
Video Upload Options
Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) is a highly important and attractive conducting polymer as well as commercially available in organic electronics, including electrochemical and electronic chemosensors, due to its unique features such as excellent solution-fabrication capability and miscibility, high and controllable conductivity, excellent chemical and electrochemical stability, good optical transparency and biocompatibility.
1. Introduction
It remains imperative to develop chemosensor techniques for environmental, health and safety monitoring [1][2]. With the technical advancement in instrumentation, microelectronics and computers, it becomes more feasible nowadays to design and construct chemosensors utilizing most of the known chemical, physical and biological properties or features of sensor materials. Chemosensors may be classified into different types according to the sensor operating principle. Typical types of chemosensors include optical [3] (based on colorimetric or fluorescence change), electrical [4] (based on measurement of resistance, capacitance, impedance or other electrical signal), IR spectrometry, mass spectrometry, chromatography, surface acoustic wave, and others. Among all these types of sensors, chemosensors based on electrical signal modulation are much more straightforward and facile for device design, signal transduction and system integration, which combined can be made to be portable and small in size, thus suited for real-time onsite operation. Electrochemical [5] and electronic signals [6] are mostly used in electrical mode chemosensors. Upon exposure to the chemical analytes, an electrochemical sensor can transform the amperometric, potentiometric, or voltammetric effect of the analyte–electrode interactions (mostly in liquid solutions) into a measurable signal that in turn can identify and quantize the presence of different analytes [4]. In comparison, an electronic chemosensor usually outputs a signal directly arising from the change of electrical properties (resistive, conductivity, etc.) of the sensor material caused by the surface charge-transfer interaction with chemical analytes (mostly in the gas phase).
The overall performance of chemosensors is determined by several factors such as chemical and physical properties of sensing material, device geometry, and signal transduction. Improvement of the sensor performance demands a synergistic optimization of the above-mentioned factors. In terms of sensing materials, diverse conductive materials such as conducting polymers, polymer/carbon composites, graphene, and metal or semiconductor nanocrystals show great promise [7]. Especially, conducting polymers such as poly(3,4-ethylenedioxythiophene) (PEDOT) [8], polypyrrole (PPy) [9] and polyaniline (PANI) [10] are among the highest attractive sensing materials owing to their intriguing features such as their all-organic nature with good designability, intrinsic electrical conductivity, high signal transduction, dimensional durability, mechanical flexibility, and chemical stability. The synthesis of the polymers, usually via convenient chemical or electrochemical polymerization methods, are simple and cost effective. The polymers thus synthesized can retain their electrical conductivity and chemical/physical stability when used in chemosensors. Such durability is crucial for chemosensors, for which the sensing repeatability is one of the most important factors affecting the real application of sensor devices.
Among the conducting polymers used for chemosensors, PEDOT exhibits a relatively high stability and adjustable conductivity (10−3–103 S cm−1) compared to PPy and PANI. The linear, rigid molecular conformation of PEDOT facilitates its charge transport [11]. To be suited for use in chemoresistive sensors, the conductivity of polymers must be at an appropriate level and, more importantly, adjustable. Indeed, if the conductivity is too low or too high, the sensor performance will be limited by the low signal-to-noise ratio [12]. PEDOT by itself is typically insoluble in water or common organic solvents [13]. However, if it is synthesized in the presence of poly(4-styrenesulfonate) (PSS), an stable aqueous dispersion containing both polymers (PEDOT:PSS) (Figure 1b) can be obtained, which is normally of a dark-blue color [14]. As a commercially available and ready-to-use waterborne dispersion, PEDOT:PSS remains attractive for development as organic electronics (including thermoelectric conversion [15], photovoltaic devices [16], supercapacitors [17] and sensors [18], etc.) because of its excellent solution-fabrication capability and miscibility into functionally films (usually by drop-casting [19], spin-coating [20], and spray-coating [21]) exhibiting a high and controllable conductivity, a high work function, excellent chemical and electrochemical stability, good optical transparency, good biocompatibility, and so on [22]. Since year 2000 when the three discoverers of conducting polymers won the Nobel Prize in Chemistry, PEDOT:PSS and its hybrid composites with metal/metal oxide nanoparticles, insulating polymers, carbon materials and others have been extensively studied and applied widely in organic electronics including electrochemical and/or electronic chemosensors as covered in this review. These research activities have produced thousands of peer-reviewed papers and patents.
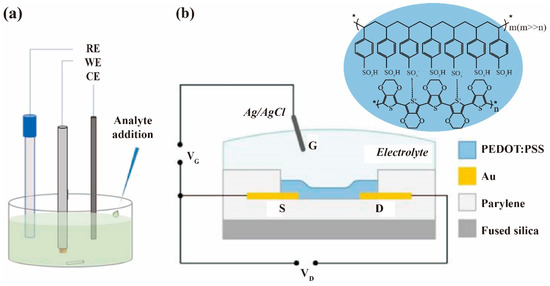
Figure 1. Schematic architectures of electrochemical chemosensors based on PEDOT:PSS: (a) three- or two-electrode electrochemical system (RE = reference electrode, WE = working electrode, CE = counter electrode) [23]; (b) OECT device [24].
In the last decade, some excellent reviews have been published on the related topics of electrical sensors based on various conducting polymers. For example, Liao et al. [25] reviewed the development of organic electrochemical transistor (OECT)-based sensors by focusing on the functionalization of PEDOT:PSS films as channel materials. Rahimzadeh et al. [13] reviewed the recent advancement in PEDOT-based electrochemical biosensors. Various PEDOT composites blended with nanomaterials such as carbon nanomaterials and metal/metal oxide nanoparticles, and the applications in electrochemical sensors were described by Kaur et al. [18] However, there still lacks a special and comprehensive review of electrochemical and electronic chemosensors based on PEDOT:PSS and its composites.
2. PEDOT:PSS-Based Electrochemical Chemosensors
A typical electrochemical sensor has been considered as electrochemical cells containing three or two electrodes (Figure 1a) immersed in an electrolyte solution. A characteristic three-electrode system contains a reference, counter, and working electrode (sensing electrode). The working electrode has a chemically stable solid conductive substance (conventionally gold, carbon, and platinum), and the reference electrode generally contains the silver wire coated with a layer of silver chloride (Ag/AgCl) to provide a fixed stable potential to other electrodes, and a platinum wire is usually employed as the counter electrode (not necessary in a two-electrode system) to the working electrode. The electrochemical procedures can usually be classified into three key categories concerning different modes of measurement: current (voltammetry, amperometry), potential difference (potentiometry), and impedance (electrochemical impedance spectroscopy) [11]. The common current mode-based sensors are to measure the current under the potential of a working electrode against a reference electrode. This electrical current results from the electrolysis of chemical analytes (e.g., molecules, ions) through either electrochemical oxidation or reduction at the working electrode surface. This procedure depends on the mass transport rate of the analytes to the electrode, as well as the rate of electron transfer at the electrode surface. In addition to the conventional electrochemical systems, OECT (Figure 1b) is another sensor architecture commonly used in chemical and biological detection. In a regular OECT configuration, the gate is immersed in an electrolyte and the active material channel is in direct contact with an electrolyte and with the source and drain [26]. The important sensing interfaces for analyte monitoring are the gate electrode surface and channel surface. Any chemical reaction occurring on these two surfaces may induce a change in interfacial potential, and result in a change in channel current, which in turn can be used as a sensor response signal [25].
It is important to note that for the development of electrochemical chemosensors to determinate different analytes, the working electrodes have often been modified with appropriate substances to achieve the desired performance such as improved selectivity, sensitivity and stability. PEDOT:PSS has not only been employed as a common material for working electrodes, but has also been proven compatible and processible with metal or carbon materials for surface modification. Besides, good biocompatibility of PEDOT:PSS makes it an ideal material for bioelectronic applications. PEDOT:PSS is an excellent matrix for functionalization with various biological components such as antibodies [27], enzymes [28], and peptides [29] to be developed as electrochemical biosensors. There are several reviews [2][5][30] focused on such electrochemical biosensors integrated with conducting polymers including PEDOT:PSS for monitoring the biological targets such as DNA, proteins [29], glucose [31], and other biomarkers. In this section, we just give a brief summary about this aspect of research, while the main attention will be laid on the issues and improvements of electrochemical chemosensors towards biological and environmental analytes such as H2O2, pH, NH4+, some inorganic ions and organic compounds, etc. (Table 1).
2.1. H2O2 Detection
Hydrogen peroxide (H2O2) has been widely used in industry as it plays crucial roles in chemical, pharmaceutical, and food manufacturing [32]. H2O2 is also an important component or synthetic precursor for some improvised explosives. Moreover, H2O2 is a key marker in biological processes as it is involved in signaling paths such as cellular growth, senescence and apoptosis, wherein it can be generated through different stimuli. Detection of H2O2 can help probe the biomolecules in different fluids as H2O2 is often a byproduct of many biochemical reactions involving oxidase enzymes such as glucose, cholesterol and lactate oxidase, etc. For these reasons, the development of chemosensors for trace level detection of H2O2 has remained the focus of chemosensor research, for which most of the sensors reported are based on electrochemical system owing to the electroactive nature of H2O2. In this section, we focus on the direct detection of H2O2, and exclude the indirect detection of H2O2 as a byproduct of a redox reaction.
As shown in Table 1, most of the electrochemical sensors developed for H2O2 are amperometric types and used glassy carbon electrode (GCE) or a screen-printed electrode (SPE) as the working electrode substrate. GCE has the advantages of wide commercial availability, a low background current, and easy surface modification, while SPE is small in volume and easy to be integrated in a sensor system for miniaturization.
The main architecture of components of a typical working electrode for electrochemical detection of H2O2 is shown in Figure 2. The electrochemistry technique based on a simple and low-cost enzyme electrode has been extensively employed for accurate determination of H2O2 due to the intrinsic selectivity and sensitivity of enzymatic reactions [33][34]. The most frequently used enzyme to decompose H2O2 is horseradish peroxidase (HRP), which catalyzes the oxidation of a substrate using H2O2 as the oxidizing agent, allowing for direct electron transfer through the electrode. In this situation, PEDOT:PSS was coated on the electrode to immobilize HRP because of its good conductivity and biocompatibility, providing an ideal microenvironment for keeping biological activity while facilitating the direct electron transfer between the enzyme’s active sites and the electrode. After the surface modification of the electrode and immobilization of the enzyme, a Nafion film is usually coated to prevent the modification layer from falling off because PEDOT:PSS is prone to swell and fall off from the substrate in an aqueous solution.
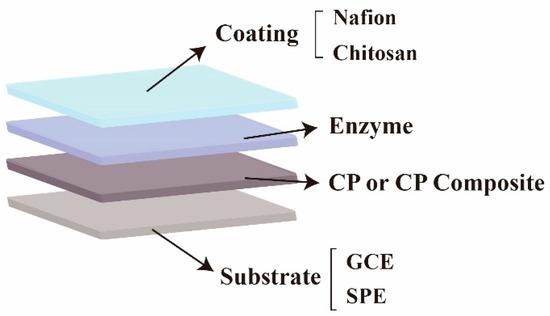
Figure 2. Component architecture of the working electrode based on PEDOT:PSS or its composite for electrochemical detection of H2O2.
As an example of success, Zhang et al. [33] fabricated a hybrid composite of PEDOT:PSS and chitosan micelles and coated it on the surface of GCE, followed by immobilization with HRP. The presence of PEDOT:PSS was found to be capable of enhancing the interfacial electron transfer between HRP and the electrode. The sensor thus fabricated showed a very low detection limit (LDL) of 0.03 nM and a dynamic linear range between 0.1 nM and 10 nM for detection of H2O2. The wide detection range was attributed to the large surface area of the hybrid film of PEDOT:PSS, which in turn facilitates the immobilization of HRP. The large surface area would also facilitate the signal transduction from the enzyme to the electrode. The similar advantage of a large surface area of electrode for improving the sensor performance was also evidenced in a sensor involving PEDOT:PSS hydrogel, which was also conducive to a high loading of enzymes like HRP [34].
In recent years, gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs) have been widely used in the fabrication of electrochemical biosensors because of their unique properties such as high biocompatibility, good conductivity, high catalytic activity, and size-dependent properties. Compared to the pristine PEDOT:PSS, the composites with an encapsulation of the metal nanoparticles could further enhance the electron exchange and realize the direct electrochemical redox reactions of enzymes since metal nanoparticles may act as electron relays in the PEDOT:PSS film. As evidenced in many studies, the GCEs modified with HRP/PEDOT:PSS/AuNPs [35] and HRP/PEDOT:PSS/AgNPs [36] showed excellent electrocatalytic ability and were thus sensitive to detection for H2O2 with a wide linear range of 0.2~380 μM and 0.05~20 μM, respectively. More sophisticated, a ternary composite [37] with PEDOT:PSS, AuNPs and reduced-graphene oxide (rGO) was assembled with HRP on a screen-printed gold electrode (SPGE) for amperometric detection of H2O2. This composite sensor exhibited a high sensitivity of up to 677 A mM−1 cm−2, with a wide linear range from 5 to 400 μM and LDL of 0.08 μM. The enhanced sensing performance could be ascribed to the intimate contact of AuNPs onto the rough surface of the PEDOT:PSS-rGO nanocomposite, which has a high electrical conductivity and a large surface area, allowing for the growth and support of nanoparticles while still maintaining the high electrical conductivity.
While PEDOT:PSS and other materials have been commonly used to immobilize enzymes on an electrode for retaining the enzymatic biologic activity and electrically connecting the enzyme with the electrode surface, the research of PEDOT:PSS-based electrochemical sensors for H2O2 has also been extended from use of enzymes to enzymeless active composites such as Meldola Blue (MDB) and Prussian Blue (PB). This alternative approach aims to overcome the technical limitations of enzymes like poor stability and the complexity of the immobilization process involving enzymes. Siao et al. [38] reported an enzymeless electrochemical sensor for H2O2, which was fabricated with PEDOT:PSS and Meldola Blue (MDB) on a GCE. The sensor thus fabricated could electrocatalytically reduce H2O2 with a low overpotential and showed a linear response in the concentration range of 5 to 120 μM with a LDL of 0.1 μM and a sensitivity of 353.9 μAmM−1 cm−2.
Although electrochemical sensors are usually suitable for detection of analytes in solutions, there exists a potential to adapt the sensor for gas phase chemical detection by combining the sensor system with an air extraction system that can concentrate airborne analytes into solution to be ready for electrochemical detection. Recently, Chen et al. [19] prepared a new composite consisting of PEDOT:PSS, PB, ethylene glycol (EG), and divinyl sulfone (DVS). After drop-casting it onto a SPGE, the sensor showed a good linear response between 0.1 μM to 25.6 μM with a sensitivity of −0.95 A M−1 cm−2 and LDL of 0.22 μM for solution phase detection. When combing the sensor with an exhaled breath condensate (EBC) device, it became capable of directing detection of H2O2 in human breath, which may provide a new way for noninvasive diagnosis or screenings of diseases (e.g., cancers) as H2O2 has been identified as one of the common breath biomarkers for humans.
2.2. pH Detection
pH is an extremely important parameter in biological processes and related chemical reactions. Detection of pH changes can provide diagnostic information about health and medical status. In particular, pH variations in human sweat have been correlated to blood glucose levels [39] and the pH of a wound exudate is indicative of the progression of the wound-healing process and the presence of bacterial colonies [40]. Real-time analysis of such biofluids can be achieved by means of portable and wearable sensing devices integrated into clothing, bandages or other everyday accessories, which imposes a great technical challenge to the traditional chemical sensors. While much effort has been put into combining flexible substrates with traditional rigid sensing materials to fabricate pH sensors with a good performance, the flexibility of PEDOT:PSS film and its biocompatibility for wearable bioelectronics are beyond the reach of these technologies. Within the PEDOT:PSS composite, the PEDOT chains are uniformly distributed along the PSS polymer chains under slightly acidic conditions. Such an optimized distribution of PEDOT chains within the colloidal PEDOT:PSS system ensures the formation of a continuous electrical connection between PEDOT segments. When the pH shifts from acidic to basic, the homogeneous distribution of PEDOT along the PSS polymer chains is interrupted by the negatively charged hydroxyl groups. Under more basic conditions, the PEDOT short chains are neutralized by the hydroxyl groups, forming a new hydrophobic phase which is covered by the long chains of PSS [41] (Figure 3a). As a result, conductivity of the polymer film declined significantly. However, swelling of the hydrophilic PSS in PEDOT:PSS could affect the resistance of the electrode, leading to poor pH sensitivity in electrochemical sensors. In general, electrochemical sensing of pH relies on the potentiometric measurement of an active working electrode with respect to the reference electrode.
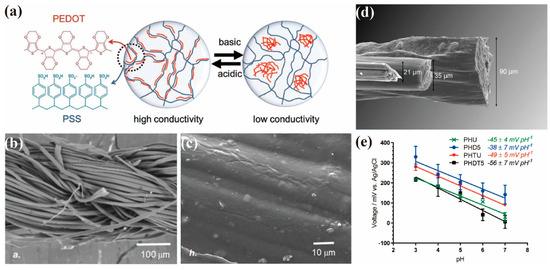
Figure 3. (a) Schematic illustration of the impact of pH on molecular structure of PEDOT:PSS [41]; SEM images of (b) uncoated cotton and (c) PEDOT:PSS-MWCNTs-cotton [40]; (d) SEM image comparing three fiber type; (e) Potentiometric (open circuit) response of PEDOT:PSS wet-spun fibers coated with PANI. (PHU: 90 µm PEDOT:PSS fibers without DMSO treatment; PHD5: 90 µm PEDOT:PSS fibers with 5 min DMSO treatment; PHTU: 20 µm PEDOT:PSS fibers without DMSO treatment; PHDT5: 20 µm PEDOT:PSS fibers with 5 min DMSO treatment) [42].
The conductivity of PEDOT:PSS used in the electrochemical sensors can be improved by various methods [43] in order to overcome the above-mentioned technical limitations. The PEDOT:PSS composite is often fabricated with other pH-sensitive materials acting as a charge transfer layer of work electrode. PANI, for example, has low conductivity, but its various oxidation states are pH-dependent, making it suitable for pH sensing. Smith et al. [40] deposited the dispersions of PEDOT:PSS and multiwalled carbon nanotubes (MWCNTs) on cotton (Figure 3b,c) by a dipping method, followed by electrochemical deposition of PANI to fabricate a fibril material to function as the working electrode. The fiber electrode showed a rapid response (−61 ± 2 mV pH−1) over a wide pH range (2–12) and a high selectivity in the presence of varying interfering ions (NH4+, Mg2+, Ca2+, K+ and Na+). The PANI/PEDOT:PSS-MWCNTs-cotton fibril composite showed intermediate values of ultimate tensile strengths (130 ± 25 MPa, 10 ± 2% strain at break) whilst maintaining sufficient flexibility. The fibril materials have an obvious ability to inhibit the growth of bacteria and has biocompatibility with skin cells. This highlights the potential to develop these fibril materials as pH-sensing fabrics for wound monitoring. Smith et al. [42] also directly prepared PEDOT:PSS fibers (Figure 3d) by wet spinning and then electrodeposited PANI onto them for pH detection. The pristine PEDOT:PSS fibers were treated with dimethyl sulfoxide (DMSO) and their diameters were controlled to obtain a high conductivity (802 ± 122 S cm−1). The thicker fibers have a higher PSS-to-PEDOT ratio, which leads to more water being absorbed by the hydrophilic PSS, resulting in more swelling during pH analysis. This would affect the resistance of the electrode and thus the potential and slope of voltage vs. pH (Figure 3e). The best pH response and lowest swelling was achieved by the thin fiber prepared from DMSO treatment, which gave a response of −56 ± 7 mV pH−1.
2.3. Ion Detection
When PEDOT:PSS is introduced into an ion sensor, it acts as an ion-to-electron transducer layer to decrease the impedance and promote the charge transfer within the sensor, which significantly improves the potentiometric stability and detection performance. The selectivity of such an ion sensor comes from the use of an ion-selective membrane (ISM). ISM allows for only specific ions to transport from the solution to the conductive layer, whereas it blocks the transport of other interfering ions, thus enabling selective detection of a variety of cations and anions such as Na+, K+ [44], Ca2+, NH4+ [45], Cu2+ [46], Mg2+, Cl− [47], etc.
For many years, direct potentiometry and ion-selective electrodes have been useful tools for determining ion concentrations in human body fluids and other areas in medical analyses. Urbanowicz et al. [47] fabricated a two-layer surface coating on the gold electrode: electrically synthesized PEDOT:PSS forming an intermediate layer and ISM, to monitor Na+, K+, Ca2+, Mg2+ and Cl− in human saliva (Figure 4a). The sensor consisted of a reference electrode and an ion selective electrode, showing a wide linear range and sensitivity (Table 1) assuring its applicability in biomedical fields.
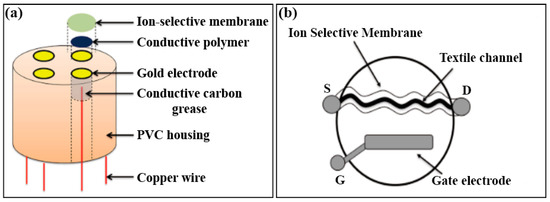
Figure 4. (a) Schematic illustration showing the architecture of working electrode for multiple ion sensing [47]; (b) sketch of a OECT type sensor fabricated with textile channel coated with ion selective membrane [44].
PEDOT:PSS is characterized by high stability, high ionic mobility and a high charge transfer rate, as well as good adhesion to the membrane material and electrode substrate. The most common ways to deposit PEDOT:PSS onto the electron conductive substrate are via drop-casting, spin-coating, or inkjet printing, etc. However, as reported in many publications, the films of the same conducting polymer fabricated with different deposition methods may show different characteristics [48]. These factors affect the reproducibility of electrical potentials, which are critical characteristics for the mass production of ion-selective electrodes. In this regard, Wang et al. [49] studied a Ca2+ sensor based on a screen-printing technique, that is, PEDOT:PSS was screen-printed on carbon paste electrodes as internal solid contacts for both ion-selective and reference electrodes. The screen-printing method provides a cost-effective and reproducible way of mass-manufacturing portable ion sensors.
Recently, Coppedè et al. [44] fabricated a textile OECT device (Figure 4b) by soaking an acrylic textile thread with PEDOT:PSS. The selectivity was improved by directly functionalizing the textile device with ISM. Membrane selectivity was tested by comparing the transistor response with interfering ions, proving successfully the selective response to Na+, K+, Ca2+. This direct functionalization of textile fibers as an active channel may find wide application in the wearable devices for analyzing liquid samples, such as sweat, enabling real-time monitoring and screening of health.
2.4. Other Analyte Detection
As described above, in electrochemical sensors, the sensing signal comes from the redox reaction of the analyte on the electrode surface. PEDOT:PSS is used to modify the bare electrode to improve the detection sensitivity due to its excellent electrochemical and environmental stability, good biocompatibility, superior film-forming properties, and fine tuning of their physicochemical properties [50][51]. In addition, researchers have often combined PEDOT:PSS with different nanoscale catalytic materials (mainly carbon materials [52][53][54][55] and metal/metal oxide nanoparticles [56][57][58][59]) to form composites in order to promote rapid electron transfer. Such an approach has great potential for application in the construction of electrochemical sensors for various biological and environmental detections [18].
The incorporation of different nanostructures into PEDOT:PSS generally improves the interaction and the electrocatalytic activity with the analyte in a synergistic way, which leads to an amplified signal output. As shown in Figure 5a, carbon black (CB), graphene oxide (GO), copper nanoparticles (CuNPs) and PEDOT:PSS can be mixed and used for surface modification of the GCE electrode [57]. The cyclic voltammograms of the GCE electrode before and after modification with a PEDOT-PSS film containing GO, CB-GO or CuNPs/CB-GO was shown in Figure 5b. It is clear that the peak current was relatively increased after each step of modification, indicating that the electroactive surface area of GCE increased after every modification. And the quinary mixtures of isoproterenol, acetaminophen, folic acid, propranolol and caffeine was determined by square-wave voltammetry (Figure 5c), resulting the linear ranges and LDL of micromolar concentration levels, as shown in Table 1.
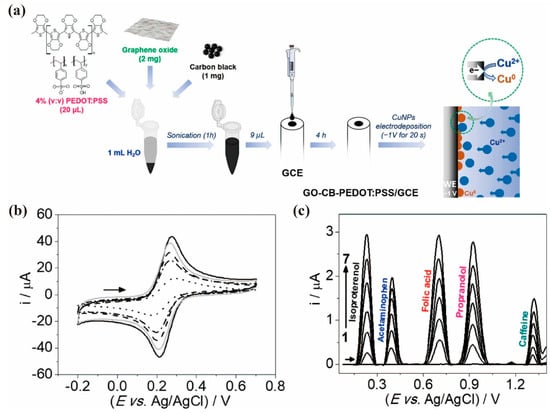
Figure 5. (a) Scheme of the fabrication of CuNPs/CB-GO-PEDOT:PSS/GCE electrode; (b) Cyclic voltammograms obtained by using unmodified GCE (….), PEDOT:PSS/GCE (— · —), GO-PEDOT:PSS/GCE (— —), CB-GO-PEDOT:PSS/GCE (——) and CuNPs-CB-GO-PEDOT:PSS/GCE (——); (c) Square-wave voltammograms of the response to diverse analytes obtained on a CuNPs/CB-GO-PEDOT:PSS/GCE [57].
In addition, various other methods have been proven to be quite useful for fabrication of low-resistance PEDOT:PSS films for electrochemical analyte detection [49][59][60]. For example, our group [60] prepared PEDOT:PSS films with enhanced conductivity by DMSO second-doping, which can be directly used as flexible electrodes for detection of p-butylhydroquinone (TBHQ), which gave a LDL of 0.15 μM. However, the poor water resistance and weather stability of the PEDOT:PSS film would limit the widespread application in electrochemical analysis. To solve this problem, Zhang et al. [61] introduced carboxymethyl cellulose (CMC) into PEDOT:PSS, which demonstrated a significant improvement of the flexibility, adhesion and long-term electrode stability in water, as well as the electrocatalytic ability for maleic hydrazide (MH), salicylic acid (SA), and sunset yellow. In their subsequent work [62], a carbon nanotube with a large rough surface area was added to the composite of PEDOT:PSS and CMC to improve the electrochemical sensing performance of PEDOT:PSS composites. The fabricated PEDOT:PSS-CMC-SWCNT/GCE exhibited enhanced electron transfer and a synergistic electrocatalytic ability towards maleic hydrazide, and displayed excellent sensing performance with a wide linear range (0.8 to 51 μM), a low LDL (0.1 μM) and good sensing stability.
Table 1. Comparison of PEDOT:PSS-based electrochemical chemosensors for different analytes.
| Working Electrode | Analyte | Model | LDL | Sensitivity | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| PEDOT:PSS-PB-EG-DVS/SPGE | H2O2 | Amperometry | 219 nM | −0.95 A M−1 cm−2 | 0.1–25.6 μM | [19] |
| Nafion/HRP/PEDOT:PSS-CS micelle/GCE | H2O2 | Amperometry | 0.03 nM | - | 0.1 nM–10 nM | [33] |
| Nafion/HRP/PEDOT:PSS hydrogel/GCE | H2O2 | Amperometry | 0.94 μM | 155 μA mM−1 | 0.0088–0.15 mM | [34] |
| 0.045 mM | 3.5 μA mM−1 | 0.4–10 mM | ||||
| HRP-PEDOT:PSS-AuNPs/GCE | H2O2 | Amperometry | 0.1 μM | - | 0.2–380 μM | [35] |
| Nafion/HRP/AgNPs/PEDOT:PSS-Nafion/GCE | H2O2 | Amperometry | 0.02 μM | - | 0.05–20 μM | [36] |
| HRP/PEDOT:PSS-rGO-AuNPs/SPGE | H2O2 | Amperometry | 0.08 μM | 677 μA mM−1 cm−2 | 0.5–400 μM | [37] |
| PEDOT:PSS-MDB/GCE | H2O2 | Amperometry | 0.1 μM | 353.9 mA mM−1 cm−2 | 5–120 μM | [38] |
| PANI/PEDOT:PSS/G | pH | Potentiometry | - | 75.06 mV pH−1 | 4–7 | [39] |
| PANI/PEDOT:PSS-MWCNTs-cotton | pH | Potentiometry | - | −61 ± 2 mV pH−1 | 2–12 | [40] |
| PANI/PEDOT:PSS fiber | pH | Potentiometry | - | −56 ± 7 mV pH−1 | 3–7 | [42] |
| K+ ISM/PEDOT:PSS-acrylic textile | K+ | OECT | - | 3.49 M−1 | 0.01–1000 mM | [44] |
| NH4+ and Ca2+ ISM/PEDOT:PSS | NH4+ Ca2+ |
OECT | - | - | 10–1000 μM | [45] |
| Cu2+-ISM/PEDOT:PSS/GCE | Cu2+ | Potentiometry | 0.5 nM | 28.1 ± 0.4 mV dec−1 | 1 nM–1 mM | [46] |
| ISMs/PEDOT:PSS/GCE | Na+ | Potentiometry | - | 56 ± 1 mV dec−1 | 0.1–100 μM | [47] |
| K+ | - | 58 ± 1 mV dec−1 | 0.01 mM–100 μM | |||
| Ca2+ | - | 29 ± 1 mV dec−1 | 0.01–100 μM | |||
| Mg2+ | - | 30 ± 1 mV dec−1 | 1–100 μM | |||
| Cl− | - | −54 ± 1 mV dec−1 | 0.1–100 μM | |||
| Pb2+-ISM/PEDOT:PSS/GCE | Pb2+ | Potentiometry | 0.1 μM | 27 mV dec−1 | 10−5–10−7 M | [49] |
| PEDOT:PSS/glassy-carbon disk | thiols | Amperometry | 0.005 mM | 0.43 A M−1 cm−2 | 0.005–0.1 mM | [50] |
| PEDOT:PSS/GCE | Tricresyl phosphate | Voltammetry | 70 ppb | - | 50–300 ppb | [51] |
| PEDOT:PSS-GO/PET | carbofuran | Voltammetry | 0.1 µM | - | 1 mM–90 mM | [52] |
| PEDOT:PSS-β-CD-SWCNT-COOH/GCE | shikonin | Voltammetry | 1.80 nM | - | 6.0–30,000 nM | [53] |
| PEDOT:PSS-rGO/GCE | nimesulide | Voltammetry | 2.4 nM | - | 80–1900 nM | [54] |
| piroxicam | 0.1 µM | 0.87–260 µM | ||||
| PEDOT:PSS-G/SPCE | 2,2-diphenyl1-picrylhydrazyl (DPPH) | Amperometry | 0.59 μM | - | 5–30 μM | [55] |
| PEDOT:PSS-MgO/GCE | bisphenol A | Voltammetry | 0.5 nM | - | 1.0 nM–0.4 μM and 0.4–10 μM | [56] |
| CuNPs/GO-CB-PEDOT:PSS/GCE | isoproterenol | Voltammetry | 1.9 μM | 0.062 μA μM−1 | 8–50 μM | [57] |
| acetaminophen | 0.23 μM | 0.29 μA μM−1 | 0.9–7 μM | |||
| folic acid | 1.0 μM | 0.098 μA μM−1 | 5–31 μM | |||
| propranolol | 0.18 μM | 0.92 μA μM−1 | 0.5–2.9 μM | |||
| caffeine | 3.4 μM | 0.028 μA μM−1 | 11–64 μM | |||
| PEDOT:PSS-ZnO/GCE | chlorogenic acid | Voltammetry | 0.02 μM | 26.38 μA mΜ−1 cm−2 | 0.03–476.2 μM | [58] |
| AgNPs/PEDOT:PSS-H2SO4/glass | nitrite | Amperometry | 0.34 μM | 0.03639 μA μM−1 cm−2 | 0.5–3400 μM | [59] |
| PEDOT:PSS-DMSO film | tert-butylhydroquinone | Voltammetry | 0.15 μM | - | 0.5–200 μM | [60] |
| PEDOT:PSS-CMC/GCE | tryptophan | Voltammetry | 0.02 μM | - | 0.05–100 mM | [61] |
| PEDOT:PSS-CMC-SWCNT/GCE | maleic hydrazide | Voltammetry | 0.1 µM | - | 0.8–51 µM | [62] |
References
- Kumar, H.; Kumari, N.; Sharma, R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: Its issues and challenges. Environ. Impact Assess. Rev. 2020, 85, 106438.
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843.
- Ramdzan, N.S.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Saleviter, S. Development of biopolymer and conducting polymer-based optical sensors for heavy metal ion detection. Molecules 2020, 25, 2548–2573.
- Wen, Y.P.; Xu, J.K. Scientific importance of water-processable PEDOT-PSS and preparation, challenge and new application in sensors of its film electrode: A review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150.
- Dubey, N.; Kushwaha, C.S.; Shukla, S.K. A review on electrically conducting polymer bionanocomposites for biomedical and other applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 709–727.
- Zamiri, G.; Haseeb, A. Recent trends and developments in graphene/conducting polymer nanocomposites chemiresistive sensors. Materials 2020, 13, 3311.
- Bae, J.; Hwang, Y.; Park, S.-H.; Park, S.J.; Lee, J.; Kim, H.J.; Jang, A.; Park, S.; Kwon, O.S. An elaborate sensor system based on conducting polymer-oligosaccharides in hydrogel and the formation of inclusion complexes. J. Ind. Eng. Chem. 2020, 90, 266–273.
- Mantione, D.; Del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) derivatives: Innovative conductive polymers for bioelectronics. Polymers 2017, 9, 354.
- Cho, K.H.; Yu, H.; Lee, J.S.; Jang, J. Facile synthesis of palladium-decorated three-dimensional conducting polymer nanofilm for highly sensitive H2 gas sensor. J. Mater. Sci. 2020, 55, 5156–5165.
- Morais, R.M.; Klem, M.d.S.; Nogueira, G.L.; Gomes, T.C.; Alves, N. Low cost humidity sensor based on PANI/PEDOT:PSS printed on paper. IEEE Sens. J. 2018, 18, 2647–2651.
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent developments in conducting polymers: Applications for electrochemistry. RSC Adv. 2020, 10, 37834–37856.
- Hakimi, M.; Salehi, A.; Boroumand, F.A. Fabrication and characterization of an ammonia gas sensor based on PEDOT-PSS with n-doped graphene quantum dots dopant. IEEE Sens. J. 2016, 16, 6149–6154.
- Rahimzadeh, Z.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. An overview on the synthesis and recent applications of conducting poly(3,4-ethylenedioxythiophene) (PEDOT) in industry and biomedicine. J. Mater. Sci. 2020, 55, 7575–7611.
- Elschner, A.; Kirchmeyer, S.; Lövenich, W.; Merker, U.; Reuter, K. PEDOT-Principles and Applications of an Intrinsically Conductive Polymer; CRC Press: New York, NY, USA, 2011.
- Zheng, Y.; Zeng, H.N.; Zhu, Q.; Xu, J.W. Recent advances in conducting poly(3,4-ethylenedioxythiophene):polystyrene sulfonate hybrids for thermoelectric applications. J. Mater. Chem. C 2018, 6, 8858–8873.
- Sun, K.; Zhang, S.P.; Li, P.C.; Xia, Y.J.; Zhang, X.; Du, D.H.; Isikgor, F.H.; Ouyang, J.Y. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J Mater. Sci. Mater Electron. 2015, 26, 4438–4462.
- Ouyang, J.Y. Recent advances of intrinsically conductive polymers. Acta Phys. Chim. Sin. 2018, 34, 1211–1220.
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Plast. Tech. Mat. 2020, 60, 502–519.
- Chen, Y.C.; O’Hare, D. Exhaled breath condensate based breath analyser-a disposable hydrogen peroxide sensor and smart analyser. Analyst 2020, 145, 3549–3556.
- Li, S.Y.; Chen, S.J.; Zhuo, B.G.; Li, Q.F.; Liu, W.J.; Guo, X.J. Flexible ammonia sensor based on PEDOT:PSS/silver nanowire composite film for meat freshness monitoring. IEEE Electr. Device L. 2017, 38, 975–978.
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive polymers and composites based on PEDOT and PEDOT: PSS. Adv. Mater. 2019, 31, 1806133.
- Reynolds, J.R.; Tompson, B.C.; Skotheim, T.A. Conjugated Polymers: Properties, Processing, and Applications; CRC Press: New York, NY, USA, 2019.
- Mariani, F.; Gualandi, I.; Tonelli, D.; Decataldo, F.; Possanzini, L.; Fraboni, B.; Scavetta, E. Design of an electrochemically gated organic semiconductor for pH sensing. Electrochem. Commun. 2020, 116, 106763–106770.
- Khodagholy, D.; Rivnay, J.; Sessolo, M.; Gurfinkel, M.; Leleux, P.; Jimison, L.H.; Stavrinidou, E.; Herve, T.; Sanaur, S.; Owens, R.M. High transconductance organic electrochemical transistors. Nat. Commun. 2013, 4, 2133.
- Liao, J.J.; Si, H.W.; Zhang, X.D.; Lin, S.W. Functional sensing interfaces of PEDOT: PSS organic electrochemical transistors for chemical and biological sensors: A mini review. Sensors 2019, 19, 218–233.
- Yan, Y.J.; Wu, X.M.; Chen, Q.Z.; Liu, Y.Q.; Chen, H.P.; Guo, T.L. High-performance low-voltage flexible photodetector arrays based on all-solid-state organic electrochemical transistors for photosensing and imaging. ACS Appl. Mater. Inter. 2019, 11, 20214–20224.
- Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. AuNPs/PpPD/PEDOT: PSS-Fc modified screen-printed carbon electrode label-free immunosensor for sensitive and selective determination of human serum albumin. Microchem. J. 2020, 155, 104709–104716.
- Abd-Wahab, F.; Abdul Guthoos, H.F.; Wan Salim, W.W.A. Solid-state rGO-PEDOT: PSS transducing material for cost-effective enzymatic sensing. Biosensors 2019, 9, 36–50.
- Bhasin, A.; Sanders, E.C.; Ziegler, J.M.; Briggs, J.S.; Drago, N.P.; Attar, A.M.; Santos, A.M.; True, M.Y.; Ogata, A.F.; Yoon, D.V.; et al. Virus bioresistor (VBR) for detection of bladder cancer marker DJ-1 in urine at 10 pM in one minute. Anal. Chem. 2020, 92, 6654–6666.
- El-Said, W.A.; Abdelshakour, M.; Choi, J.H.; Choi, J.W. Application of conducting polymer nanostructures to electrochemical biosensors. Molecules 2020, 25, 307–317.
- Amirzadeh, Z.; Javadpour, S.; Shariat, M.H.; Knibbe, R. Non-enzymatic glucose sensor based on copper oxide and multi-wall carbon nanotubes using PEDOT: PSS matrix. Synth. Met. 2018, 245, 160–166.
- Borras-Brull, M.; Blondeau, P.; Riu, J. The use of conducting polymers for enhanced electrochemical determination of hydrogen peroxide. Crit. Rev. Anal. Chem. 2020, 1–14.
- Zhang, R.L.; Xu, X.F.; Fan, X.X.; Yang, R.C.; Wu, T.; Zhang, C.G. Application of conducting micelles self-assembled from commercial poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) and chitosan for electrochemical biosensor. Colloid Polym. Sci. 2018, 296, 495–502.
- Słoniewska, A.; Kasztelan, M.; Berbeć, S.; Pałys, B. Influence of buffer solution on structure and electrochemical properties of poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) hydrogels. Synth. Met. 2020, 263, 116363–116369.
- Xu, J.J.; Peng, R.; Ran, Q.; Xian, Y.Z.; Tian, Y.; Jin, L.T. A highly soluble poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid)/Au nanocomposite for horseradish peroxidase immobilization and biosensing. Talanta 2010, 82, 1511–1515.
- Yao, Y.Y.; Wen, Y.P.; Zhang, L.; Xu, J.K.; Wang, Z.F.; Duan, X.M. A stable sandwich-type hydrogen peroxide sensor based on immobilizing horseradish peroxidase to a silver nanoparticle monolayer supported by PEDOT: PSS-nafion composite electrode. Int. J. Electrochem. Sci. 2013, 8, 9348–9359.
- Mercante, L.A.; Facure, M.H.M.; Sanfelice, R.C.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. One-pot preparation of PEDOT: PSS-reduced graphene decorated with Au nanoparticles for enzymatic electrochemical sensing of H2O2. Appl. Surf. Sci. 2017, 407, 162–170.
- Siao, H.-W.; Chen, S.-M.; Lin, K.-C. Electrochemical study of PEDOT-PSS-MDB-modified electrode and its electrocatalytic sensing of hydrogen peroxide. J. Solid State Electr. 2010, 15, 1121–1128.
- Zahed, M.A.; Barman, S.C.; Das, P.S.; Sharifuzzaman, M.; Yoon, H.S.; Yoon, S.H.; Park, J.Y. Highly flexible and conductive poly (3, 4-ethylene dioxythiophene)-poly (styrene sulfonate) anchored 3-dimensional porous graphene network-based electrochemical biosensor for glucose and pH detection in human perspiration. Biosens. Bioelectron. 2020, 160, 112220–112229.
- Smith, R.E.; Totti, S.; Velliou, E.; Campagnolo, P.; Hingley-Wilson, S.M.; Ward, N.I.; Varcoe, J.R.; Crean, C. Development of a novel highly conductive and flexible cotton yarn for wearable pH sensor technology. Sens. Actuator B Chem. 2019, 287, 338–345.
- Naficy, S.; Oveissi, F.; Patrick, B.; Schindeler, A.; Dehghani, F. Printed, flexible pH sensor hydrogels for wet environments. Adv. Mater. Technol. US 2018, 3, 1800137–1800146.
- Reid, D.O.; Smith, R.E.; Garcia-Torres, J.; Watts, J.F.; Crean, C. Solvent treatment of wet-spun PEDOT:PSS fibers for fiber-based wearable pH sensing. Sensors 2019, 19, 4213–4222.
- Shi, H.; Liu, C.C.; Jiang, Q.L.; Xu, J.K. Effective approaches to improve the electrical conductivity of PEDOT:PSS: A review. Adv. Electron. Mater. 2015, 1, 1500017–1500032.
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2020, 78, 105579–105584.
- Keene, S.T.; Fogarty, D.; Cooke, R.; Casadevall, C.D.; Salleo, A.; Parlak, O. Wearable organic electrochemical transistor patch for multiplexed sensing of calcium and ammonium ions from human perspiration. Adv. Healthc. Mater. 2019, 8, 1901321–1901328.
- Abd, E.A.; Mohamed, A.-O.; Ayman, H.K.; Elsayed, A.E. Single-piece solid contact Cu(2+)-selective electrodes based on a synthesized macrocyclic calix[4]arene derivative as a neutral carrier ionophore. Molecules 2019, 24, 920–931.
- Urbanowicz, M.; Pijanowska, D.G.; Jasiński, A.; Ekman, M.; Bocheńska, M.K. A miniaturized solid-contact potentiometric multisensor platform for determination of ionic profiles in human saliva. J. Solid State Electr. 2019, 23, 3299–3308.
- Ocana, C.; Munoz-Correas, M.; Abramova, N.; Bratov, A. Comparison of different commercial conducting materials as ion-to-electron transducer layers in low-cost selective solid-contact electrodes. Sensors 2020, 20, 1348–1359.
- Wagner, M.; Lisak, G.; Ivaska, A.; Bobacka, J. Durable PEDOT: PSS films obtained from modified water-based inks for electrochemical sensors. Sens. Actuator B Chem. 2013, 181, 694–701.
- Shadrina, A.A.; Nikiforova, T.G.; Poturai, D.O. Fabrication of electrodes modified with poly-3,4-ethylenedioxythiophene-polystyrene sulfonate film and study of their applicability in thiol-sensitive sensors. Russ. J. Appl. Chem. 2015, 88, 423–429.
- Yang, X.; Kirsch, J.; Olsen, E.V.; Fergus, J.W.; Simonian, A.L. Anti-fouling PEDOT: PSS modification on glassy carbon electrodes for continuous monitoring of tricresyl phosphate. Sens. Actuator B Chem. 2013, 177, 659–667.
- Chekol, F.; Mehretie, S.; Hailu, F.A.; Tolcha, T.; Megersa, N.; Admassie, S. Roll-to-roll printed PEDOT/PSS/GO plastic film for electrochemical determination of carbofuran. Electroanalysis 2019, 31, 1104–1111.
- Chai, J.D.; Zhang, J.; Wen, Y.P.; Zou, L.; Zhang, X.X.; Xin, X.; Zhou, M.H.; Xu, J.K.; Zhang, G. Highly sensitive electrochemical sensor based on PEDOT:PSS-β-CD-SWCNT-COOH modified glassy carbon electrode enables trace analysis shikonin. J. Electrochem. Soc. 2019, 166, B388–B394.
- Wong, A.; Santos, A.M.; Fatibello-Filho, O. Determination of piroxicam and nimesulide using an electrochemical sensor based on reduced graphene oxide and PEDOT: PSS. J. Electroanal. Chem. 2017, 799, 547–555.
- Tirawattanakoson, R.; Rattanarat, P.; Ngamrojanavanich, N.; Rodthongkum, N.; Chailapakul, O. Free radical scavenger screening of total antioxidant capacity in herb and beverage using graphene/PEDOT:PSS-modified electrochemical sensor. J. Electroanal. Chem. 2016, 767, 68–75.
- Zheng, L. An electrochemical sensor on the novel MgO-PEDOT: PSS platform for sensitive bisphenol a determination. Int. J. Electrochem. Sc. 2019, 14, 9030–9041.
- Wong, A.; Santos, A.M.; Silva, T.A.; Fatibello-Filho, O. Simultaneous determination of isoproterenol, acetaminophen, folic acid, propranolol and caffeine using a sensor platform based on carbon black, graphene oxide, copper nanoparticles and PEDOT:PSS. Talanta 2018, 183, 329–338.
- Manivannan, K.; Sivakumar, M.; Cheng, C.-C.; Lu, C.-H.; Chen, J.-K. An effective electrochemical detection of chlorogenic acid in real samples: Flower-like ZnO surface covered on PEDOT: PSS composites modified glassy carbon electrode. Sensor. Actuat. B-Chem. 2019, 301, 127002–127009.
- Pang, D.; Ma, C.; Chen, D.Z.; Shen, Y.L.; Zhu, W.Q.; Gao, J.J.; Song, H.O.; Zhang, X.M.; Zhang, S.P. Silver nanoparticle-functionalized poly (3, 4-ethylenedioxythiophene): Polystyrene film on glass substrate for electrochemical determination of nitrite. Org. Electron. 2019, 75, 105374–105380.
- Tian, Q.Y.; Xu, J.K.; Xu, Q.; Duan, X.M.; Jiang, F.X.; Lu, L.M.; Jia, H.Y.; Jia, Y.H.; Li, Y.Y.; Yu, Y.F. A poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)-based electrochemical sensor for tert.-butylhydroquinone. Mikrochim. Acta 2019, 186, 772–779.
- Zhang, H.; Xu, J.K.; Wen, Y.P.; Wang, Z.F.; Zhang, J.; Ding, W.C. Conducting poly(3,4-ethylenedioxythiophene):poly(styrene-sulfonate) film electrode with superior long-term electrode stability in water and synergistically enhanced electrocatalytic ability for application in electrochemical sensors. Synth. Met. 2015, 204, 39–47.
- Zhang, J.; Xu, J.K.; Wen, Y.P.; Wang, Z.F.; Zhang, H.; Ding, W.C. Voltammetric determination of phytoinhibitor maleic hydrazide using PEDOT:PSS composite electrode. J. Electroanal. Chem. 2015, 751, 65–74.




