mutans and polymicrobial biofilms [1,2,20,37,47]. Lactic acid is a known byproduct of caries-causing bacteria, and coupled with their aciduric properties, allows them to survive in low pH conditions [47]. By reducing the amount of lactic acid, root dentine demineralization at the restoration margins may be reduced [47], which can improve the longevity of these restorations.
Combining MPC with DMAHDM was effective in inhibiting extracellular matrix synthesis of root caries pathogens by having substantially reduced polysaccharide production [47]. Polysaccharides are a key component of the extracellular polymeric substances (EPS) that surrounds biofilms and protects pathogens from antibacterial agents. Reducing polysaccharide production reduces this protection and the virulence of these pathogens, potentially reducing caries and inhibiting local periodontitis [50].
AgNPs have been associated with many dental applications such as acrylic resins for dentures [51], endodontic irrigants and intracanal medications [52,53] and other restorative materials [15,54]. The antibacterial properties of AgNPs is hypothesized to be due to the release of silver ions to the bacterial environment where the nanoparticle promotes infiltration into the bacterial cell membranes and affects intracellular processes [41]. Furthermore, the incorporation of AgNPs at 0.12% concentration with MPC and DMAHDM composite was shown to have even greater synergistic mechanisms of action, with further reductions of CFU counts, metabolic activity, protein adsorption and polysaccharide production [38]. AgNPs are particularly effective in inhibiting S. mutans and F. nucleatum, and are also capable of long-distance bacterial killing [38]. Incorporating small particles sizes of AgNPs increases the surface area, thus achieving a strong antimicrobial function with a relatively low filler level of AgNPs without compromising the mechanical properties or aesthetics of the resin [38,41].
Despite the interdisciplinary role AgNP has in modern medicine, recent reviews have discussed the possible environmental and economic impacts of AgNPs that are synthesized either naturally (green synthesis) or chemically [55,56,57,58]. Moreover, development of AgNPs using the biological method of synthesis with the use of bacteria, fungi and plant extracts [55,56] have shown to be ecofriendlier, cost effective and energy efficient [59], with reports of greater antimicrobial activity against pathogenic bacteria [55]. All three studies that experimented with AgNPs in this review synthesized the nanoparticles through chemical reduction. As such, further research is required to examine the effects of incorporating biosynthesized AgNPs on the efficacy of DMAHDM nanocomposite.
While NACP did not exhibit any antibacterial effect and showed comparable results to commercial control composites, NACP was effective on remineralization of tooth structure and pH neutralization [1,2,3,20,37,38,39,42,44,47]. The addition of NACP enables the release of Ca and P ions to increase the pH during cariogenic challenges, thereby, preventing demineralization and facilitating remineralization [38] and thus, reducing the potential for secondary caries development. Recent studies found no significant difference between the NACP and DMAHDM + NACP composites, meaning that DMAHDM did not impact the release of ions and can therefore be incorporated at no disadvantage [1,2,38]. Furthermore, the addition of NACP in the composite resin allows for repeated recharges, facilitates ion rerelease and remineralization over a longer period of time [1]. DMAHDM maintained potent antibiofilm properties after 12 cycles of recharge and did not affect ion re-release concentrations [1,2]. The findings from Bhadila et al. (2020) indicate that the concentration of Ca and P ion releases from NACP and DMAHDM combinations surpassed the required levels for tooth remineralization [60]. These results are very promising for improving the longevity and prognosis of current restorative materials.
Xiao et al. (2017) reported synergistic remineralizing effects such as greater acid neutralization, dentine hardness and mineral growth when combining NACP and third generation PAMAM. PAMAM exhibits the ability to remineralize tooth lesions through its role as an excellent nucleation template whereby Ca and P ions are rapidly absorbed leading to remineralization [23,61,62]. PAMAM has also shown lasting dentine mineral regeneration when incorporated with recharged NACP after prolonged fluid exposure [63], which indicates successful long-term therapeutic effects in reducing demineralization and aiding in the reduction of secondary caries.
Xiao et al. (2017) suggested that the incorporation of MPC, AgNPs, MPC and PAMAM with DMAHDM yielded the maximum antibacterial and remineralization capacity. The addition of MPC and AgNPs with DMAHDM produced significant synergistic antibacterial effects, while NACP and PAMAM provided continuous ion release and combined remineralization mechanisms of action [23]. Therefore, this novel bioactive composite combination shows promising results that may adjunctively reduce the rate of secondary caries and increase the longevity of these restorations.
The findings of this systematic review suggest that incorporation of antibacterial and remineralizing biomaterials have the potential to aid in the prevention of secondary caries. However, caries is a complex multifactorial disease and other factors must be considered when determining the clinical success of composite resins. These include but are not limited to, quality of the restoration such as the presence of microgaps and patient caries risk including oral hygiene habits, salivary flow and composition, consumption of dietary sugars and exposure to fluoride [64].
The limitations of this review include medium risk of bias and in vitro conditions in all studies. Additionally, there were wide variations of comparison groups between the studies. This study only focused on antibacterial and remineralization properties and did not consider mechanical qualities during water-aging. The bacterial incubation period for samples tested for antibacterial efficacy were heterogeneous across the studies, ranging from two days in some studies to 185 days in one study. The differences in incubation protocols may have depended on the manufacturer’s instructions, pH of biofilm culture medium and that different types of bacteria required different incubation times. Therefore, it is important to note that these variations were adjusted accordingly to target specific bacterial species for different studies.
This entry is adapted from the peer-reviewed paper 10.3390/ma14071688
This entry is offline, you can click here to edit this entry!
