
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Ali | + 1958 word(s) | 1958 | 2021-03-22 04:53:28 | | | |
| 2 | Lily Guo | Meta information modification | 1958 | 2021-04-06 03:36:08 | | |
Video Upload Options
Carbon dots have gained tremendous interest attributable to their unique features. Two approaches are involved in the fabrication of quantum dots (Top-down and Bottom-up). Most of the synthesis methods are usually multistep, required harsh conditions, and costly carbon sources that may have a toxic effect, therefore green synthesis is more preferable. Herein, the current review presents the green synthesis of carbon quantum dots (CQDs) and graphene quantum dots
1. Introduction
In recent years, carbonaceous and carbon-based nanomaterials have gained great attention owing to their relevant properties [1][2][3][4]. In particular, these substances are characterized by high biocompatibility, less toxicity, significant thermal and mechanical features, and can functionalize easily [5][6][7][8]. Fluorescent carbons are commonly known as carbon dots because of their unique properties that revealed strong fluorescence [9]. In addition, carbon dots are distinguished by high stability, reducing toxic activity, water solubility, and derivatization availability. All of these unique features support their applications in several disciplines as shown in Figure 1 [10][11][12][13][14]. Carbon dots are relatively new and considered one of the most promising nanomaterials ever recognized to humanity, mainly composed of the heteroatoms (functional groups) attached with carbonized core [15]. Carbon dots including different types of nanomaterials such as polymer dots, carbon nanodots, and graphene quantum dots (GQDs). It defined as nanoparticles with small size (<10 nm) that consist of sp2 hybrid conjugated of carbon core-shell between carbon (core) and organic functional groups (shell) such as N–H,–OH,–C = O, COOH, C−O, and C–N or polymer aggregates [16]. Several studies have reported that different techniques and carbon sources are employed in the fabrication of carbon dots with different structures [17].
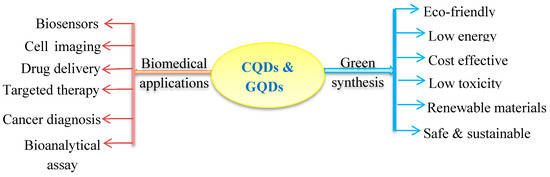
Figure 1. Chart of green synthetic methods of quantum dots (carbon quantum dots (CQDs) and graphene quantum dots (GQDs)) and their biomedical applications.
Typically, two techniques are commonly used in the formation of carbon dots; top-down and bottom-up as described in Figure 2 [18][19]. Usually in the first process “top-down” carbon dots are fabricated by chemical and physical cutting approaches; laser ablation/passivation [20], chemical oxidation, and electrochemical synthesis [21]. In the second method, “bottom-up” carbon dots are converted from appropriate molecular precursors with specific conditions represented by combustion, hydrothermal and thermal [22], and ultrasonic irradiation [23] in which the conditions required fewer amount of carbon sources. It is noteworthy to mention that bottom-up strategy is more preferable to top-down because some limitations are related to this technique including the high cost of the required materials, long time, and harsh conditions [24]. Further, the fabrication of carbon dots via top-down approaches usually needs a separate step for functionalization and passivation of the surface but the second method” bottom-up” does not require that [15]. In addition, different more approaches have been reported such as plasma treatment [25], cage-opening of fullerenes [26], and solution chemistry approaches [27]. The formed carbon dots, nevertheless, of its production methods, have different sizes that required complicated separation technique to get a mono-dispersed carbon dot. There are several post-synthesis separation processes such as chromatography [28], dialysis [29], and gel electrophoresis [30]. On the other hand, the characterized composition of carbon dots gave it significant points for several applications like bioimaging, label-free detection, photocatalysis, and sensing. The current review discussed synthetic approaches for the fabrication of carbon dots and the most significant properties. The biotechnological and biomedical applications are also highlighted.
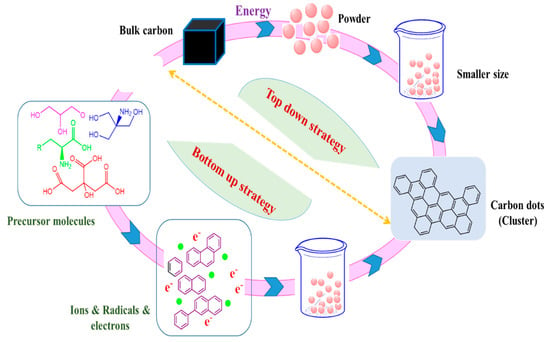
Figure 2. Generation of carbon dots by well-known Top-down and bottom-up approaches.
2. Unique Features of Quantum Dots (CQDs and GQDs)
Carbon dots are considered one of the recently discovered materials having promising and unique properties [31]. The chemical composition of carbon dots containing several function groups on the surface such as amino groups, oxygen, and polymer chains is highly supported by their remarkable features. These functionals have a significant effect on photoluminescence activity and also enhanced the energy gap and energy level of the surface [32]. Such substances have gained great attention because of their significant tunable optical properties, less toxic, simplicity, and low cost, which support them as perfect candidates for use in optical sensors [33]. The aptness of emission of light through carbon dots near the Infra-red area is of particular prominence because the light in this region has deeper tissue penetration proficiency and biological systems are transparent to these wavelengths [34]. Typically, CQDs and GQDs exhibit effectiveness in the short-wavelength area for photon-harvesting that caused by π–π* transition of C = C bonds and n-π* transitions of the groups; C–N, C = O, and C−S for example. Significant optical absorption was demonstrated in the ultraviolet region expanded to the visible range. The region between 230 and 270 nm appeared absorption owing to π–π* transition related to C = C bonds, while the peak shoulder in the range of 300–390 nm is attributed to n–π* transition of C = O bonds [35]. The absorbance can be modified by different types of surface passivation and functionalization methods [36]. For example, multimode emissive carbon dots with high fluorescent were prepared using D-cysteine and l-cysteine. Two absorption bands appeared at the same time related to L-carbon dots at 243 and 300 nm with the low band at 400 nm. The absorbance was displayed due to π-π* transition of the aromatic sp2 domains (243 nm) and n-π* transition of C = O, C–N, C–S (300 nm). However, D-cysteine was not showed any band above 240 nm [37]. The results reported that several function groups (e.g., NH2 and COOH) were found on the surface of L-carbon dots and hence the band gap increased due to the surface interfacial excitation. In addition, Lin et al. have recently investigated the synthesis of other carbon dots from poly (vinyl alcohol) and phenylenediamine. The formed composite exposed two different bands at 247 and 355 nm, matching to π–π* transition of C = C bonds and n-π* transition of C–N, C = N, respectively [38]. Commonly, CQDs have been evaluated successfully in surface passivation as they have the ability for improving brightness because of long wavelengths and decreasing quantum yield. On the opposite, the quantum yield of graphene dots was more than carbon quantum dots because their structures appeared as layers and crystalline phases [32]. The color of the fabricated carbon dots was changed between red, green, and blue. It was not recommended for multi-color imaging, due to the differences in chemical composition, size, and increasing heterogeneity of carbon dots. Most of these particles appeared wide emission spectra originating from difficulty controlling the synthesis processes. Interestingly, carbon dots have several attractive optical properties, but photoluminescence is the most significant one, including phosphorescence and fluorescence. The property of electrochemiluminescence plays an important role in surface passivation, whereas CQDs that passivated have a strong fluorescence and weak electrochemiluminescence [39]. For example, methyl parathion sensors were fabricated by the hydrothermal reaction between tyrosine methyl ester and carbon dots with citric acid employed as a resource of carbon. These types of sensors revealed high and stable photoluminescence and the yield of quantum was approximately 3.8%. This could be successfully developed to determine organophosphorus compound [40].
In addition, most studies revealed that carbon dots have excitation-dependent fluorescence features, although, the excitation-independent emission in S, N-co-doped carbon dots have been investigated [41]. For instance, excitation-independent carbon dots with tunable fluorescent colors have been synthesized through a well-controlled wet oxidative process whereas the results displayed that the photoluminescent properties of carbon dots were principally detected by surface oxidation degree and their molecular weight [42]. The fluorescent carbon dots having fluorescence wavelength can be tuned across the visible spectrum with varying the passivation or functionalization substances, the molar mass ratio of the precursors, and the different synthetic factors. The emission of CQDs can be also influenced by an assortment of adaptable solvents. Subsequently, the performance of excitation dependent/independent photoluminescence is mostly originating from the surface states of carbon dots [43]. It is worth mentioning that the emission mechanism of carbon dots is still unclear. Currently, some expected theoretical explanations may be acceptable including surface state electron-hole radiation rearrangement, quantum size effect, and molecular state luminescence emission mechanism [44]. Consequently, the preparation of monochromatic fluorescent carbon dots and the study of the fluorescence mechanism is an imperative research area for developing the applicability of carbon dots.
On the other hand, biocompatibility is one of the most important features that showed a considerable influence on the application of carbon dots particularly in bio-imaging and cellular imaging [33]. GQDs having an excess of oxygen groups which showed high biocompatibility, low toxicity and enhanced for use in radiotherapy [45][46]. The cytotoxic effect of GQDs was caused by reactive oxygen species generated from the function groups. For example, the in vivo studies of GQDs exhibit low toxicity, no accumulation in the basic organs, and the kidney can dispose of it quickly. By investigation, it has appeared that the mice were not affected by injection with GQDs whereas the graphene oxide showed toxic activity until its death. This happens because graphene oxide can aggregate in the organs.
3. Fundamental Approaches of Carbon Dots Fabrication (Green Synthesis)
Green synthesis is Avery important topic matching with sustainability in our daily life [47][48][49][50][51]. Relevant studies have reported that small organic precursors can be polymerized and carbonized for the synthesis of carbon dots such as ammonium citrate [52], ethylene glycol [53], citric acid [54], phenylenediamine [55], graphite [56], and carbon nanotube [57]. To make them potential fluorescent materials with unique surface functionalities, two main approaches are widely investigated for the generation of ultra-small fluorescent carbon dots. Among these synthetic approaches, the colloidal synthetic methodology has received significant interest due to the generation of large quantities with a tightly controlled size of carbon dots [58]. For example, GQDs were fabricated from small aromatic molecules by stepwise solution chemistry and characterized by significant size uniformity and well-defined structures as presented in Figure 3 [59]. The structure controlling could be enhanced through the covalently bonding between 2′,4′,6′-trialkyl-substituted phenyl moieties (at the 1′-position) to the edges of graphene. The peripheral phenyl groups twisted from the plane of the graphene due to the crowding on the edges, and then the alkyl chains forming a three-dimensional cage around it (Figure 3). This action caused increasing distance between the conjugated systems in all three dimensions and consequently critically decreases the intermolecular π–π* attraction. The well-defined colloidal quantum dots have some unique characteristics that make them excellent model systems for studying fundamental processes in complex carbon materials.
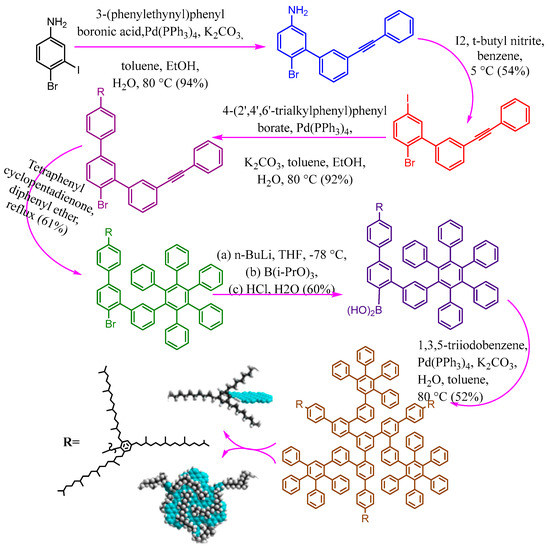
Figure 3. The synthetic procedures of colloidal GQDs with well-defined structures.
Green chemistry is one of the fundamental branches of chemistry that provide golden solves for most problems with significant properties. Green chemistry has several advantages including, safe, environmentally friendly, no need for hazardous materials, and can occur under normal conditions [60]. Green chemical procedures have been engaged in the formation of carbon dots from several natural sources counting chicken eggs, animals [61], different plant species including fruits and vegetables [62], and waste materials like waste paper and frying oil [63]. There is an exponential increase in the number of research articles with both carbon quantum dots and green synthesis content. The fabrication processes can be achieved by different types of methods including hydrothermal/solvothermal, microwave-assisted polymerization, pyrolysis, and carbonization [64]. These approaches are widely used in the synthesis of carbon dots and having several advantages as displayed in Figure 4. In Table 1, conventional CQDs production methods, and maximum emission wavelength, quantum yield and reported CQDs dimensions of CQDs produced by these methods were compared.
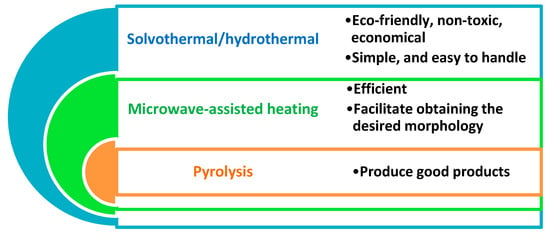
Figure 4. Advantages of different current routes used for the preparation of carbon dots.
Table 1. Recent green quantum dots produced by the bottom-up approach (Hydrothermal, microwave-assisted, and pyrolysis) and their applications.
| Synthetic Approach |
Source | Quantum Yield (%) | Size Range (nm) | λem Max | Application | Ref. |
|---|---|---|---|---|---|---|
| Hydrothermal | Banana peel waste | 5 | 4–6 | 355–429 | Bio imaging | [65] |
| Hydrothermal | Cambuci juice (Campomanesia phaea) | 21.3 | 3.7 | 270, 283 | Sensing of Zn2+ | [66] |
| Hydrothermal | Biomass waste | 4.3–8.2 | 1.3 and 4.9 | 445, 435, 43, 435 | Detection of Fe3+ | [67] |
| Hydrothermal | Biomass waste | 14–3.5 | 6 | 205, 260 | Bio imaging | [68] |
| Hydrothermal | Manilkara zapota fruits | 5.7, 7.9, 5.2 | 1.9 ± 0.3, 2.9 ± 0.7, 4.5 ± 1.25 | 405, 488, 561 | Bio imaging | [69] |
| Hydrothermal | Broccoli | - | 2–6 | 330–470 | Ag+ sensing | [70] |
| Hydrothermal | Lemon juice | 79 | 4.5 | 540 | Biosensors | [71] |
| Hydrothermal | Cherry tomatoes | 9.7 | 7 | 430 | Biosensors | [72] |
| Microwave-assisted | ND | 26 | ~10 | ND | sensor of Hg2+ detection | [73] |
| Microwave-assisted | Cotton linter waste | ND | 10.1 | 420 | Bioimaging | [74] |
| Microwave-assisted | Quince fruit | 8.6 | 4.9 | 450 | Bioimaging | [75] |
| Microwave-assisted | Roasted–Chickpeas | 1.8 | 4.5–10.3 | 435 | Detection of Fe3+ | [76] |
| Pyrolysis | Chia seeds | ND | 4 | ND | Sensors | [77] |
| Pyrolysis | Finger millet ragi | ND | 6 | ND | Biosensor | [78] |
| Pyrolysis | Mango | 18.2 | 6 | 525 | Biosensor | [79] |
References
- Mohanty, A.; Janowska, I. Tuning the structure of in-situ synthesized few layer graphene/carbon composites into nanoporous vertically aligned graphene electrodes with high volumetric capacitance. Electrochim. Acta. 2019, 308, 206–216.
- Pirzado, A.A.; Le Normand, F.; Romero, T.; Paszkiewicz, S.; Papaefthimiou, V.; Ihiawakrim, D.; Janowska, I. Few-layer graphene from mechanical exfoliation of graphite-based materials: Structure-dependent characteristics. Chem. Eng. 2019, 3, 37.
- Mohanty, W.; Baaziz, M.; Lafjah, M.; Da Costa, V.; Janowska, I. Few layer graphene as a template for Fe-based 2D nanoparticles. Flat Chem. 2018, 9, 15–20.
- Atta, A.M.; El-Mahdy, G.A.; Al-Lohedan, H.A.; Shoueir, K.R. Electrochemical behavior of smart N-isopropyl acrylamide copolymer nanogel on steel for corrosion protection in acidic solution. Int. J. Electrochem. Sci. 2015, 10, 870.
- Aljohani, H.; Ahmed, Y.; El-Shafey, O.; El-Shafey, S.; Fouad, R.; Shoueir, K. Decolorization of turbid sugar juice from sugar factory using waste powdered carbon. Appl. Water Sci. 2018, 8, 1–10.
- Trache, D.; Thakur, V.K.; Boukherroub, R. Cellulose nanocrystals/graphene hybrids—A promising new class of materials for advanced applications. Nanomaterials 2020, 10, 1523.
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250.
- Lee, K.-C.; Lo, P.-Y.; Lee, G.-Y.; Zheng, J.-H.; Cho, E.-C. Carboxylated carbon nanomaterials in cell cycle and apoptotic cell death regulation. J. Biotechnol. 2019, 296, 14–21.
- Thulasi, S.; Kathiravan, A.; Asha Jhonsi, M. Fluorescent carbon dots derived from vehicle exhaust soot and sensing of tartrazine in soft drinks. ACS Omega 2020, 5, 7025–7031.
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205.
- Lv, K.; Suo, W.; Shao, M.; Zhu, Y.; Wang, X.; Feng, J.; Fang, M. Nitrogen doped MoS2 and nitrogen doped carbon dots composite catalyst for electroreduction CO2 to CO with high Faradaic efficiency. Nano Energy 2019, 63, 103834.
- Hu, M.; Li, M.; Qiu, J.; Sun, Y.-P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337.
- Cai, H.; Ma, J.; Xu, X.; Chu, H.; Zhang, D.; Li, J. Sulfonated glycosaminoglycan bioinspired carbon dots for effective cellular labelling and promotion on the differentiation of mesenchymal stem cells. J. Mater. Chem. B. 2020, 8, 5655–5666.
- Zhao, J.; Li, F.; Zhang, S.; An, Y.; Sun, S. Preparation of N-doped yellow carbon dots and N, P co-doped red carbon dots for bioimaging and photodynamic therapy of tumors. New J. Chem. 2019, 43, 6332–6342.
- Wang, J.; Wei, J.; Su, S.; Qiu, J. Novel fluorescence resonance energy transfer optical sensors for vitamin B 12 detection using thermally reduced carbon dots. New J. Chem. 2015, 39, 501–507.
- Shi, Y.; Liu, X.; Wang, M.; Huang, J.; Jiang, X.; Pang, J.; Xu, F.; Zhang, X. Synthesis of N-doped carbon quantum dots from bio-waste lignin for selective irons detection and cellular imaging. Int. J. Biol. Macromol. 2019, 128, 537–545.
- Liu, P.; Zhang, X.; Zhai, F.; Tian, F.; Li, W.; Yang, J.; Liu, Y.; Wang, H.; Wang, W.; Liu, W. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613.
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429.
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542.
- Hang, D.-R.; Pan, Y.-Q.; Sharma, K.H.; Chou, M.; Islam, S.E.; Wu, H.-F.; Liang, C.-T. 2D CTAB-MoSe2 nanosheets and 0D MoSe2 quantum dots: Facile top-down preparations and their peroxidase-like catalytic activity for colorimetric detection of hydrogen peroxide. Nanomaterials 2020, 10, 2045.
- Ming, H.; Ma, Z.; Liu, Y.; Pan, K.; Yu, H.; Wang, F.; Kang, Z. Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property. Dalt. Trans. 2012, 41, 9526–9531.
- Zhang, M.; Zhao, X.; Fang, Z.; Niu, Y.; Lou, J.; Wu, Y.; Zou, S.; Xia, S.; Sun, M.; Du, F. Fabrication of HA/PEI-functionalized carbon dots for tumor targeting, intracellular imaging and gene delivery. RSC Adv. 2017, 7, 3369–3375.
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano. 2012, 6, 5102–5110.
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92.
- Ma, X.; Li, S.; Hessel, V.; Lin, L.; Meskers, S.; Gallucci, F. Synthesis of luminescent carbon quantum dots by microplasma process. Chem. Eng. Process. Intensif. 2019, 140, 29–35.
- Kaciulis, S.; Mezzi, A.; Soltani, P.; Pizzoferrato, R.; Ciotta, E.; Prosposito, P. Graphene quantum dots obtained by unfolding fullerene. Thin. Solid Films 2019, 673, 19–25.
- Khan, Z.M.S.H.; Saifi, S.; Aslam, Z.; Khan, S.A.; Zulfequar, M. A facile one step hydrothermal synthesis of carbon quantum dots for label-free fluorescence sensing approach to detect picric acid in aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112201.
- Vinci, J.C.; Ferrer, I.M.; Seedhouse, S.J.; Bourdon, A.K.; Reynard, J.M.; Foster, B.A.; Bright, F.V.; Coloón, L.A. Hidden properties of carbon dots revealed after HPLC fractionation. J. Phys. Chem. Lett. 2013, 4, 239–243.
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chemie 2007, 119, 6593–6595.
- Tao, H.; Yang, K.; Ma, Z.; Wan, J.; Zhang, Y.; Kang, Z.; Liu, Z. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 2012, 8, 281–290.
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67.
- Tan, Q.; Kong, X.; Guan, X.; Wang, C.; Xu, B. Crystallization of zinc oxide quantum dots on graphene sheets as an anode material for lithium ion batteries. Cryst. Eng. Comm. 2020, 22, 320–329.
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748.
- Guo, L.; Li, L.; Liu, M.; Wan, Q.; Tian, J.; Huang, Q.; Wen, Y.; Liang, S.; Zhang, X.; Wei, Y. Bottom-up preparation of nitrogen doped carbon quantum dots with green emission under microwave-assisted hydrothermal treatment and their biological imaging. Mater. Sci. Eng. C. 2018, 84, 60–66.
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 1–25.
- Gai, W.; Zhao, D.L.; Chung, T.-S. Thin film nanocomposite hollow fiber membranes comprising Na+-functionalized carbon quantum dots for brackish water desalination. Water Res. 2019, 154, 54–61.
- Wang, X.; Hao, J.; Cheng, J.; Li, J.; Miao, J.; Li, R.; Li, Y.; Li, J.; Liu, Y.; Zhu, X. Chiral CdSe nanoplatelets as an ultrasensitive probe for lead ion sensing. Nanoscale 2019, 11, 9327–9334.
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19.
- Dong, Y.; Zhou, N.; Lin, X.; Lin, J.; Chi, Y.; Chen, G. Extraction of electrochemiluminescent oxidized carbon quantum dots from activated carbon. Chem. Mater. 2010, 22, 5895–5899.
- Hou, J.; Dong, J.; Zhu, H.; Teng, X.; Ai, S.; Mang, M. A simple and sensitive fluorescent sensor for methyl parathion based on l-tyrosine methyl ester functionalized carbon dots. Biosens. Bioelectron. 2015, 68, 20–26.
- Zou, S.; Hou, C.; Fa, L.; Zhang, Y.; Ma, L.; Dong, D.; Li, D.; Huo, D.; Yang, M. An efficient fluorescent probe for fluazinam using N, S co-doped carbon dots from L-cysteine. Sensors Actuators B Chem. 2017, 239, 1033–1041.
- Lu, Y.-C.; Chen, J.; Wang, A.-J.; Bao, N.; Feng, J.-J.; Wang, W.; Shao, L. Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury (II) detection and bioimaging. J. Mater. Chem. C. 2015, 3, 73–78.
- Gu, C.; Guo, Z.; Li, Z.; Wang, M.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic ZrHf-based metal-organic framework embedded with carbon dots: Ultra-sensitive platform for early diagnosis of HER2 and HER2-overexpressed living cancer cells. Biosens. Bioelectron. 2019, 134, 8–15.
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104.
- Yuan, X.; Liu, Z.; Guo, Z.; Ji, Y.; Jin, M.; Wang, X. Cellular distribution and cytotoxicity of graphene quantum dots with different functional groups. Nanoscale Res. Lett. 2014, 9, 1–9.
- Zhou, Y.; Sun, H.; Wang, F.; Ren, J.; Qu, X. How functional groups influence the ROS generation and cytotoxicity of graphene quantum dots. Chem. Commun. 2017, 53, 10588–10591.
- Al Jahdaly, B.A.; Al-Radadi, N.S.; Eldin, G.M.G.; Almahri, A.; Ahmed, M.K.; Shoueir, K.; Janowska, I. Selenium nanoparticles synthesized using an eco-friendly method: Dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol. 2021, 11, 85–97.
- El-Naggar, M.E.; Wassel, A.R.; Shoueir, K. Visible-light driven photocatalytic effectiveness for solid-state synthesis of ZnO/natural clay/TiO2 nanoarchitectures towards complete decolorization of MB from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100425.
- El-Desouky, N.; Shoueir, K.R.; El-Mehasseb, I.; El-Kemary, M. Bio-inspired green manufacturing of plasmonic silver nanoparticles/degussa using banana waste peduncles: Photocatalytic, antimicrobial, and cytotoxicity evaluation. J. Mater. Res. Technol. 2020, 10, 671–686.
- Teaima, M.H.; Elasaly, M.K.; Omar, S.A.; El-Nabarawi, M.A.; Shoueir, K.R. Eco-friendly synthesis of functionalized chitosan-based nanoantibiotic system for potential delivery of linezolid as antimicrobial agents. Saudi Pharm. J. 2020, 28, 859–868.
- Shoueir, K.R. Green microwave synthesis of functionalized chitosan with robust adsorption capacities for Cr (VI) and/or RHB in complex aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 1–12.
- Fang, L.; Zhang, L.; Chen, Z.; Zhu, C.; Liu, J.; Zheng, J. Ammonium citrate derived carbon quantum dot as on-off-on fluorescent sensor for detection of chromium (VI) and sulfites. Mater. Lett. 2017, 191, 1–4.
- Wang, B.; Tang, W.; Lu, H.; Huang, Z. Ionic liquid capped carbon dots as a high-performance friction-reducing and antiwear additive for poly (ethylene glycol). J. Mater. Chem. A. 2016, 4, 7257–7265.
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; von Seckendorff, M.; Susha, A.S.; Kasaók, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C. 2017, 121, 2014–2022.
- Vedamalai, M.; Periasamy, A.P.; Wang, C.-W.; Tseng, Y.-T.; Ho, L.-C.; Shih, C.-C.; Chang, H.-T. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu 2+ ions in cells. Nanoscale 2014, 6, 13119–13125.
- Joseph, J.; Anappara, A.A. White-light-emitting carbon dots prepared by the electrochemical exfoliation of graphite. Chem. Phys. Chem. 2017, 18, 292–298.
- Shinde, D.B.; Pillai, V.K. Electrochemical preparation of luminescent graphene quantum dots from multiwalled carbon nanotubes. Chem. Eur. J. 2012, 18, 12522–12528.
- Yan, X.; Li, B.; Li, L. Colloidal graphene quantum dots with well-defined structures. Acc. Chem. Res. 2013, 46, 2254–2262.
- Li, Q.; Noffke, B.W.; Liu, Y.; Li, L.S. Understanding fundamental processes in carbon materials with well-defined colloidal graphene quantum dots. Curr. Opin. Colloid Interface Sci. 2015, 20, 346–353.
- El-Shabasy, R.; Yosri, N.; El-Seedi, H.; Shoueir, K.; El-Kemary, M. A green synthetic approach using chili plant supported Ag/ P25 heterostructure with enhanced photocatalytic properties under solar irradiation. Optik 2019, 192, 162943.
- Sharma, V.; Orejon, D.; Takata, Y.; Krishnan, V.; Harish, S. Gladiolus dalenii based bioinspired structured surface via soft lithography and its application in water vapor condensation and fog harvesting. ACS Sustain. Chem. Eng. 2018, 6, 6981–6993.
- Mehta, V.N.; Jha, S.; Basu, H.; Singhal, R.K.; Kailasa, S.K. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens. Actuators B Chem. 2015, 2013, 434–443.
- Wei, J.; Zhang, X.; Sheng, Y.; Shen, J.; Huang, P.; Guo, S.; Pan, J.; Liu, B.; Feng, B. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J. Chem. 2014, 385, 906–909.
- Wang, L.; Zhou, H.S. Green synthesis of luminescent nitrogen-doped carbon dots from milk and its imaging application. Anal. Chem. 2014, 86, 8902–8905.
- Atchudan, R.; Edison, T.N.J.I.; Shanmugam, M.; Perumal, S.; Somanathan, T.; Lee, Y.R. Sustainable synthesis of carbon quantum dots from banana peel waste using hydrothermal process for in vivo bioimaging. Phys. E Low. Dimens. Syst. Nanostruct. 2020, 126, 114417.
- Da Silva Júnior, A.H.; Macuvele, D.L.P.; Riella, H.G.; Soares, C.; Padoin, N. Novel carbon dots for zinc sensing from Campomanesia phaea. Mater. Lett. 2021, 283, 128813.
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730.
- Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Facile synthesis of surface-modified carbon quantum dots (CQDs) for biosensing and bioimaging. Materials 2020, 13, 3313.
- Bhamore, J.R.; Jha, S.; Park, T.J.; Kailasa, S.K. Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells. J. Photochem. Photobiol. B Biol. 2019, 191, 150–155.
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40.
- Hoan, B.T.; Tam, P.D.; Pham, V.-H. Green synthesis of highly luminescent carbon quantum dots from lemon juice. J. Nanotechnol. 2019, 2019.
- Lai, Z.; Guo, X.; Cheng, Z.; Ruan, G.; Du, F. Green synthesis of fluorescent carbon dots from cherry tomatoes for highly effective detection of trifluralin herbicide in soil samples. Chem. Select. 2020, 5, 1956–1960.
- Pajewska-Szmyt, M.; Buszewski, B.; Gadzała-Kopciuch, R. Sulphur and nitrogen doped carbon dots synthesis by microwave assisted method as quantitative analytical nano-tool for mercury ion sensing. Mater. Chem. Phys. 2020, 242, 122484.
- Eskalen, H.; Uruş, S.; Cömertpay, S.; Kurt, A.H.; Özgan, Ş. Microwave-assisted ultra-fast synthesis of carbon quantum dots from linter: Fluorescence cancer imaging and human cell growth inhibition properties. Ind. Crops Prod. 2020, 147, 112209.
- Tadesse, M.; Hagos, D.; RamaDevi, K.; Basavaiah, N.; Belachew, N. Fluorescent-nitrogen-doped carbon quantum dots derived from citrus lemon juice: Green synthesis, mercury (II) ion sensing, and live cell imaging. ACS Omega 2020, 5, 3889–3898.
- Başoğlu, Ü.; Ocak, A.; Gümrükçüoğlu, A. Synthesis of microwave-assisted fluorescence carbon quantum dots using roasted–chickpeas and its applications for sensitive and selective detection of Fe3+ ions. J. Fluoresc. 2020, 30, 1–12.
- Wesoły, M.; Cetó, X.; Del Valle, M.; Ciosek, P.; Wróblewski, W. Quantitative analysis of active pharmaceutical ingredients (APIs) using a potentiometric electronic tongue in a SIA flow system. Electroanalysis 2016, 28, 626–632.
- Murugan, N.; Prakash, M.; Jayakumar, M.; Sundaramurthy, A.; Sundramoorthy, A.K. Green synthesis of fluorescent carbon quantum dots from Eleusine coracana and their application as a fluorescence ‘turn-off’ sensor probe for selective detection of Cu 2+. Appl. Surf. Sci. 2019, 476, 468–480.
- Thomas, K.V.; Bijlsma, L.; Castiglioni, S.; Covaci, A.; Emke, E.; Grabic, R.; Hernández, F.; Karolak, S.; Kasprzyk-Hordern, B.; Lindberg, R.H. Comparing illicit drug use in 19 European cities through sewage analysis. Sci. Total Environ. 2012, 432, 432–439.




