
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liang Zhao | + 7353 word(s) | 7353 | 2021-05-19 05:09:30 | | | |
| 2 | Bruce Ren | -21 word(s) | 7332 | 2021-05-26 03:31:41 | | |
Video Upload Options
Alcoholic liver disease (ALD) is one type of liver disease, causing a global healthcare problem and mortality. The liver undergoes tissue damage by chronic alcohol consumption because it is the main site for metabolism of ethanol. Chronic alcohol exposure progresses from alcoholic fatty liver (AFL) to alcoholic steatohepatitis (ASH), which further lead to fibrosis, cirrhosis, and even hepatocellular cancer. Therapeutic interventions to combat ALD are very limited such as use of corticosteroids. However, these therapeutic drugs are not effective for long-term usage. Therefore, additional effective and safe therapies to cope with ALD are urgently needed. Previous studies confirmed that edible food plants and their bioactive compounds exert a protective effect against ALD.
1. ALD: Epidemiology and Risk Factors
1.1. Alcohol Metabolism
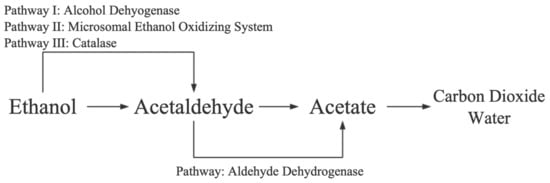
1.2. Mechanisms of Alcoholic Liver Disease
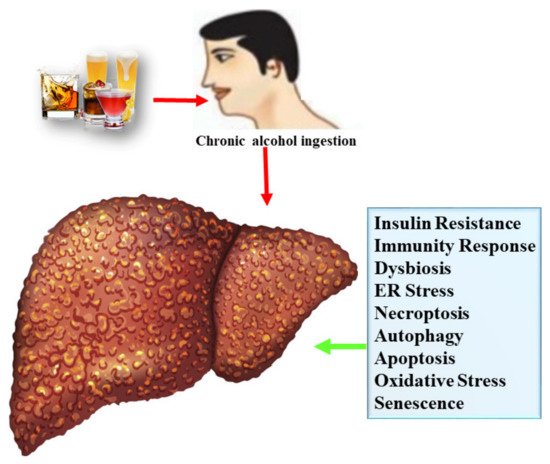
1.2.1. Lipogenesis
1.2.2. Oxidative Stress
1.2.3. Inflammatory Response
1.2.4. Gut Microflora
1.2.5. Endoplasmic Reticulum Stress
1.2.6. Apoptosis
2. Multicomponent Food Plant Extracts against ALD
2.1. Fruits
2.2. Vegetables
2.3. Spices
2.4. Cereals and Grains
2.5. Tea and Coffee
| Edible Food Plant Category | Source | Bioactive Compounds | Study Design | Major Findings | Ref. |
|---|---|---|---|---|---|
| Fruits | Blueberry | ND | Blueberry juice combined with mixed probiotics containing (Bifidobacterium, Lactobacillus bulgaricus, and Streptococcus thermophilus; blueberry juice: 1.5 mL/100 g; 20 mL/100 g probiotics) for 10 days were given to ethanol-induced mice. | Blueberry juice and probiotics increased SOD, GSH, and HDL-C levels, decreased AST, ALT, TG, TC, LDL-C, and MDA, suppressed acetylated FOXO1, FOXO1, FasL, and caspase-3, and increased the SIRT1 in ethanol-exposed mice. | [69] |
| Mango | Mangiferin | Mangiferin (50 and 100 mg/kg bw) was orally given to ethanol-exposed rats for 12 weeks. | Mangiferin effectively regulated metal elements and FFA in serum, modulated specific alcohol-hepatitis-related genes, metabolic pathways, and potential biomarkers in alcoholic hepatitis rats. | [67] | |
| Grape | Quercetin, myricetin, rosmarinic acid, catechin, b-type procyanidin trimer, caffeic acid-O-hexoside, epicatechin | Grape-leaf extract (250–500 mg/kg) was orally given to ethanol-induced rats for 12 days. | Grape leaf extract attenuated liver injury by improving antioxidant activities, suppressed NF-κB p65 and proinflammatory cytokines (TNF-α), and normalized histopathological changes in liver. | [68] | |
| Pomegranate | ND | Pomegranate (600 mg/kg bw) was orally given to ethanol-induced female Fischer wild-type rats for 10 days. | Pomegranate pretreatment markedly reduced alcohol-mediated plasma endotoxin, gut barrier dysfunction, and inflammatory biomarkers and inhibited elevated oxidative and nitrative stress marker proteins. Moreover, pomegranate also restored the levels of intestinal tight junction proteins (claundin-3, ZO-1, occludin, and claudin-1). | [72] | |
| Cranberry | Cyanidin 3-O-galactoside, peonidin 3-O-galactoside and peonidin 3-O-arabinoside, (+)-catechin, (−)-epicatechin and (−)-epicatechin 3-gallate, procyanidin oligomers, myricetin aglycone, quercetin derivatives, benzoic acid, hydroxycinnamic acid derivatives, and hydroxybenzoic acids | Male albino Wistar rats were received cranberry polyphenols daily, 4 mg/kg bw, along with 4 g/kg bw for 8 weeks | Cranberry polyphenols ameliorated alcoholic liver damage and hepatic steatosis, decreased TG, AST, and ALT activities, diminished TNF-α, TGF-β levels, and free radical generation in mitochondria during intoxication. | [73] | |
| Wolfberry | Zeaxanthin dipalmitate | BRL-3A cells were treated with ethanol (250 mM) or Wolfberry-derived zeaxanthin dipalmitate (1 µM). Wolfberry-derived zeaxanthin dipalmitate (10 mg/kg bw) was administered to ethanol-induced rats for 4 weeks. |
Wolfberry-derived zeaxanthin dipalmitate attenuated hepatocyte and whole-liver injury in both ethanol-treated cells and rat model. The underlying mechanism was mainly due to Wolfberry-derived zeaxanthin dipalmitate directly targeted on cell membrane and including receptor P2 × 7 and adipoR1 which further modulate PI3K/AMP-FoXO3 pathways to restore mitochondrial autophagy. Moreover, WZD also alleviates hepatic inflammation by suppressing NLRP3 inflammasome. | [74] | |
| Mango | Mangiferin | Mangiferin (100 and 200 mg/kg bw) was orally given to ethanol-exposed rats for 11 days. | Mangiferin attenuated liver injury induced by chronic plus a single binge ethanol by restoring PDE3B stability, which further activated the AMPK/TBK1 signaling and inhibited NF-κB activation, leading to decreased FFA. | [75] | |
| Lychee | Procyanidin B2, quercetin, 3-O-rutinoside-7-O-a-L-rhamnosidase, isorhamnetin-3-O-rutinoside, (−)-epicatechin, rutin | Lychee pulp (0.4 to 0.8 g/L) was given to mice along with ethanol-containing liquid diet (4%) for 8 weeks. | Lychee pulp ameliorated ALD by decreasing TG, improved the antioxidant status, reduced Nrf2, suppressed lipid synthesis genes, elevated fatty acid β-oxidation expression, and decreased the serum endotoxin level. | [77] | |
| Lychee | Lychee pulp (0.2 and 0.4 g/kg bw) was given to mice along with ethanol-containing liquid diet for 8 weeks. | Lychee pulp supplementation decreased ALT and AST levels, inhibited serum and hepatic oxidative stress, suppressed mitochondrial 8-hydroxy-2’-deoxyguanosine level, and elevated the hepatic ATP level, mitochondrial membrane potential, activities of mitochondrial complexes I and IV, and mitochondrial DNA content. | [78] | ||
| Lychee | Lychee pulp (0.2 and 0.4 g/kg bw) was given to mice along with ethanol-containing liquid diet for 8 weeks. | Lychee pulp phenolic extract alleviated ethanol-induced liver injury in treated mice via reversed alteration of intestinal microbiota composition, downregulated inflammation markers, increased the expression of intestinal tight junction proteins, antimicrobial proteins, and mucus protecting proteins, repressed NF-κB p65, and suppressed CD14 and TLR4 expression. | [79] | ||
| Blueberry | ND | Blueberry polyphenols extract (100 and 200 mg/kg bw) was orally given to ethanol-exposed mice for 30 days. | Blueberry polyphenols decreased the TG lipid droplet content in liver and serum TG and TC levels and decreased lipogenic and increased lipodieretic mRNA levels. Blueberry polyphenols promoted autophagy to accelerate lipid metabolism and thus protect from ALD. | [80] | |
| Mulberry | Water extracts of mulberry (0.3 g/kg bw) were orally administered to chronic ethanol-induced rats. | Water extracts of mulberry decreased TG level and MDA contents, increased glycogen deposits, prevented the disruption of the hepatic cells and nuclei, and decreased Firmicutes to Bacteroidetes ratio. | [82] | ||
| Indian gooseberry | ND | Indian gooseberry was administered (250 mg/kg bw) to alcohol-exposed rats. | Indian gooseberry significantly reduced lipid peroxidation levels and restored antioxidant level. | [84] | |
| Ginseng berry | Ginsenoside F5, ginsenoside Rd, ginsenoside F3, and ginsenoside Re | Ginseng berry extract at the dosage of 0.5–5 mg/mouse along with ethanol was given to mice for 10 days. | Ginseng berry attenuated ALD by improving antioxidant level and reducing inflammatory mediators. | [85] | |
| Apricot | 3-caffeoylshikimic acid, 3-feruloylquinic acid, 3-hydroxy-3-methoxycarbonyl glutaric acid, 1,5-dimethyl citrate, 3,4,5-trimethoxyphenyl-β-D-glucopyranoside, prunate, methyl 3-caffeoylquinate, 3-O- caffeoylquinic acid |
AML-12 cells were treated with ethanol or chlorogenic acid. Apricot extract (100 mg/kg bw) along with alcohol (1 g/kg bw) was orally given to mice for 5 days. |
Chlorogenic acid derived from apricot extract ameliorated ALD in AML-12 cells by inhibiting alcohol-induced apoptosis, MAPK activation, and antioxidant activities. Apricot extract protected ALD by suppressing lipogenesis in liver tissue, inhibiting activation of SREBP-1, and suppressing hepatic apoptosis and inflammation via ROS-mediated p53 signaling pathway in mice with alcohol-induced liver injury. |
[86] | |
| Lemon | ND | Lemon juice (10 mL/kg bw) was orally given to alcohol-induced C57BL/6 mice for 15 days. | Lemon juice markedly inhibited alcohol-induced increase of ALT, AST, lipid peroxidation levels, and hepatic TG, improved antioxidant capacity (SOD and CAT), and improved histopathological changes in ALD mice. | [88] | |
| Citrus depressa | 5-O-demethylnobiletin, sinensetin, tangeretin, and nobiletin | Citrus depressa extract (300 mg/kg) was orally administered to ethanol-induced mice for 8 weeks. | Citrus depressa extract remarkably decreased AST, ALT, TNF-α levels, hepatic MDA, and CYP2E1 expression, and increased glutathione in ALD mice. | [89] | |
| Noni fruit | ND | Noni fruit was orally given to ethanol-exposed mice. | Noni fruit reversed the ethanol-induced changes in mice such as ALT, AST, gamma-glutamyl transferase, LDL-C, HDL-C, TG, and TC. | [90] | |
| Vegetables | Purple potato | Petunidin-3-glucoside, Petunidin-3-rutinoside-5-glucoside, Petunidin-3-caffeoyl-rutinoside-5-glucoside | Purple potato extract was administered at the dosage of 5 and 10 mg/kg bw to ethanol-exposed mice for 5 weeks. | Purple potato extract ameliorated ALD by decreasing ALT, AST, TG, and TC, reducing MDA contents and CYP2E1 protein expression, and increasing GSH and SOD levels in ethanol-exposed mice. | [94] |
| Garlic oil | ND | Human normal cell LO2 was treated with ethanol (100 mM). Garlic oil was administered (50 to 200 mg/kg bw) to ethanol-exposed male Kunming mice. |
Garlic oil decreased n-SREBP-1c and CYP2E1 and increased PPAR-α protein levels in human normal cell L02. Garlic oil decreased n-SREBP-1c and CYP2E1 and increased PPAR-α protein levels in ethanol-induced mice. Additionally, garlic oil decreased FAS and inhibited ethanol-induced hepatic mitochondrial dysfunction. |
[99] | |
| Asparagus officinalis | ND | Asparagus extracts (400 mg/kg bw) were orally administered to male Wistar rats for 70 connective days. | Edible asparagus protected from toxicity mediated by alcohol by improving antioxidant status. | [102] | |
| Okra seed oil | Polyunsaturated fatty acids; ROS: reactive oxygen species; short-chain fatty acids; monounsaturated fatty acids | Okra seed oil (400 and 800 mg/kg bw) was given to mice for 8 weeks. | Okra seed oil attenuated alcohol-induced liver damage via inhibition of liver fat accumulation, decreased MDA content, decreased hepatic pro-inflammatory cytokines (IL-6, TNF-α, and IL-1), increased SOD and GSH levels, and attenuated lipid metabolic disorder. Furthermore, okra seed oil also modulated gut microbiota dysbiosis by enhancing the Bacteroidetes population and reducing the Proteobacteria proportion, Staphylococcus, and Clostridium XlVa. | [103] | |
| Artichoke | ND | Ethanolic extract of artichoke (0.4 to 1.6 g/kg) was given to ethanol-induced ICR mice for 10 days. | Artichoke remarkably attenuated ALD by preventing elevated levels of ALT, AST, TG, and TC, increased SOD and GSH, decreased MDA level, and suppressed inflammatory pathway (TLR4/NF-κB) in ethanol-induced ICR mice. | [104] | |
| Rhubarb | ND | Rhubarb extract (0.3%) was given to C57BL/6J mice for 17 days. | Rhubarb extracts protected alcohol-induced liver injury by modulating intestinal microflora, improving antioxidant level, and reducing inflammatory response. | [105] | |
| Bitter gourd | ND | Bitter gourd was administered (500 mg/kg bw) to C57BL/6 mice fed an alcohol-containing liquid diet for 30 days. | Bitter gourd supplementation reduced the steatotic alternation of liver histopathology, decreased AST, ALT, hepatic TG level, and MDA content, improved antioxidant defense system (SOD, GSH, GRd, GPx, and CAT), reduced pro-inflammatory cytokine levels (IL-6, TNF-α, and IL-1β), and suppressed ACC, CYP2E1, FAS, and SREBP-1 protein expression in alcohol-induced mice. | [106] | |
| Spices | Cinnamon | ND | Cinnamon bark extract (0.5 mL) was administered for 4 days prior to ethanol, and on 5th day, ethanol (6 g/kg bw) was administered. Murine RAW 264.7 macrophage-like cells were treated with cinnamon bark extract (4 µL). |
Cinnamon bark extract protected liver from alcohol via the inhibition of MyD88 expression both in vitro and in vivo. | [107] |
| Fenugreek | ND | Fenugreek seed polyphenol extract (200 mg/kg bw) and ethanol (6 g/kg per day) were fed to rats for 30 days. | Fenugreek seed polyphenol extract inhibited lipid accumulation in ethanol-induced rats. | [108] | |
| Crocus sativus L. | Safranal, crocin, myricetin, and quercetin | Crocus sativus L. (saffron) petal extract was administered (167.5 and 335 mg/kg/day) to ethanol-induced rats for 30 days. | Saffron polyphenolic extract protected liver from ethanol by reducing inflammation in ethanol-administered rats. | [109] | |
| Parsley oil | ND | Parsley oil (50 mg/kg bw) was given to adult male albino rats for 4 weeks. | Parsley oil attenuated alcohol-induced liver injury by oxidative stress mechanism. | [110] | |
| Syzygium aromaticum L. | ND | Polyphenol-rich extract of clove buds (Clovinol) (100 mg/kg bw) was given to ethanol-induced rats for 30 days. | Clovinol decreased alcohol-associated oxidative stress and inflammatory changes in ethanol-induced rats. | [111] | |
| Thymus vulgaris | ND | Thymus vulgaris leaves were orally given (500 mg/kg bw) to ethanol-induced rats for 21 days. | Co-administration (Thymus vulgaris and ethanol) modulated several biomarkers such as ALP, AST, albumin, CAT, MDA, SOD, GST, and lipid profile. | [112] | |
| Peppers | Capsaicin | Capsaicin was given (10 and 20 mg/kg) to ethanol-induced rats. | Capsaicin ameliorated alcohol-induced liver injury by modulating matrix metalloproteinases and suppressing free radical formation and oxidative stress. | [113] | |
| Cereals | Black rice | Cyanidin-3,5-diglucoside, cyanidin-3-glucoside, cyanidin-3-rutinoside, and peonidin-3-glucoside | Alcohol (3.7 g/kg bw) and anthocyanin-rich black rice extract (125, 250, and 500 mg/kg bw) dissolved in water was administered using an intragastric tube for 45 days. | Anthocyanin-rich black rice extract attenuated ALD by decreasing serum AST, ALT, TCH, TG, and GGT levels and improving antioxidant levels. | [116] |
| Rice | Acacetin, caffeic acid, ferulic acid, sinapic acid, p-coumaric acid, quercitrin, vitexin, rutin, hesperidin, ethyl caffeate, and ethyl coumarate | Rice bran phenolic extract (0.25 or 0.50 g/L) was fed along with alcohol-containing liquid diet (4%) to mice for 8 weeks. | Anthocyanin-rich black rice extract supplementation ameliorated ALD by repressing inflammatory responses in liver, intestinal microbiota dysbiosis, and barrier dysfunction and inactivated the endotoxin-TLR4-NF-κB pathway. | [114] | |
| Rice | Acacetin, caffeic acid, ferulic acid, sinapic acid, p-coumaric acid, quercitrin, vitexin, rutin, hesperidin, ethyl caffeate, and ethyl coumarate | Rice bran phenolic extract (0.25 or 0.50 g/L) was fed along with alcohol-containing liquid diet (4%) to mice for 8 weeks. | Rice bran phenolic extract exerted protective effect against ALD in mice fed with an ethanol-containing diet via microRNAs-PGC-1α-TFAM signal pathway. |
[115] | |
| Tartary buckwheat | ND | Acute liver injury model group: buckwheat ethanol extracts (8.35, 16.70 and 41.75 mL/kg bw) and ethanol (4 g/kg bw) were intragastrically administered to rats for 7 consecutive days. Chronic alcoholic liver injury: buckwheat ethanol extracts (8.35, 16.70 and 41.75 mL/kg bw) and ethanol (3 g/kg/day bw; 37.5% volume fraction) intragastrically administered to SD rats for 8–9 consecutive weeks. |
Tartary buckwheat extract administration significantly decreased serum ALT, AST, and hepatic MDA and improved hepatic GSH level. | [117] | |
| Mung bean extract | Vitexin and isovitexin | Mung bean extract (containing 15 mg vitexin and 13 mg isovitexin, respectively, per kg bw) was given along with spirit (56% alcohol, 16 mL/kg bw) 2 h after the doses of mung bean extract for 14 days. | Mung bean extract decreased ALT and AST and improved antioxidant levels. | [122] | |
| Tea | Pu-erh tea | Gallocatechin, gallic acid, and caffeine | Pu-erh tea extract (1 or 4 g/L w/v added into drinking water) and ethanol solution (10% w/v) were administered by gavage for 30 days. | Pu-erh tea extract contributed to the protective effect against ALD by improving oxidative stress, reducing lipid accumulation, reducing inflammation, and modulating microbiomic and metabolomic responses. | [129] |
References
- Bloomfield, K.; Stockwell, T.; Gmel, G.; Rehn, N. International Comparisons of Alcohol Consumption. Alcohol Res. Health 2003, 27, 95–109.
- Faiad, Y.; Khoury, B.; Daouk, S.; Maj, M.; Keeley, J.; Gureje, O.; Reed, G. Frequency of use of the international classification of diseases ICD-10 diagnostic categories for mental and behavioural disorders across world regions. Epidemiol. Psychiatr. Sci. 2018, 27, 568–576.
- Probst, C.; Manthey, J.; Merey, A.; Rylett, M.; Rehm, J. Unrecorded alcohol use: A global modelling study based on nominal group assessments and survey data. Addiction 2018, 113, 1231–1241.
- Room, R. The relation between blood alcohol content and clinically assessed intoxication: Lessons from applying the ICD-10 Y90 and Y91 codes in the emergency room. In Alcohol and Injuries: Emergency Department Studies in an International Perspective; Cherpitel, C.J., Borges, G., Hungerford, D., Peden, M., Poznyak, V., Room, R., Stockwell, T., Eds.; WHO: Geneva, Switzerland, 2009; pp. 135–146.
- World Health Organization. Global Status Report on Alcohol and Health 2014. Available online: (accessed on 6 April 2021).
- Peacock, A.; Leung, J.; Larney, S.; Colledge, S.; Hickman, M.; Rehm, J.; Giovino, G.A.; West, R.; Hall, W.; Griffiths, P.; et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018, 113, 1905–1926.
- Kim, W.R.; Brown, R.S.; Terrault, N.A.; El-Serag, H. Burden of liver disease in the United States: Summary of a workshop. Hepatology 2002, 36, 227–242.
- Nephew, T.M.; Williams, G.D.; Yi, H.; Hoy, A.K.; Stinson, F.S.; Dufour, M.C. Surveillance Report #59: Apparent Per Capita Alcohol Consumption: National, State, and Regional Trends, 1977–2000; NIAAA, Division of Biometry and Epidemiology, Alcohol Epidemiologic Data System: Rockville, MD, USA, 2003.
- Jiang, H.; Xiang, X.; Hao, W.; Room, R.; Zhang, X.; Wang, X. Measuring and preventing alcohol use and related harm among young people in Asian countries: A thematic review. Glob. Health Res. Policy 2018, 3, 14.
- Kourkoumpetis, T.; Sood, G. Pathogenesis of Alcoholic Liver Disease: An Update. Clin. Liver Dis. 2019, 23, 71–80.
- Edenberg, H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 2007, 30, 5–13.
- Purohit, V.; Gao, B.; Song, B.J. Molecular mechanisms of alcoholic fatty liver. Alcohol. Clin. Exp. Res. 2009, 33, 191–205.
- Craemer, D.D.; Pauwels, M.; Branden, C.V. Morphometric characteristics of human hepatocellular peroxisomes in alcoholic liver disease. Alcohol. Clin. Exp. Res. 1996, 20, 908–913.
- You, M.; Matsumoto, M.; Pacold, C.M.; Cho, W.K.; Crabb, D.W. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 2004, 127, 1798–1808.
- Israel, Y.; Videla, L.; Fernandez Videla, V.; Bernstein, J. Effects of chronic ethanol treatment and thyroxine administration on ethanol metabolism and liver oxidative capacity. J. Pharmacol. Exp. Ther. 1975, 192, 565–574.
- Amet, Y.; Lucas, D.; Zhang-Gouillon, Z.Q.; French, S.W. P-450-dependent metabolism of lauric acid in alcoholic liver disease: Comparison between rat liver and kidney microsomes. Alcohol. Clin. Exp. Res. 1998, 22, 455–462.
- Watkins, P.B. Role of cytochromes P450 in drug metabolism and hepatotoxicity. Semin. Liver Dis. 1990, 10, 235–250.
- McKillop, I.H.; Schrum, L.W.; Thompson, K.J. Role of alcohol in the development and progression of hepatocellular carcinoma. Hepatic Oncol. 2016, 3, 29–43.
- Teschke, R. Alcoholic steatohepatitis (ASH) and alcoholic hepatitis (AH): Cascade of events, clinical aspects, and pharmacotherapy options. Expert Opin. Pharmacother. 2018, 19, 779–793.
- Baraona, E.; Lieber, C.S. Effects of ethanol on lipid metabolism. J. Lipid Res. 1979, 20, 289–315.
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347.
- Galli, A.; Pinaire, J.; Fischer, M.; Dorris, R.; Crabb, D.W. The transcriptional and DNA binding activity of peroxisome proliferator-activated receptor α is inhibited by ethanol metabolism. A novel mechanism for the development of ethanol-induced fatty liver. J. Biol. Chem. 2001, 276, 68–75.
- Parker, R.; Kim, S.J.; Gao, B. Alcohol, adipose tissue and liver disease: Mechanistic links and clinical considerations. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 50–59.
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585.
- Lackner, C.; Tiniakos, D. Fibrosis and alcohol-related liver disease. J. Hepatol. 2019, 70, 294–304.
- Sancho-Bru, P.; Altamirano, J.; Rodrigo-Torres, D.; Coll, M.; Millán, C.; José Lozano, J.; Miquel, R.; Arroyo, V.; Caballería, J.; Ginès, P.; et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 2012, 55, 1931–1941.
- IARC (WHO). A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 377–503.
- Testino, G. Alcoholic hepatitis. J. Med. Life 2013, 6, 161–167.
- Gudowska, M.; Wojtowicz, E.; Cylwik, B.; Gruszewska, E.; Chrostek, L. The distribution of liver steatosis, fibrosis, steatohepatitis and inflammation activity in alcoholics according to FibroMax test. Adv. Clin. Exp. Med. 2015, 24, 823–827.
- Osna, N.A.; Donohue, T.M.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 2017, 38, 147–161.
- Fischer, M.; You, M.; Matsumoto, M.; Crabb, D.W. Peroxisome proliferator activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J. Biol. Chem. 2003, 278, 27997–28004.
- Albano, E.; Clot, P.; Morimoto, M.; Tomasi, A.; Ingelman-Sundberg, M.; French, S.W. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology 1996, 23, 155–163.
- Mueller, S.; Peccerella, T.; Qin, H.; Glassen, K.; Waldherr, R.; Flechtenmacher, C.; Straub, B.K.; Millonig, G.; Stickel, F.; Bruckner, T.; et al. Carcinogenic Etheno DNA Adducts in Alcoholic Liver Disease: Correlation with Cytochrome P-4502E1 and Fibrosis. Alcohol. Clin. Exp. Res. 2018, 42, 252–259.
- Linhart, K.; Bartsch, H.; Seitz, H.K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox Biol. 2014, 3, 56–62.
- Leung, T.M.; Nieto, N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 395–398.
- Bailey, S.M.; Cunningham, C.C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 2002, 32, 11–16.
- García-Ruiz, C.; Colell, A.; París, R.; Fernández-Checa, J.C. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J. 2000, 14, 847–858.
- Chamulitrat, W.; Spitzer, J.J. Nitric oxide and liver injury in alcohol-fed rats after lipopolysaccharide administration. Alcohol. Clin. Exp. Res. 1996, 20, 1065–1070.
- Lieber, C.S.; Cao, Q.; Decarli, L.M.; Leo, M.A.; Mak, K.M.; Ponomarenko, A.; Ren, C.; Wang, X. Role of medium-chain triglycerides in the alcohol-mediated cytochrome P450 2E1 induction of mitochondria. Alcohol. Clin. Exp. Res. 2007, 31, 1660–1668.
- Harrison-Findik, D.D.; Schafer, D.; Klein, E.; Timchenko, N.A.; Kulaksiz, H.; Clemens, D.; Fein, E.; Andriopoulos, B.; Pantopoulos, K.; Gollan, J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 2006, 281, 22974–22982.
- Wang, H.J.; Gao, B.; Zakhari, S.; Nagy, L.E. Inflammation in alcoholic liver disease. Annu. Rev. Nutr. 2012, 32, 343–368.
- Pritchard, M.T.; Mcmullen, M.R.; Stavitsky, A.B.; Cohen, J.I.; Lin, F.; Medof, M.E.; Nagy, L.E. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology 2007, 132, 1117–1126.
- Maltby, J.; Wright, S.; Bird, G.; Sheron, N. Chemokine levels in human liver homogenates: Associations between GRO α and histopathological evidence of alcoholic hepatitis. Hepatology 1996, 24, 1156–1160.
- Petrasek, J.; Mandrekar, P.; Szabo, G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroent. Res. Pract. 2010, 2010, 710381.
- Lawrence, T.; Bebien, M.; Liu, G.Y.; Nizet, V.; Karin, M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 2005, 434, 1138–1143.
- Park, C.M.; Youn, H.J.; Chang, H.K.; Song, Y.S. TOP1 and 2, polysaccharides from Taraxacum officinale, attenuate CCl4-induced hepatic damage through the modulation of NF-κB and its regulatory mediators. Food Chem. Toxicol. 2010, 48, 1255–1261.
- Bukong, T.N.; Iracheta-Vellve, A.; Gyongyosi, B.; Ambade, A.; Catalano, D.; Kodys, K.; Szabo, G. Therapeutic Benefits of Spleen Tyrosine Kinase Inhibitor Administration on Binge Drinking-Induced Alcoholic Liver Injury, Steatosis, and Inflammation in Mice. Alcohol. Clin. Exp. Res. 2016, 40, 1524–1530.
- Lee, J.E.; Ha, J.S.; Park, H.Y.; Lee, E. Alteration of gut microbiota composition by short-term low-dose alcohol intake is restored by fermented rice liquor in mice. Food Res. Int. 2020, 128, 108800.
- Meroni, M.; Longo, M.; Dongiovanni, P. Alcohol or Gut Microbiota: Who Is the Guilty? Int. J. Mol. Sci. 2019, 20, 4568.
- Bjorkhaug, S.T.; Aanes, H.; Neupane, S.P.; Bramness, J.G.; Malvik, S.; Henriksen, C.; Skar, V.; Medhus, A.W.; Valeur, J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes 2019, 10, 663–675.
- Guerra Ruiz, A.; Casafont, F.; Crespo, J.; Cayón, A.; Mayorga, M.; Estebanez, A.; Fernadez-Escalante, J.C.; Pons-Romero, F. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: Evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes. Surg. 2007, 17, 1374–1380.
- Yang, S.Q.; Lin, H.Z.; Lane, M.D.; Clemens, M.; Diehl, A.M. Obesity increases sensitivity to endotoxin liver injury: Implications for the pathogenesis of steatohepatitis. Proc. Natl. Acad. Sci. USA 1997, 94, 2557–2562.
- Staun-Olsen, P.; Bjorneboe, M.; Prytz, H.; Thomsen, A.C.; Orskov, F. Escherichia coli antibodies in alcoholic liver disease. Correlation to alcohol consumption, alcoholic hepatitis, and serum IgA. Scand. J. Gastroenterol. 1983, 18, 889–896.
- Bode, C.; Kugler, V.; Bode, J.C. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 1987, 4, 8–14.
- Rao, R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009, 50, 638–644.
- Kosnicki, K.L.; Penprase, J.C.; Cintora, P.; Torres, P.J.; Harris, G.L.; Brasser, S.M.; Kelley, S.T. Effects of moderate, voluntary ethanol consumption on the rat and human gut microbiome. Addict. Biol. 2019, 24, 617–630.
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809.
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242.
- Ji, C. Dissection of endoplasmic reticulum stress signaling in alcoholic and non-alcoholic liver injury. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. 1), S16–S24.
- Kaplowitz, N.; Ji, C. Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum. J. Gastroenterol. Hepatol. 2006, 21 (Suppl. 3), S7–S9.
- Ji, C.; Chan, C.; Kaplowitz, N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J. Hepatol. 2006, 45, 717–724.
- Ji, C.; Kaplowitz, N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003, 124, 1488–1499.
- Decker, R.H.; Dai, Y.; Grant, S. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in human leukemia cells (U937) through the mitochondrial rather than the receptor-mediated pathway. Cell Death Differ. 2001, 8, 715–724.
- Hao, F.; Cubero, F.J.; Ramadori, P.; Liao, L.; Haas, U.; Lambertz, D.; Sonntag, R.; Bangen, J.M.; Gassler, N.; Hoss, M.; et al. Inhibition of Caspase-8 does not protect from alcohol-induced liver apoptosis but alleviates alcoholic hepatic steatosis in mice. Cell Death Dis. 2017, 8, e3152.
- Castilla, R.; González, R.; Fouad, D.; Fraga, E.; Muntané, J. Dual effect of ethanol on death in primary culture of human and rat hepatocytes. Alcohol Alcohol. 2004, 39, 290–296.
- Thurman, R.G.; Bradford, B.U.; Iimuro, Y.; Frankenberg, M.V.; Knecht, K.T.; Connor, H.D.; Adachi, Y.; Wall, C.; Arteel, G.E.; Raleigh, J.A.; et al. Mechanisms of alcohol-induced hepatotoxicity: Studies in rats. Front. Biosci. 1999, 4, e42–e46.
- Li, M.; Wu, C.; Guo, H.; Chu, C.; Hu, M.; Zhou, C. Mangiferin improves hepatic damage-associated molecular patterns, lipid metabolic disorder and mitochondrial dysfunction in alcohol hepatitis rats. Food Funct. 2019, 10, 3514–3534.
- Amen, Y.; Sherif, A.E.; Shawky, N.M.; Abdelrahman, R.S.; Wink, M.; Sobeh, M. Grape-Leaf Extract Attenuates Alcohol-Induced Liver Injury via Interference with NF-κB Signaling Pathway. Biomolecules 2020, 10, 558.
- Zhu, J.; Ren, T.; Zhou, M.; Cheng, M. The combination of blueberry juice and probiotics reduces apoptosis of alcoholic fatty liver of mice by affecting SIRT1 pathway. Drug Des. Dev. Ther. 2016, 10, 1649–1661.
- Liang, H.W.; Yang, T.Y.; Teng, C.S.; Lee, Y.J.; Yu, M.H.; Lee, H.J.; Hsu, L.S.; Wang, C.J. Mulberry leaves extract ameliorates alcohol-induced liver damages through reduction of acetaldehyde toxicity and inhibition of apoptosis caused by oxidative stress signals. Int. J. Med. Sci. 2021, 18, 53–64.
- Nam, K.S.; Kim, J.; Noh, S.K.; Park, J.H.; Sung, E.G. Effect of Sweet Persimmon Wine on Alcoholic Fatty Livers in Rats. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1548–1555.
- Cho, Y.E.; Song, B.J. Pomegranate prevents binge alcohol-induced gut leakiness and hepatic inflammation by suppressing oxidative and nitrative stress. Redox Biol. 2018, 18, 266–278.
- Zavodnik, I.; Buko, V.; Lukivskaya, O.; Lapshina, E.; Ilyich, T.; Belonovskaya, E.; Kirko, S.; Naruta, E.; Kuzmitskaya, I.; Budryn, G.; et al. Cranberry (Vaccinium macrocarpon) peel polyphenol-rich extract attenuates rat liver mitochondria impairments in alcoholic steatohepatitis in vivo and after oxidative treatment in vitro. J. Funct. Foods 2019, 57, 83–94.
- Gao, H.; Lv, Y.; Liu, Y.; Li, J.; Wang, X.; Zhou, Z.; Tipoe, G.L.; Ouyang, S.; Guo, Y.; Zhang, J.; et al. Wolfberry-Derived Zeaxanthin Dipalmitate Attenuates Ethanol-Induced Hepatic Damage. Mol. Nutr. Food Res. 2019, 63, e1801339.
- Dong, M.; Li, L.; Li, G.; Song, J.; Liu, B.; Liu, X.; Wang, M. Mangiferin protects against alcoholic liver injury: Via suppression of inflammation-induced adipose hyperlipolysis. Food Funct. 2020, 11, 8837–8851.
- Pari, L.; Suresh, A. Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem. Toxicol. 2008, 46, 1627–1634.
- Xiao, J.; Zhang, R.; Huang, F.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, Y.; Liu, D.; Zhang, M. The biphasic dose effect of lychee (Litchi chinensis Sonn.) pulp phenolic extract on alcoholic liver disease in mice. Food Funct. 2017, 8, 189–200.
- Xiao, J.; Zhang, R.; Huang, F.; Liu, L.; Deng, Y.; Ma, Y.; Tang, X.; Zhang, Y.; Zhang, M. Lychee (Litchi chinensis Sonn.) pulp phenolic extract confers a protective activity against alcoholic liver disease in mice by alleviating mitochondrial dysfunction. J. Agric. Food Chem. 2017, 65, 5000–5009.
- Xiao, J.; Zhang, R.; Zhou, Q.; Liu, L.; Huang, F.; Deng, Y.; Ma, Y.; Wei, Z.; Tang, X.; Zhang, M. Lychee (Litchi chinensis Sonn.) pulp phenolic extract provides protection against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, intestinal barrier dysfunction, and liver inflammation. J. Agric. Food Chem. 2017, 65, 9675–9684.
- Zhuge, Q.; Zhang, Y.; Liu, B.; Wu, M. Blueberry polyphenols play a preventive effect on alcoholic fatty liver disease C57BL/6 J mice by promoting autophagy to accelerate lipolysis to eliminate excessive TG accumulation in hepatocytes. Ann. Cardiothorac. Surg. 2020, 9, 1045–1054.
- Adedosu, O.T.; Oyedeji, A.T.; Iwaku, T.; Ehigie, A.F.; Olorunsogo, O.O. Hepatoprotective activity and inhibitory effect of flavonoid-rich extract of Brysocarpus Coccineus leaves on mitochondrial membrane permeability transition pore. Asian J. Nat. Appl. Sci. 2014, 3, 92–100.
- Park, S.; Kim, D.S.; Wu, X.; Yi, Q.J. Mulberry and dandelion water extracts prevent alcohol-induced steatosis with alleviating gut microbiome dysbiosis. Exp. Biol. Med. 2018, 243, 882–894.
- Szachowicz-Petelska, B.; Dobrzyńska, I.; Skrzydlewska, E.; Figaszewski, Z. Protective effect of blackcurrant on liver cell membrane of rats intoxicated with ethanol. J. Membr. Biol. 2012, 245, 191–200.
- Damodara Reddy, V.; Padmavathi, P.; Gopi, S.; Paramahamsa, M.; Varadacharyulu, N.C. Protective effect of Emblica officinalis against alcohol-induced hepatic injury by ameliorating oxidative stress in rats. Indian J. Clin. Biochem. 2010, 25, 419–424.
- Lee, D.Y.; Kim, M.J.; Yoon, D.; Lee, Y.S.; Kim, G.S.; Choon Yoo, Y. Ginseng berry prevents alcohol-induced liver damage by improving the anti-inflammatory system damage in mice and quality control of active compounds. Int. J. Mol. Sci. Artic. 2019, 20, 3522.
- Pan, J.H.; Lee, K.Y.; Kim, J.H.; Shin, H.; Lee, J.H.; Kim, Y.J. Prunus mume Sieb. et Zucc. fruit ameliorates alcoholic liver injury in mice by inhibiting apoptosis and inflammation through oxidative stress. J. Funct. Foods 2016, 25, 135–148.
- Abozid, M.M.; Farid, H.E. The anti-fatty liver effects of guava leaves and pomegranate peel extracts on ethanol-exposed rats. J. Biol. Chem. Environ. Sci. 2013, 8, 83–104.
- Zhou, T.; Zhang, Y.J.; Xu, D.-P.; Wang, F.; Zhou, Y.; Zheng, J.; Li, Y.; Zhang, J.J.; Li, H.B. Protective effects of lemon juice on alcohol-induced liver injury in mice. BioMed Res. Int. 2017, 2017, 1–8.
- Lee, E.Y.; Kim, S.H.; Chang, S.N.; Lee, J.H.; Hwang, B.S.; Woo, J.T.; Kang, S.C.; Lee, J.; Park, J.G. Efficacy of polymethoxylated flavonoids from citrus depressa extract on alcohol-induced liver injury in mice. Biotechnol. Bioprocess Eng. 2019, 24, 907–914.
- Guo, M.; Mao, B.; Ahmed Sadiq, F.; Hao, Y.; Cui, S.; Yi, M.; Hong, Q.; Lee, Y.K.; Zhao, J. Effects of noni fruit and fermented noni juice against acute alcohol induced liver injury in mice. J. Funct. Foods 2020, 70, 103995.
- Wiese, J.; McPherson, S.; Odden, M.C.; Shlipak, M.G. Effect of Opuntia ficus indica on Symptoms of the Alcohol Hangover. Arch. Intern. Med. 2004, 164, 1334–1340.
- Cheng, N.; Du, B.; Wang, Y.; Gao, H.; Cao, W.; Zheng, J.; Feng, F. Antioxidant properties of jujube honey and its protective effects against chronic alcohol-induced liver damage in mice. Food Funct. 2014, 5, 900–908.
- Sun, H.; Mu, T.; Liu, X.; Zhang, M.; Chen, J. Purple Sweet Potato (Ipomoea batatas L.) Anthocyanins: Preventive Effect on Acute and Subacute Alcoholic Liver Damage and Dealcoholic Effect. J. Agric. Food Chem. 2014, 62, 2364–2373.
- Jiang, Z.; Chen, C.; Wang, J.; Xie, W.; Wang, M.; Li, X.; Zhang, X. Purple potato (Solanum tuberosum L.) anthocyanins attenuate alcohol-induced hepatic injury by enhancing antioxidant defense. J. Nat. Med. 2016, 70, 45–53.
- Kim, J.; Seo, Y.; Park, J.H.; Noh, S.K. Protective Effect of Onion Wine on Alcoholic Fatty Liver in Rats. J. Korean Soc. Food Sci. Nutr. 2016, 45, 467–473.
- Guan, M.J.; Zhao, N.; Xie, K.Q.; Zeng, T. Hepatoprotective effects of garlic against ethanol-induced liver injury: A mini-review. Food Chem. Toxicol. 2018, 111, 467–473.
- Zeng, T.; Guo, F.F.; Zhang, C.L.; Zhao, S.; Dou, D.D.; Gao, X.C.; Xie, K.Q. The anti-fatty liver effects of garlic oil on acute ethanol-exposed mice. Chem. Biol. Interact. 2008, 176, 234–242.
- Zeng, T.; Zhang, C.L.; Pan, G.B.; Zhao, S.; Dou, D.D.; Xin, X.; Xie, K.Q. The protective effects of garlic oil on acute ethanol-induced oxidative stress in the liver of mice. J. Sci. Food Agric. 2008, 88, 2238–2243.
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Xie, K.Q. Garlic oil alleviated ethanol-induced fat accumulation via modulation of SREBP-1, PPAR-α, and CYP2E1. Food Chem. Toxicol. 2012, 50, 485–491.
- Zeng, T.; Zhang, C.L.; Song, F.Y.; Zhao, X.L.; Yu, L.H.; Zhu, Z.P.; Xie, K.Q. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4848–4859.
- Zeng, T.; Zhang, C.L.; Zhu, Z.P.; Yu, L.H.; Zhao, X.L.; Xie, K.Q. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxicology 2008, 252, 86–91.
- Kim, B.Y.; Cui, Z.G.; Lee, S.R.; Kim, S.J.; Kang, H.K.; Lee, Y.K.; Park, D.B. Effects of Asparagus officinalis Extracts on Liver Cell Toxicity and Ethanol Metabolism. J. Food Sci. 2009, 74, H204–H208.
- Zhang, J.; Lu, Y.; Yang, X.; Zhao, Y. Supplementation of okra seed oil ameliorates ethanol-induced liver injury and modulates gut microbiota dysbiosis in mice. Food Funct. 2019, 10, 6385–6398.
- Tang, X.; Wei, R.; Deng, A.; Lei, T. Protective Effects of Ethanolic Extracts from Artichoke, an Edible Herbal Medicine, against Acute Alcohol-Induced Liver Injury in Mice. Nutrients 2017, 9, 1000.
- Neyrinck, A.M.; Etxeberria, U.; Taminiau, B.; Daube, G.; Van Hul, M.; Everard, A.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol. Nutr. Food Res. 2017, 61, 1500899.
- Lu, K.H.; Tseng, H.C.; Liu, C.T.; Huang, C.J.; Chyuan, J.H.; Sheen, L.Y. Wild bitter gourd protects against alcoholic fatty liver in mice by attenuating oxidative stress and inflammatory responses. Food Funct. 2014, 5, 1027–1037.
- Kanuri, G.; Weber, S.; Volynets, V.; Spruss, A.; Bischoff, S.C.; Bergheim, I. Cinnamon extract protects against acute alcohol-induced liver steatosis in mice. J. Nutr. 2009, 139, 482–487.
- Kaviarasan, S.; Viswanathan, P.; Anuradha, C. Fenugreek seed (Trigonella foenum graecum) polyphenols inhibit ethanol-induced collagen and lipid accumulation in rat liver. Cell Biol. Toxicol. 2007, 23, 373–383.
- Azizi, M.; Abbasi, N.; Mohamadpour, M.; Bakhtiyari, S.; Asadi, S.; Shirzadpour, E.; Aidy, A.; Mohamadpour, M.; Amraei, M. Investigating the effect of Crocus sativus L. petal hydroalcoholic extract on inflammatory and enzymatic indices resulting from alcohol use in kidney and liver of male rats. J. Inflamm. Res. 2019, 12, 269–283.
- Abou Seif, H.S. Ameliorative effect of parsley oil (Petroselinum crispum) against alcohol-induced hepatotoxicity and oxidative stress. Med. Res. J. 2014, 13, 100–107.
- Jose, S.P.; Ratheesh, M.; Asha, S.; Krishnakumar, I.M.; Sandya, S.; Kumar, G. Hepato-protective Effect of Clove Bud Polyphenols (Syzygium aromaticum L.) (Clovinol®) by Modulating Alcohol Induced Oxidative Stress and Inflammation. J. Food Res. 2018, 7, 10–20.
- El-Newary, S.A.; Shaffie, N.M.; Omer, E.A. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac. J. Trop. Med. 2017, 10, 361–371.
- Koneru, M.; Dhar Sahu, B.; Mir, S.M.; Ravuri, H.G.; Kuncha, M.; Kumar, J.M.; Kilari, K.; Sistla, R. Capsaicin, the pungent principle of peppers, ameliorates alcohol-induced acute liver injury in mice via modulation of matrix metalloproteinases. Can. J. Physiol. Pharmacol. 2018, 96, 419–427.
- Xiao, J.; Zhang, R.; Wu, Y.; Wu, C.; Jia, X.; Dong, L.; Liu, L.; Chen, Y.; Bai, Y.; Zhang, M. Rice Bran Phenolic Extract Protects against Alcoholic Liver Injury in Mice by Alleviating Intestinal Microbiota Dysbiosis, Barrier Dysfunction, and Liver Inflammation Mediated by the Endotoxin-TLR4-NF-κB Pathway. J. Agric. Food Chem. 2020, 68, 1237–1247.
- Xiao, J.; Wu, C.; He, Y.; Guo, M.; Peng, Z.; Liu, Y.; Liu, L.; Dong, L.; Guo, Z.; Zhang, R.; et al. Rice Bran Phenolic Extract Confers Protective Effects against Alcoholic Liver Disease in Mice by Alleviating Mitochondrial Dysfunction via the PGC-1α-TFAM Pathway Mediated by microRNA-494-3p. J. Agric. Food Chem. 2020, 68, 12284–12294.
- Hou, Z.; Qin, P.; Ren, G. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. Japonica) on chronically alcohol-induced liver damage in rats. J. Agric. Food Chem. 2010, 58, 3191–3196.
- Yang, Q.; Luo, C.; Zhang, X.; Liu, Y.; Wang, Z.; Cacciamani, P.; Shi, J.; Cui, Y.; Wang, C.; Sinha, B.; et al. Tartary buckwheat extract alleviates alcohol-induced acute and chronic liver injuries through the inhibition of oxidative stress and mitochondrial cell death pathway. Am. J. Transl. Res. 2020, 12, 70–89.
- Li, H.M.; Guo, P.; Hu, X.; Xu, L.; Zhang, X.Z. Preparation of corn (Zea mays) peptides and their protective effect against alcohol-induced acute hepatic injury in NH mice. Biotechnol. Appl. Biochem. 2007, 47, 169–174.
- Wu, Y.; Pan, X.; Zhang, S.; Wang, W.; Cai, M.; Li, Y.; Yang, F.; Guo, H. Protective effect of corn peptides against alcoholic liver injury in men with chronic alcohol consumption: A randomized double-blind placebo-controlled study. Lipids Health Dis. 2014, 13, 192.
- Lee, Y.H.; Kim, J.H.; Kim, S.H.; Oh, J.Y.; Seo, W.D.; Kim, K.M.; Jung, J.C.; Jung, Y.S. Barley sprouts extract attenuates alcoholic fatty liver injury in mice by reducing inflammatory response. Nutrients 2016, 8, 440.
- Li, Y.; Sun, Y.; Zang, Y.; Su, Y.; Zhou, H.; Wang, J.; Xie, M.; Chen, G.; Liu, L.; Mei, Q. GanMeijian ameliorates lipid accumulation and oxidative damage in alcoholic fatty liver disease in Wistar rats. Life Sci. 2020, 255, 117721.
- Liu, T.; Yu, X.H.; Gao, E.Z.; Liu, X.N.; Sun, L.J.; Li, H.L.; Wang, P.; Zhao, Y.L.; Yu, Z.G. Hepatoprotective Effect of Active Constituents Isolated from Mung Beans (Phaseolus radiatus L.) in an Alcohol-Induced Liver Injury Mouse Model. J. Food Biochem. 2014, 38, 453–459.
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123.
- Arteel, G.E.; Uesugi, T.; Bevan, L.N.; Gäbele, E.; Wheeler, M.D.; McKim, S.E.; Thurman, R.G. Green tea extract protects against early alcohol-induced liver injury in rats. Biol. Chem. 2002, 383, 663–670.
- Chen, K.H.; Li, P.C.; Lin, W.H.; Chien, C.T.; Low, B.H. Depression by a green tea extract of alcohol-induced oxidative stress and lipogenesis in rat liver. Biosci. Biotechnol. Biochem. 2011, 75, 1668–1676.
- Lodhi, P.; Tandan, N.; Singh, N.; Kumar, D.; Kumar, M. Camellia sinensis (L.) kuntze extract ameliorates chronic ethanol-induced hepatotoxicity in albino rats. Evidence-based Complement. Evid. Based Complement. Altern. Med. 2014, 2014, 787153.
- Park, J.H.; Kim, Y.; Kim, S.H. Green tea extract (Camellia sinensis) fermented by lactobacillus fermentum attenuates alcohol-induced liver damage. Biosci. Biotechnol. Biochem. 2012, 76, 2294–2300.
- Wang, R.; Xiao, R.; Yang, L.; Liu, W.; Shi, H.; Hou, Y. The Effects of Fermented Pu-erh Tea on the Dynamic Pathological Changes of the Alcoholic Liver Injury Rats. J. Yunnan Agric. Univ. 2015, 30, 408–412.
- Liu, Y.; Luo, Y.; Wang, X.; Luo, L.; Sun, K.; Zeng, L. Gut Microbiome and Metabolome Response of Pu-erh Tea on Metabolism Disorder Induced by Chronic Alcohol Consumption. J. Agric. Food Chem. 2020, 68, 6615–6627.
- McKim, S.E.; Konno, A.; Gäbele, E.; Uesugi, T.; Froh, M.; Sies, H.; Thurman, R.G.; Arteel, G.E. Cocoa extract protects against early alcohol-induced liver injury in the rat. Arch. Biochem. Biophys. 2002, 406, 40–46.
- Hu, C.M.; Cao, Q.; Lv, X.W.; Cheng, W.M.; Li, R.; Li, J. Protective effects of total flavonoids from Litsea coreana on alcoholic fatty liver in rats associated with down-regulation adipose differentiation-related protein expression. Am. J. Chin. Med. 2012, 40, 599–610.
- Zhang, X.; Wu, Z.; Weng, P. Antioxidant and hepatoprotective effect of (-)-epigallocatechin 3- O -(3- O -Methyl) gallate (EGCG3Me) from chinese oolong tea. J. Agric. Food Chem. 2014, 62, 10046–10054.




