Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Industrial
Carbon dots have gained tremendous interest attributable to their unique features. Two approaches are involved in the fabrication of quantum dots (Top-down and Bottom-up). Most of the synthesis methods are usually multistep, required harsh conditions, and costly carbon sources that may have a toxic effect, therefore green synthesis is more preferable. Herein, the current review presents the green synthesis of carbon quantum dots (CQDs) and graphene quantum dots
- carbon dots
- optical properties
1. Introduction
In recent years, carbonaceous and carbon-based nanomaterials have gained great attention owing to their relevant properties [1,2,3,4]. In particular, these substances are characterized by high biocompatibility, less toxicity, significant thermal and mechanical features, and can functionalize easily [5,6,7,8]. Fluorescent carbons are commonly known as carbon dots because of their unique properties that revealed strong fluorescence [9]. In addition, carbon dots are distinguished by high stability, reducing toxic activity, water solubility, and derivatization availability. All of these unique features support their applications in several disciplines as shown in Figure 1 [10,11,12,13,14]. Carbon dots are relatively new and considered one of the most promising nanomaterials ever recognized to humanity, mainly composed of the heteroatoms (functional groups) attached with carbonized core [15]. Carbon dots including different types of nanomaterials such as polymer dots, carbon nanodots, and graphene quantum dots (GQDs). It defined as nanoparticles with small size (<10 nm) that consist of sp2 hybrid conjugated of carbon core-shell between carbon (core) and organic functional groups (shell) such as N–H,–OH,–C = O, COOH, C−O, and C–N or polymer aggregates [16]. Several studies have reported that different techniques and carbon sources are employed in the fabrication of carbon dots with different structures [17].
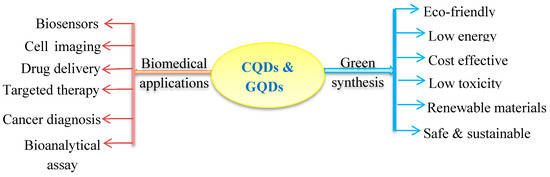
Figure 1. Chart of green synthetic methods of quantum dots (carbon quantum dots (CQDs) and graphene quantum dots (GQDs)) and their biomedical applications.
Typically, two techniques are commonly used in the formation of carbon dots; top-down and bottom-up as described in Figure 2 [18,19]. Usually in the first process “top-down” carbon dots are fabricated by chemical and physical cutting approaches; laser ablation/passivation [20], chemical oxidation, and electrochemical synthesis [21]. In the second method, “bottom-up” carbon dots are converted from appropriate molecular precursors with specific conditions represented by combustion, hydrothermal and thermal [22], and ultrasonic irradiation [23] in which the conditions required fewer amount of carbon sources. It is noteworthy to mention that bottom-up strategy is more preferable to top-down because some limitations are related to this technique including the high cost of the required materials, long time, and harsh conditions [24]. Further, the fabrication of carbon dots via top-down approaches usually needs a separate step for functionalization and passivation of the surface but the second method” bottom-up” does not require that [15]. In addition, different more approaches have been reported such as plasma treatment [25], cage-opening of fullerenes [26], and solution chemistry approaches [27]. The formed carbon dots, nevertheless, of its production methods, have different sizes that required complicated separation technique to get a mono-dispersed carbon dot. There are several post-synthesis separation processes such as chromatography [28], dialysis [29], and gel electrophoresis [30]. On the other hand, the characterized composition of carbon dots gave it significant points for several applications like bioimaging, label-free detection, photocatalysis, and sensing. The current review discussed synthetic approaches for the fabrication of carbon dots and the most significant properties. The biotechnological and biomedical applications are also highlighted.
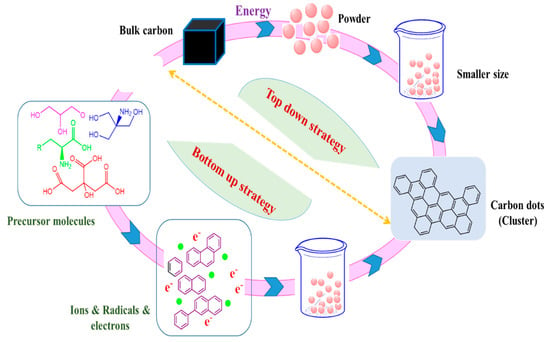
Figure 2. Generation of carbon dots by well-known Top-down and bottom-up approaches.
2. Unique Features of Quantum Dots (CQDs and GQDs)
Carbon dots are considered one of the recently discovered materials having promising and unique properties [31]. The chemical composition of carbon dots containing several function groups on the surface such as amino groups, oxygen, and polymer chains is highly supported by their remarkable features. These functionals have a significant effect on photoluminescence activity and also enhanced the energy gap and energy level of the surface [32]. Such substances have gained great attention because of their significant tunable optical properties, less toxic, simplicity, and low cost, which support them as perfect candidates for use in optical sensors [33]. The aptness of emission of light through carbon dots near the Infra-red area is of particular prominence because the light in this region has deeper tissue penetration proficiency and biological systems are transparent to these wavelengths [34]. Typically, CQDs and GQDs exhibit effectiveness in the short-wavelength area for photon-harvesting that caused by π–π* transition of C = C bonds and n-π* transitions of the groups; C–N, C = O, and C−S for example. Significant optical absorption was demonstrated in the ultraviolet region expanded to the visible range. The region between 230 and 270 nm appeared absorption owing to π–π* transition related to C = C bonds, while the peak shoulder in the range of 300–390 nm is attributed to n–π* transition of C = O bonds [35]. The absorbance can be modified by different types of surface passivation and functionalization methods [36]. For example, multimode emissive carbon dots with high fluorescent were prepared using D-cysteine and l-cysteine. Two absorption bands appeared at the same time related to L-carbon dots at 243 and 300 nm with the low band at 400 nm. The absorbance was displayed due to π-π* transition of the aromatic sp2 domains (243 nm) and n-π* transition of C = O, C–N, C–S (300 nm). However, D-cysteine was not showed any band above 240 nm [37]. The results reported that several function groups (e.g., NH2 and COOH) were found on the surface of L-carbon dots and hence the band gap increased due to the surface interfacial excitation. In addition, Lin et al. have recently investigated the synthesis of other carbon dots from poly (vinyl alcohol) and phenylenediamine. The formed composite exposed two different bands at 247 and 355 nm, matching to π–π* transition of C = C bonds and n-π* transition of C–N, C = N, respectively [38]. Commonly, CQDs have been evaluated successfully in surface passivation as they have the ability for improving brightness because of long wavelengths and decreasing quantum yield. On the opposite, the quantum yield of graphene dots was more than carbon quantum dots because their structures appeared as layers and crystalline phases [32]. The color of the fabricated carbon dots was changed between red, green, and blue. It was not recommended for multi-color imaging, due to the differences in chemical composition, size, and increasing heterogeneity of carbon dots. Most of these particles appeared wide emission spectra originating from difficulty controlling the synthesis processes. Interestingly, carbon dots have several attractive optical properties, but photoluminescence is the most significant one, including phosphorescence and fluorescence. The property of electrochemiluminescence plays an important role in surface passivation, whereas CQDs that passivated have a strong fluorescence and weak electrochemiluminescence [39]. For example, methyl parathion sensors were fabricated by the hydrothermal reaction between tyrosine methyl ester and carbon dots with citric acid employed as a resource of carbon. These types of sensors revealed high and stable photoluminescence and the yield of quantum was approximately 3.8%. This could be successfully developed to determine organophosphorus compound [40].
In addition, most studies revealed that carbon dots have excitation-dependent fluorescence features, although, the excitation-independent emission in S, N-co-doped carbon dots have been investigated [41]. For instance, excitation-independent carbon dots with tunable fluorescent colors have been synthesized through a well-controlled wet oxidative process whereas the results displayed that the photoluminescent properties of carbon dots were principally detected by surface oxidation degree and their molecular weight [42]. The fluorescent carbon dots having fluorescence wavelength can be tuned across the visible spectrum with varying the passivation or functionalization substances, the molar mass ratio of the precursors, and the different synthetic factors. The emission of CQDs can be also influenced by an assortment of adaptable solvents. Subsequently, the performance of excitation dependent/independent photoluminescence is mostly originating from the surface states of carbon dots [43]. It is worth mentioning that the emission mechanism of carbon dots is still unclear. Currently, some expected theoretical explanations may be acceptable including surface state electron-hole radiation rearrangement, quantum size effect, and molecular state luminescence emission mechanism [44]. Consequently, the preparation of monochromatic fluorescent carbon dots and the study of the fluorescence mechanism is an imperative research area for developing the applicability of carbon dots.
On the other hand, biocompatibility is one of the most important features that showed a considerable influence on the application of carbon dots particularly in bio-imaging and cellular imaging [33]. GQDs having an excess of oxygen groups which showed high biocompatibility, low toxicity and enhanced for use in radiotherapy [45,46]. The cytotoxic effect of GQDs was caused by reactive oxygen species generated from the function groups. For example, the in vivo studies of GQDs exhibit low toxicity, no accumulation in the basic organs, and the kidney can dispose of it quickly. By investigation, it has appeared that the mice were not affected by injection with GQDs whereas the graphene oxide showed toxic activity until its death. This happens because graphene oxide can aggregate in the organs.
3. Fundamental Approaches of Carbon Dots Fabrication (Green Synthesis)
Green synthesis is Avery important topic matching with sustainability in our daily life [47,48,49,50,51]. Relevant studies have reported that small organic precursors can be polymerized and carbonized for the synthesis of carbon dots such as ammonium citrate [52], ethylene glycol [53], citric acid [54], phenylenediamine [55], graphite [56], and carbon nanotube [57]. To make them potential fluorescent materials with unique surface functionalities, two main approaches are widely investigated for the generation of ultra-small fluorescent carbon dots. Among these synthetic approaches, the colloidal synthetic methodology has received significant interest due to the generation of large quantities with a tightly controlled size of carbon dots [58]. For example, GQDs were fabricated from small aromatic molecules by stepwise solution chemistry and characterized by significant size uniformity and well-defined structures as presented in Figure 3 [59]. The structure controlling could be enhanced through the covalently bonding between 2′,4′,6′-trialkyl-substituted phenyl moieties (at the 1′-position) to the edges of graphene. The peripheral phenyl groups twisted from the plane of the graphene due to the crowding on the edges, and then the alkyl chains forming a three-dimensional cage around it (Figure 3). This action caused increasing distance between the conjugated systems in all three dimensions and consequently critically decreases the intermolecular π–π* attraction. The well-defined colloidal quantum dots have some unique characteristics that make them excellent model systems for studying fundamental processes in complex carbon materials.
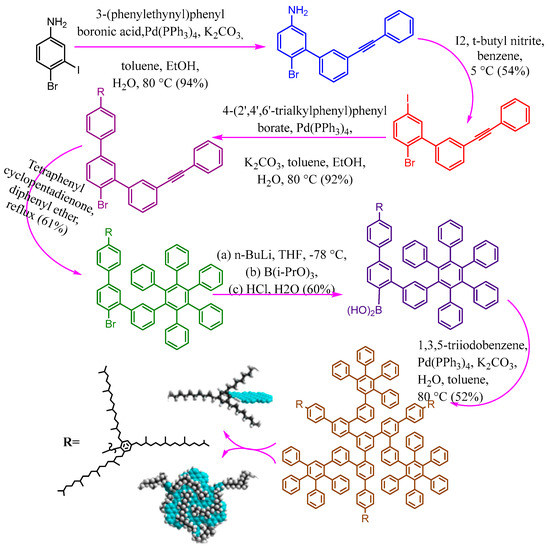
Figure 3. The synthetic procedures of colloidal GQDs with well-defined structures.
Green chemistry is one of the fundamental branches of chemistry that provide golden solves for most problems with significant properties. Green chemistry has several advantages including, safe, environmentally friendly, no need for hazardous materials, and can occur under normal conditions [60]. Green chemical procedures have been engaged in the formation of carbon dots from several natural sources counting chicken eggs, animals [61], different plant species including fruits and vegetables [62], and waste materials like waste paper and frying oil [63]. There is an exponential increase in the number of research articles with both carbon quantum dots and green synthesis content. The fabrication processes can be achieved by different types of methods including hydrothermal/solvothermal, microwave-assisted polymerization, pyrolysis, and carbonization [64]. These approaches are widely used in the synthesis of carbon dots and having several advantages as displayed in Figure 4. In Table 1, conventional CQDs production methods, and maximum emission wavelength, quantum yield and reported CQDs dimensions of CQDs produced by these methods were compared.
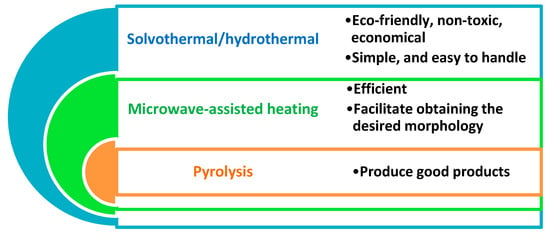
Figure 4. Advantages of different current routes used for the preparation of carbon dots.
Table 1. Recent green quantum dots produced by the bottom-up approach (Hydrothermal, microwave-assisted, and pyrolysis) and their applications.
| Synthetic Approach |
Source | Quantum Yield (%) | Size Range (nm) | λem Max | Application | Ref. |
|---|---|---|---|---|---|---|
| Hydrothermal | Banana peel waste | 5 | 4–6 | 355–429 | Bio imaging | [65] |
| Hydrothermal | Cambuci juice (Campomanesia phaea) | 21.3 | 3.7 | 270, 283 | Sensing of Zn2+ | [66] |
| Hydrothermal | Biomass waste | 4.3–8.2 | 1.3 and 4.9 | 445, 435, 43, 435 | Detection of Fe3+ | [67] |
| Hydrothermal | Biomass waste | 14–3.5 | 6 | 205, 260 | Bio imaging | [68] |
| Hydrothermal | Manilkara zapota fruits | 5.7, 7.9, 5.2 | 1.9 ± 0.3, 2.9 ± 0.7, 4.5 ± 1.25 | 405, 488, 561 | Bio imaging | [69] |
| Hydrothermal | Broccoli | - | 2–6 | 330–470 | Ag+ sensing | [70] |
| Hydrothermal | Lemon juice | 79 | 4.5 | 540 | Biosensors | [71] |
| Hydrothermal | Cherry tomatoes | 9.7 | 7 | 430 | Biosensors | [72] |
| Microwave-assisted | ND | 26 | ~10 | ND | sensor of Hg2+ detection | [73] |
| Microwave-assisted | Cotton linter waste | ND | 10.1 | 420 | Bioimaging | [74] |
| Microwave-assisted | Quince fruit | 8.6 | 4.9 | 450 | Bioimaging | [75] |
| Microwave-assisted | Roasted–Chickpeas | 1.8 | 4.5–10.3 | 435 | Detection of Fe3+ | [76] |
| Pyrolysis | Chia seeds | ND | 4 | ND | Sensors | [77] |
| Pyrolysis | Finger millet ragi | ND | 6 | ND | Biosensor | [78] |
| Pyrolysis | Mango | 18.2 | 6 | 525 | Biosensor | [79] |
ND: Not defined.
This entry is adapted from the peer-reviewed paper 10.3390/pr9020388
This entry is offline, you can click here to edit this entry!
