
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fumio Fukai | + 3580 word(s) | 3580 | 2021-04-08 06:06:05 | | | |
| 2 | Nora Tang | -1 word(s) | 3579 | 2021-04-13 11:10:45 | | | | |
| 3 | Vicky Zhou | -2946 word(s) | 633 | 2022-04-13 11:57:38 | | |
Video Upload Options
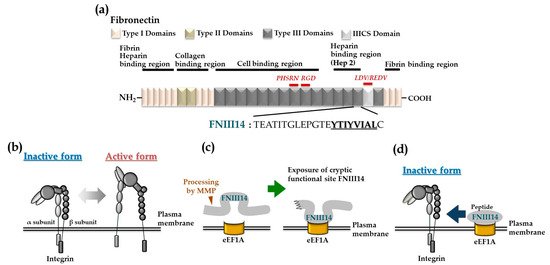
Biofunctional peptide FNIII14, which is derived from the 14th fibronectin (FN) type III-like (FN-III) repeat of FN molecule, is capable of inhibiting cell adhesion to the extracellular matrix (ECM). This functional site is usually buried within the molecular structure of FN, but can be exposed by conformational changes and proteolytic cleavage. Peptide FNIII14 can induce a conformational change in β1-integrin from the active to the inactive form, causing functional inactivation. Based on this anti-adhesive activity, peptide FNIII14 exhibits therapeutic potential for several diseases such as metabolic diseases, organ fibrosis, and malignant tumors. Peptide FNIII14 blocks integrin-mediated signaling by a mechanism entirely distinct from that of conventional antagonisitic peptides, including Arg-Gly-Asp peptides that competitively inhibit the ECM binding of integrin.
References
- de Castro Brás, L.E.; Frangogiannis, N.G. Extracellular Matrix-Derived Peptides in Tissue Remodeling and Fibrosis. Matrix Biol. 2020, 91–92, 176–187.
- Miller, L.M.; Pritchard, J.M.; Macdonald, S.J.F.; Jamieson, C.; Watson, A.J.B. Emergence of Small-Molecule Non-RGD-Mimetic Inhibitors for RGD Integrins. J. Med. Chem. 2017, 60, 3241–3251.
- Davis, G.E.; Bayless, K.J.; Davis, M.J.; Meininger, G.A. Regulation of Tissue Injury Responses by the Exposure of Matricryptic Sites within Extracellular Matrix Molecules. Am. J. Pathol. 2000, 156, 1489–1498.
- Ricard-Blum, S.; Salza, R. Matricryptins and Matrikines: Biologically Active Fragments of the Extracellular Matrix. Exp. Dermatol. 2014, 23, 457–463.
- Pankov, R.; Yamada, K.M. Fibronectin at a Glance. J. Cell Sci. 2002, 115, 3861–3863.
- Johansson, S.; Svineng, G.; Wennerberg, K.; Armulik, A.; Lohikangas, L. Fibronectin-Integrin Interactions. Front. Biosci. J. Virtual Libr. 1997, 2, d126–d146.
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.-J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers 2017, 9, 116.
- Fujita, M.; Sasada, M.; Iyoda, T.; Fukai, F. Involvement of Integrin-Activating Peptides Derived from Tenascin-C in Cancer Aggression and New Anticancer Strategy Using the Fibronectin-Derived Integrin-Inactivating Peptide. Molecules 2020, 25, 3239.

