| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mosab Kaseem | + 2903 word(s) | 2903 | 2021-02-22 07:16:56 | | | |
| 2 | Rita Xu | -1614 word(s) | 1289 | 2021-03-02 02:28:08 | | |
Video Upload Options
This entry presents an overview of the recent developments in the synthesis of layered double hydroxide (LDH) on the anodized films of Mg alloys prepared by either conventional anodizing or plasma electrolytic oxidation (PEO) and the applications of the formed composite ceramics as smart chloride traps in corrosive environments.
1. Introduction
Material degradation, specifically corrosion, is a serious issue limiting the use of active metals like magnesium in advanced applications [1]. Mg and its alloys have high specific strengths by which they can replace heavy metals in different technological sectors [1]. Therefore, it is highly important to improve the electrochemical stability of these materials in corrosive environments to extend their applications. To date, several methods, such as sol-gel coating, chemical vapor deposition, anodizing, and plasma electrolytic oxidation (PEO) have been utilized to enhance the protective properties of light metals and their alloys [2][3][4][5][6][7][8][9][10]. Among them, the anodizing method discovered in 1923 has been used extensively to form thin protective anodic films [7]. As an updated version of anodizing, PEO is an emerging method because of its unique plasma-in-water system. Typically, PEO transforms metal surfaces into a robust layer of their corresponding oxides using numerous micro-sized plasma discharges, which are generated as the result of electrical breakdown events at high overvoltages [7]. These micro-sized plasma discharges induce a high-temperature environment (T > ~3500 K) that is above the melting point of most metals and oxides [11], leading to a dynamic surface topography comprising micropores due to local and repetitive melting-solidification cycles. However, due to the thin layer and/or the porous structure of the anodic films produced via anodizing and PEO, the corrosive species would reach the metallic substrate, leading to its corrosion in extreme environments [7]. An additional treatment, therefore, must be applied to the anodic films towards achieving higher electrochemical stability for large-scale applications [7][12]. Several research groups have used several approaches, such as the typical sealing by boiling water [13], post-treatment using polymers or organic compounds [7][14], post-treatment by sol-gel coatings [15] in order to enhance the stability of anodic films of Mg alloys. However, the boiling water approach would not be desirable on account of the slight improvements in the protective properties as well as the high energy consumption associated with this method [7]. Moreover, the application of polymers and organic compounds to seal the anodic films would be limited due to the susceptibly of these materials to degradation at elevated temperatures. Also, the use of sol-gel coatings led to the formation of many cracks as a result of mismatching between the metal oxides incorporated by the sol-gel approach and MgO which is known to be the main component of the anodic films produced on Mg alloy via anodizing and PEO [7].
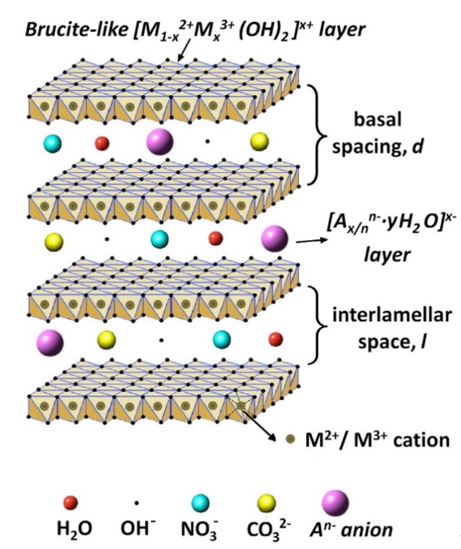
In addition to the approaches described above, layered double hydroxides (LDHs) can provide another approach to improve the protective properties by increasing the barrier properties [16][17][18][19][20][21]. LDHs are lamellar crystals with positively charged brucite-like host layers with interlayer regions containing charge-compensating anions and solvation molecules [22][23]. The typical formula of these materials can be described as [M1−x2+Mx3+(OH)2] (An−)x/n·mH2O, where M2+ and M3+ are the divalent and trivalent cations, respectively, while, An− is the interlayer anion (Figure 1) [18]. Such inorganic nano-containers have been widely proposed to improve the corrosion resistance of Mg and its alloys on account of their merits, such as small size, high loading capacity, and simple modification [24][25][26][27][28][29][30][31][32][33][34][35]. Moreover, such materials have an excellent anion-exchange capability by the simultaneous release of interlayer anions and the adsorption of aggressive species from the corrosive environment. Therefore, LDH-based protective films can be considered as smart coatings, meaning that they can control the liberation of corrosion inhibitors and improve the long-term corrosion performance.
Figure 1. The general crystal structure of layered double hydroxide (LDH) film. Reprinted with permission from ref. [18]. Elsevier 2019.
Anodization of the Mg alloys surface results in the formation of magnesium oxide (MgO) that acts as the major source of Mg2+ for the dense growth of LDHs, while LDH precursors are found to seal the anodic surface and can provide better active and passive corrosion protection with long-term stability. Moreover, the LDH films made on the anodic coating of Mg alloys can also increase the thickness of the protective film and, therefore, such factors could effectively stop corrosive species from reaching the metallic substrate. Thus, a review on the evolution of LDH materials made on the anodic films of Mg alloys focusing in depth on corrosion performance by covering the recent evaluation perspectives, trends in the synthesis methods, a deep insight into the mechanism, and the structure–corrosion correlation is urgently required. To the best of our knowledge, a review discussing the aforementioned aspects has not been undertaken.
2. Synthesis of LDH Films on Anodized Mg Alloys
To date, several methods have been utilized to produce LDHs on the Al, Mg, and Ti alloy substrates, such as the co-deposition method [36][37], hydrothermal process [38][39], steam coatings method [40][41], electrodeposition [42], etc. These techniques were highlighted recently by Tabish et al. [43] and Guo et al. [44]. However, the methods that are usually employed to fabricate LDH films on the anodic films of Mg alloys are hydrothermal treatment and the co-precipitation method or a combination of both methods. Additional procedures, such as anion exchange reaction, and LDH reconstruction can be used to modify the LDH films to improve the protective properties of the LDH-based composites [43].
2.1. Co-Precipitation Method
Generally, LDHs can be fabricated by immersion of the anodic films of Mg alloys in a solution containing a selected ratio of divalent and trivalent metallic salts in the presence of the desired interlayer anion. Based on the type of metallic ions, the pH of the reaction medium during the synthesis process is usually controlled to be in the range of 7–11. However, several problems, such as the weak adhesion strength between the LDH film and the underlying substrate, complexity, time-consuming, low crystallization, and formation of large amounts of waste would be the main drawbacks of the co-precipitation method [43].
2.2. In Situ Hydrothermal Treatment
This feasible method has been employed in many studies to prepare homogenous LDH films on the anodic films of Mg alloys. Briefly, LDHs film can be obtained by immersing the anodic film in an aqueous solution containing NO3− anions followed by hydrothermal treatment in a Teflon-lined autoclave at temperatures over 383 K. It is important to point out that autoclave conditions would limit the industrial applications of these materials, in particular the transport applications. Moreover, it is worth mentioning that the absence of autoclave conditions leads to the development of LDH films in carbonated electrolytes and the CO2-containing environment owing to the high sorption ability of LDH towards CO2 [45][46][47][48]. This led to the formation of so-called “dead” LDH film in which the intercalation of corrosion inhibitors became very difficult owing to the high charge density of CO32− anions, reducing the smart protection property of the film [49].
2.3. Anion Exchange
The LDH films are usually subjected to anion-exchange reactions to intercalate new anions into the gallery of LDH films. Therefore, it can be considered as an indirect approach to modify the structure and composition of LDH films. Anions of corrosion inhibitors, such as vanadate (VO3−) [50], and molybdate (MoO42−) [51] are usually intercalated into the LDH films formed on the anodic films of Mg alloys. The LDH films intercalated with corrosion inhibitors would have a dual function: (i) entrapment of corrosive species and (ii) a controlled liberation of corrosion inhibitors. To sum up, although significant advances are achieved in the fabrication of LDH/anodic film composites of Mg alloys, two main challenges should be considered. First, how to increase the low adhesion strength between LDH coating and anodic films. Second, the formation of LDH films that occurs usually under autoclave conditions would significantly limit the industrial applications of these materials.
References
- Esmaily, M.; Svensson, J.; Fajardo, S.; Birbilis, N.; Frankel, G.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L. Fundamentals and advances in magnesium alloy corrosion. Mater. Sci. 2017, 89, 92–193, doi:10.1016/j.pmatsci.2017.04.011.
- Balaji, J.; Roh, S.H.; Edison, T.N.J.I.; Jung, H.Y.; Sethuraman, M.G. Sol-gel based hybrid silane coatings for enhanced corrosion protection of copper in aqueous sodium chloride. Mol. Liq. 2020, 302, 112551.
- Kuznetsov, B.; Serdechnova, M.; Tedim, J.; Starykevich, M.; Kallip, S.; Oliveira, M.P.; Hack, T.; Nixon, S.; Ferreira, M.G.S.; Zheludkevich, M.L. Sealing of tartaric sulfuric (TSA) anodized AA2024 with nanostructured LDH layers. Rsc Adv. 2016, 6, 13942–13952.
- Li, Y.; Li, S.; Zhang, Y.; Yu, M.; Liu, J. Enhanced protective Zn–Al layered double hydroxide film fabricated on anodized 2198 aluminum alloy. Alloy. Compd. 2015, 630, 29–36, doi:10.1016/j.jallcom.2014.12.176.
- Hussain, T.; Kaseem, M.; Ko, Y.G. Hard acid–hard base interactions responsible for densification of alumina layer for superior electrochemical performance. Sci. 2020, 170, 108663, doi:10.1016/j.corsci.2020.108663.
- Kaseem, M.; Choe, H.C. Triggering the hydroxyapatite deposition on the surface of PEO-coated Ti-6Al-4V alloy via the dual incorporation of Zn and Mg ions. Alloy. Compd. 2020, 819, 153038.
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Mater. Sci. 2020, 100735, doi:10.1016/j.pmatsci.2020.100735.
- Kaseem, M.; Kamil, M.; Ko, Y. Electrochemical response of MoO2-Al2O3 oxide films via plasma electrolytic oxidation. Coatings Technol. 2017, 322, 163–173, doi:10.1016/j.surfcoat.2017.05.051.
- Kaseem, M.; Ko, Y.G. Morphological modification and corrosion response of MgO and Mg3(PO4)2 composite formed on magnesium alloy. Part B Eng. 2019, 176, 107225, doi:10.1016/j.compositesb.2019.107225.
- Cui, L.-Y.; Zeng, R.-C.; Guan, S.-K.; Qi, W.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Degradation mechanism of micro-arc oxidation coatings on biodegradable Mg-Ca alloys: The influence of porosity. Alloy. Compd. 2017, 695, 2464–2476, doi:10.1016/j.jallcom.2016.11.146.
- Stojadinović, S.; Vasilić, R.; Perić, M. Investigation of plasma electrolytic oxidation on valve metals by means of molecular spectroscopy—A review. RSC Adv. 2014, 4, 25759–
- Narayanan, T.; Song, P.; Lee, M. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Mater. Sci. 2014, 60, 1–71.
- Kaseem, M.; Kim, M.J.; Ko, Y.G. Hydration-dehydration behavior induced densification of porous plasma electrolysis coating. Alloy. Compd. 2019, 798, 220–226, doi:10.1016/j.jallcom.2019.05.242.
- Narayanan, T.S.N.S.; Lee, M.H. A simple strategy to modify the porous structure of plasma electrolytic oxidation coatings on magnesium. RSC Adv. 2016, 6, 16100–16114, doi:10.1039/c5ra20647b.
- Li, N.; Chen, Y.; Deng, B.; Yue, J.; Qu, W.; Yang, H.; Xia, W.; Li, L. Low temperature UV assisted sol-gel preparation of ZrO2 pore-sealing films on micro-arc oxidized magnesium alloy AZ91D and their electrochemical corrosion behaviors. Alloy. Compd. 2019, 792, 1036–1044.
- Kaseem, M.; Ko, Y.G. Benzoate intercalated Mg-Al-layered double hydroxides (LDHs) as efficient chloride traps for plasma electrolysis coatings. Alloy. Compd. 2019, 787, 772–778, doi:10.1016/j.jallcom.2019.02.124.
- Kaseem, M.; Ko, Y.G. A novel composite system composed of zirconia and LDHs film grown on plasma electrolysis coating: Toward a stable smart coating. Sonochemistry 2018, 49, 316–324, doi:10.1016/j.ultsonch.2018.08.023.
- Scarpellini, D.; Falconi, C.; Gaudio, P.; Mattoccia, A.; Medaglia, P.G.; Orsini, A.; Pizzoferrato, R.; Richetta, M. Morphology of Zn/Al layered double hydroxide nanosheets grown onto aluminum thin films. Eng. 2014, 126, 129–133.
- Bouali, A.; Serdechnova, M.; Blawert, C.; Tedim, J.; Ferreira, M.; Zheludkevich, M. Layered double hydroxides (LDHs) as functional materials for the corrosion protection of aluminum alloys: A review. Mater. Today 2020, 21, 100857, doi:10.1016/j.apmt.2020.100857.
- Chen, F.; Yu, P.; Zhang, Y. Healing effects of LDHs nanoplatelets on MAO ceramic layer of aluminum alloy. Alloy. Compd. 2017, 711, 342–348.
- Serdechnova, M.; Mohedano, M.; Kuznetsov, B.; Mendis, C.L.; Starykevich, M.; Karpushenkov, S.; Tedim, J.; Ferreira, M.G.S.; Blawert, C.; Zheludkevich, M.L. PEO Coatings with Active Protection Based on In-Situ Formed LDH-Nanocontainers. Electrochem. Soc. 2017, 164, C36–C45, doi:10.1149/2.0301702jes.
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al- layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat. Sci. 2017, 115, 159–174, doi:10.1016/j.corsci.2016.12.001.
- Sun, Z.; Gu, L.; Zheng, J.; Zhang, J.; Wang, L.; Xu, F.; Lin, C. A controlled release strategy of antifouling agent in coating based on intercalated layered double hydroxides. Lett. 2016, 172, 105–108, doi:10.1016/j.matlet.2016.02.151.
- Lin, J. K.; Uan, J. Y. Formation of Mg, Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3−/CO32− and corresponding protection against corrosion by the coating. Sci. 2009, 51, 1181–1188.
- Yu, B.-L.; Lin, J.-K.; Uan, J.-Y. Applications of carbonic acid solution for developing conversion coatings on Mg alloy. Nonferrous Met. Soc. China 2010, 20, 1331–1339, doi:10.1016/s1003-6326(09)60300-9.
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. In Situ Growth Process of Mg–Al Hydrotalcite Conversion Film on AZ31 Mg Alloy. Mater. Sci. Technol. 2015, 31, 384–390, doi:10.1016/j.jmst.2014.09.016.
- Wu, H.; Zhang, L.; Zhang, Y.; Long. S.; Jie, X.; Corrosion behavior of Mg-Al LDH film in-situ assembled with graphene on Mg alloy pre-sprayed Al layer, Alloys. Compd. 2020, 834, 155107
- Wang, L.; Zhang, K.; Sun, W.; Wu, T.; He, H.; Liu, G. Hydrothermal synthesis of corrosion resistant hydrotalcite conversion coating on AZ91D alloy. Lett. 2013, 106, 111–114, doi:10.1016/j.matlet.2013.05.018.
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg–Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044, doi:10.1021/acsami.6b12974.
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Influence of alloying elements and microstructure on the formation of hydrotalcite film on Mg alloys. Sci. 2015, 93, 90–99, doi:10.1016/j.corsci.2015.01.008.
- Lin, J. K.; Hsia, C. L.; Uan, J. Y. Characterization of Mg, Alhydrotalcite conversion film on Mg alloy and Cl− and CO32− anion-exchangeability of the film in a corrosive environment. Mater. 2007, 56, 927–930.
- Zeng, R.-C.; Li, X.-T.; Liu, Z.-G.; Zhang, F.; Li, S.-Q.; Cui, H.-Z. Corrosion resistance of Zn–Al layered double hydroxide/poly (lactic acid) composite coating on magnesium alloy AZ31. Mater. Sci. 2015, 9, 355–365, doi:10.1007/s11706-015-0307-7.
- Zhang, F.; Zhang, C.; Zeng, R.; Song, L.; Guo, L.; Huang, X. Corrosion Resistance of the Superhydrophobic Mg(OH)2/Mg-Al Layered Double Hydroxide Coatings on Magnesium Alloys. Metals 2016, 6, 85, doi:10.3390/met6040085.
- Zeng, R.-C.; Liu, L.-J.; Pang, T.-T.; Zhang, F.; Zhang, W.-W.; Li, S.-Q.; Cui, H.-Z.; Han, E.-H. Corrosion Resistance of Silane-Modified Hydroxide Zinc Carbonate Film on AZ31 Magnesium Alloy. Acta Met. Sin. 2015, 28, 373–380, doi:10.1007/s40195-015-0208-x.
- Ba, Z.; Dong, Q.; Zhang, X.; Qiang, X.; Cai, Z.; Luo, X. Cerium-based modification treatment of Mg-Al hydrotalcite film on AZ91D Mg alloy assisted with alternating electric field. Alloy. Compd. 2017, 695, 106–113, doi:10.1016/j.jallcom.2016.10.139.
- Lai, F.; Miao, Y.-E.; Zuo, L.; Lu, H.; Huang, Y.; Liu, T. Biomass-Derived Nitrogen-Doped Carbon Nanofiber Network: A Facile Template for Decoration of Ultrathin Nickel-Cobalt Layered Double Hydroxide Nanosheets as High-Performance Asymmetric Supercapacitor Electrode. Small 2016, 12, 3235–3244, doi:10.1002/smll.201600412.
- Mallakpour, S.; Dinari, M.; Behranvand, V. Ultrasonic-assisted synthesis and characterization of layered double hydroxides intercalated with bioactive N,N′-(pyromellitoyl)-bis-l-α-amino acids. RSC Adv. 2013, 3, 23303–23308, doi:10.1039/c3ra43645d.
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Polym. Sci. 2014, 39, 594–626, doi:10.1016/j.progpolymsci.2013.07.011.
- Iqbal, M. A.; Sun, L.; la Chance, A.M.; Ding, H.; Fedel, M. In situ growth of a CaAl-NO3-layered double hydroxide film directly on an aluminum alloy for corrosion resistance. Dalton Trans. 2020, 49, 3956–
- Ishizaki, T.; Kamiyama, N.; Watanabe, K.; Serizawa, A. Corrosion resistance of Mg(OH)2/Mg–Al layered double hydroxide composite film formed directly on combustion-resistant magnesium alloy AMCa602 by steam coating. Sci. 2015, 92, 76–84.
- Kamiyama, N.; Panomsuwan, G.; Yamamoto, E.; Sudare, T.; Saito, N.; Ishizaki, T. Effect of treatment time in the Mg(OH)2/Mg–Al LDH composite film formed on Mg alloy AZ31 by steam coating on the corrosion resistance. Coat. Technol. 2016, 286, 172–177.
- Wu, F.; Liang, J.; Peng, Z.; Liu, B. Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDHs) films on magnesium alloy. Surf. Sci. 2014, 313, 834–840, doi:10.1016/j.apsusc.2014.06.083.
- Tabish, M.; Yasin, G.; Anjum, M.J.; Malik, M.U.; Zhao, J.; Yang, Q.; Manzoor, S.; Murtaza, H.; Khan, W.Q. Reviewing the current status of layered double hydroxide-based smart nanocontainers for corrosion inhibiting applications. Mater. Res. Technol. 2021, 10, 390–421, doi:10.1016/j.jmrt.2020.12.025.
- Guo, L.; Wu, W.; Zhou, Y.; Zhang, F.; Zeng, R.; Zeng, J. Layered double hydroxide coatings on magnesium alloys: A review. Mater. Sci. Technol. 2018, 34, 1455–1466, doi:10.1016/j.jmst.2018.03.003.
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. Study of the in situ growth mechanism of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Sci. 2012, 63, 148–158, doi:10.1016/j.corsci.2012.05.022.
- Chen, J.; Song, Y.; Shan, D.; Han, E.-H. In situ growth of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Sci. 2011, 53, 3281–3288, doi:10.1016/j.corsci.2011.06.003.
- Shulha, T.N.; Serdechnova, M.; Lamaka, S.V.; Wieland, D.C.F.; Lapko, K.N.; Zheludkevich, M.L. Chelating agent-assisted in situ LDH growth on the surface of magnesium alloy. Rep. 2018, 8, 1–10, doi:10.1038/s41598-018-34751-7.
- Zhao, Y.; Waterhouse, G.I.N.; Chen, G.; Xiong, X.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Two-dimensional-related catalytic materials for solar-driven conversion of COx into valuable chemical feedstocks. Soc. Rev. 2019, 48, 1972–2010, doi:10.1039/c8cs00607e.
- Nakayama, H.; Hayashi, A. Mixing Acid Salts and Layered Double Hydroxides in Nanoscale under Solid Condition. Pharmaceutics 2014, 6, 436–446, doi:10.3390/pharmaceutics6030436.
- Zhang, G.; Wu, L.; Tang, A.; Zhang, S.; Yuan, B.; Zheng, Z.; Pan, F. A novel approach to fabricate protective layered double hydroxide films on the surface of anodized Mg-Al alloy. Mater. Interfaces 2017, 4, 1700163.
- Zeng, R.-C.; Liu, Z.-G.; Zhang, F.; Li, S.-Q.; Cui, H.-Z.; Han, E.-H. Corrosion of molybdate intercalated hydrotalcite coating on AZ31 Mg alloy. Mater. Chem. A 2014, 2, 13049–13057, doi:10.1039/c4ta01341g.