
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rebeca André | + 3721 word(s) | 3721 | 2021-02-04 03:31:58 | | | |
| 2 | Dean Liu | -1707 word(s) | 2014 | 2021-02-19 03:04:39 | | |
Video Upload Options
Brown algae have been part of the human diet for hundreds of years, however, in recent years, commercial and scientific interest in brown algae has increased due to the growing demand for healthier diet by the world population. Brown algae and its metabolites, such as carotenoids, polysaccharides, phlorotannins, and proteins, have been associated with multiple beneficial health effects for different diseases, such as cardiovascular diseases, one of the main causes of death in Europe.
1. Introduction
Seaweeds are macroalgae used in different sectors, such as agricultural, horticultural, cosmetics, and food industries. It has been recognized that the novel and potentially bioactive components that algae present make them a good source of healthy food[1][2] World seaweed production doubled between 2005 and 2015. Globally, in 2016, seaweed products were valued at USD 10.6 million, and it is estimated that in 2025 the value of global seaweed products will reach USD 26 million[3]. Asia and the Pacific region dominate 60% of the world algae market, followed by Europe and the Americas[3]. Seaweeds have been used as part of the human diet for thousands of years. Archaeological evidence shows that in Chile it has been used for the last 14,000 years[1], and in Japan and China, there are written records describing the use of seaweed that date back over 2000 years[4][5]. Nowadays, in Europe, seaweed consumption is increasing, not only because people are becoming interested in the uses of natural products, but also because it is seen as a healthy and nutritious “superfood”, which is sold preserved dry, fresh, frozen, canned, or salted[3][6]. Algae are used both as a food supplement and as an addition to functional food. Meat products, cereal-based products, and fermented functional foods, such as cheeses, are the main products in the market supplemented with algae[7]. Statistics from the 2012 global harvest demonstrated that 38% of the 23.8 million seaweed harvest was used for human consumption, without counting the consumption of hydrocolloids derived from algae as agars, alginates, and carrageenans[1][8]. Currently, more than 10,000 species of algae are known, but only about 200 species are consumed worldwide, with the brown algae species being the most consumed, followed by red algae species and then the green algae species[6][9]. Despite the considerable number of brown algae species consumed worldwide, under the European regulation there are only about 23 brown seaweed species authorized for food applications.
Table 1. List of brown algae species for human food applications in Europe under the regulation (UE) 2015/2283 [10].
| Brown Algae Species |
|---|
| Ascophyllum nodosum |
| Alaria esculenta |
| Eisenia bicyclis |
| Fucus vesiculosus |
| Fucus serratus |
| Fucus spiralis |
| Himanthalia elongata |
| Laminaria digitata |
| Saccharina japonica |
| Saccharina latissima |
| Saccharina longicruris |
| Sargassum fusiforme |
| Undaria pinnatifida |
2. Hypercholesterolemia and the Actual Treatment
Cholesterol is an essential factor for cell homeostasis, having an important role in the synthesis of hormones and bile acids and in membrane structures[20][21]. Whole-body cholesterol homeostasis is a tightly regulated process that involves de novo biosynthesis, dietary cholesterol absorption, and biliary clearance and excretion[16][22]. An imbalance of these processes may lead to hypercholesterolemia, which is characterized by high levels of total cholesterol (Tc), low-density lipoprotein cholesterol (LDL-c), or triglycerides (TGs), and/or a decrease in high-density lipoprotein cholesterol (HDL-c)[23].
Excess cholesterol in tissues is removed by a pathway known as reverse cholesterol transport (RCT) that transports cholesterol from non-hepatic tissues to the liver for metabolism and bile excretion[24]. The principal lipoprotein involved in the cholesterol efflux from peripheral tissues is the HDL-c and its apolipoprotein (apo) A-I, the major protein in HDL particles[25]. Moreover, there are also three important membrane cholesterol transporters that have a major role in the RCT process: ATP-binding cassette (ABC) transporters A1 (ABCA1) and G1 (ABCG1) and scavenger receptor class B type I (SR-B1)[26][27][28]. The ABCA1 transports the cholesterol to apoA-I, leading to the formation of the HDL-c particles (Figure 1). The HDL particles interact with ABCG1 to incorporate more cholesterol, which is then esterified by the enzyme lecithin-cholesterol acyl transferase (LCAT) (Figure 1)[24][25].
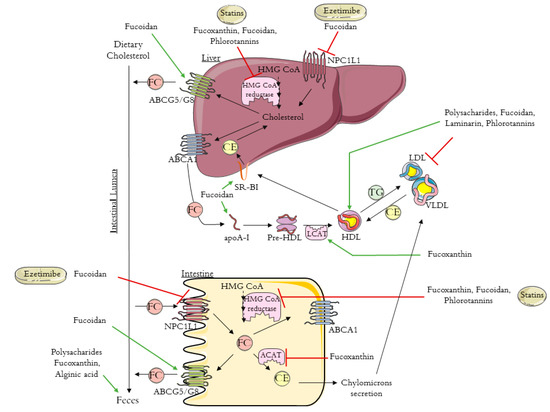
Figure 1. Schematic diagram of reverse cholesterol transport (RCT), intestinal cholesterol absorption, and the mechanisms of action of ezetimibe, statins, and brown algae compounds on cholesterol metabolism (polysaccharides, fucoxanthin, fucoidan, alginic acid, laminarin, and phlorotannins). HMG-CoA: 3-hydroxy-3-methyl-glutaryl coenzyme A; NPC1L1: Niemann–Pick C1-like 1 protein; ABCA1: ATP-binding cassette transporter A1; ABCG5/8: ATP-binding cassette transporters G5/8; SR-BI: scavenger receptor class B type I; ACAT: acyl-CoA:cholesterol acyltransferase; apoA-I: apolipoprotein A-I; HDL: high-density lipoprotein; VLDL: very low density lipoprotein; LDL: low-density lipoprotein; LCAT: lecithin-cholesterol acyl transferase; TG: triglyceride; FC: free cholesterol; CE: esterified cholesterol. The figure was designed using images from Servier Medical Art Commons Attribution 3.0 Unported License. (http://smart.servier.com). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Next, the cholesterol is delivered to the liver by a direct or an indirect pathway. In the direct pathway, HDL binds with high affinity to SR-B1 in the liver and transfers its cholesterol content (Figure 1). By the indirect pathway, the esterified cholesterol (CE) is transferred to apolipoproteins B-100 (apoB-100), to very low density lipoprotein (VLDL), or especially to LDL, in exchange for TG molecules (Figure 1)[24][25]. After this process, HDL-c molecules can re-enter the RCT cycle. Based on this knowledge, it has been proven that the plasma concentration levels of the HDL-c are inversely related to the risk of CHD[24][25][29]. The final step in RCT is the most important mechanism for cholesterol body elimination, since the hepatic cholesterol, free or converted into bile acids, is secreted in the bile and then eliminated via the feces (Figure 1)[29][30].
Whole-body cholesterol homeostasis is largely affected by the intestinal cholesterol absorption once its absorption percentage averages 56%[31][32]. Niemann–Pick C1-like 1 protein (NPC1L1) is the key player in dietary cholesterol intake, transporting dietary and bile cholesterol from the intestinal lumen to the enterocyte. Afterwards, the cholesterol can be esterified by acyl-CoA:cholesterol acyltransferase (ACAT), assembled into chylomicrons, and exported into circulation (Figure 1)[33]. Likewise, the unesterified cholesterol can also be exported to the blood stream through the protein transporter ABCA1, localized in the basolateral membrane of the enterocyte (Figure 1)[34]. The unesterified cholesterol is also effluxed back into the intestinal lumen through the transporter proteins localized in the apical membrane of the enterocytes, ABCG5/ABCG8 (Figure 1)[35].
The first approach to preventing cardiovascular disease and lowering cholesterol levels is lifestyle changes together with drug-based therapy[17]. The two main lipid-lowering drugs used are statins and ezetimibe, which act according to different mechanisms, via the de novo cholesterol synthesis and via the dietary cholesterol absorption, respectively [18][20].
Statins are the first-line drugs for lowering cholesterol levels, and there are currently seven different types approved in the United States: simvastatin, atorvastatin, lovastatin, pravastatin, fluvastatin, rosuvastatin, and pitavastatin. Statins bind to the active site of 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis, leading to its inhibition (Figure 1)[36][37]. Consequently, statins cause the upregulation of the expression of LDL receptors, improving the clearance of LDL in circulation[23][33]. It was previously demonstrated that statins increase HDL levels by 5% to 10%[38]. The mechanism that leads to this increase is still not very clear, however, it is already known that some statins improve ABCA1 expression and hepatic apoA-I production, which consequently increase HDL-c formation, leading to the association of statin therapy with enhanced RCT [38][39][40]. Despite the good results obtained with statins therapy, these drugs can cause some adverse effects. There are cases for which the statins treatment is not recommend; for example, some people are intolerant to statins, specifically patients with high cardiovascular risk. One approach to avoid the side effects of statins is to use the combination of statins with non-statin lipid-modifying drugs.
Ezetimibe (trademark name Zetia), the first drug approved after statins, is a potent intestinal cholesterol absorption inhibitor that targets NPC1L1[20][41]. This therapy also presents good results in terms of decreasing LDL-c[42]. Beyond the use of ezetimibe alone, another treatment approach to primary hyperlipidemia is its use in combination with statins, which has a synergistic effect with greater results for the reduction of blood Tc and LDL-c[42].
To prevent hypercholesterolemia and complement the drug treatments with statins, ezetimibe, or other drugs, it is important to look for new potent drugs and/or new therapeutic strategies, such as the use of dietary supplements derived from natural products[16].
3. The Anti-Hypercholesterolemic Effect of Brown Algae
The search for new drugs, nutraceuticals, and dietary supplements based on natural compounds has led to a growing interest in the study of the composition and bioactivity of marine algae[2][43]. Around the world, there are about 1800 species of brown algae (Phaeophyta), showing a variety of size and forms range from small filamentous to large and complex structures[44][45]. Most species of brown algae are exclusively found in oceans dispersed in cold waters along continental coasts and can also be found attached to rocky coasts in temperate zones or floating freely [46][47]. Just 1% of brown algae species can be found in freshwater, which are usually attached to rocks[47]. Brown algae species contain a variety of compounds, such as proteins, minerals, vitamins, alkaloids, sterols, fatty acids, carotenoids, non-digestible polysaccharides, and phenolic compounds, mostly phlorotannins[48][49]. However, the composition of algae is greatly affected by the geographic and atmospheric conditions of their habitat[50]. It is also necessary to take into account that post-harvesting storage and processing, such as extraction using different solvents (water, organic solvent, water–organic solvent mixtures) can also affect the composition of the obtained algae extract[51]. These algae species have been intensively studied not only for their anti-hyperlipidaemic activity [52][53], but also for their anti-tumorigenic[54][55], anti-diabetic[56][57], antiviral[58], anti-inflammatory[59][60], and antioxidant [48][60]capacities, among others.
Different in vivo studies, summarized in Table 2, have reported the hypocholesterolemic effect of different brown algae species[10][14][52][53][54][55][56]. In studies where brown algae extracts were used for diet supplementation or in oral administration, it was reported that, in general, there is a decrease in Tc, TG, and LDL-c levels and an increase in HDL-c levels. In these reports, the effects of brown algae on lipid levels were associated with a possible impact on the cholesterol biosynthesis due to a modulation effect on the high affinity receptor of lipoprotein metabolism[53][61]. In addition, brown algae have been reported to have the capacity to increase cholesterol excretion in feces due to the ability of the algae compounds to bind dietary cholesterol[62]. The mechanisms for the cholesterol-lowering effects of brown algae have not been clarified yet, but have often been associated with the presence of the diversity of compounds identified in these species, which led to the study of the hypocholesterolemic effect of isolated compounds obtained from different brown algae species. The most studied brown algae compounds in this area have been carotenoids, polysaccharides, and phlorotannins, and more recently interest has emerged in the hypocholesterolemic potential of alga-derived proteins and peptides.
Table 2. Overview of the in vivo hypocholesterolemic effect of different brown algae species under different extraction conditions.
| Brown Algae Specie | Algae Preparation and Administration Mode | Effect | Ref. |
|---|---|---|---|
| Ecklonia stolonifera | Oral administration of EtOAc and n-BuOH fractions derived from EtOH seaweed extract at a dose of 100 mg/kg of body weight |
↓ Tc, TGs, LDL-c ↑ HDL-c |
[13] |
| Ecklonia cava | Capsules, twice per day (200 mg seaweed powder per tablet) | ↓ Tc, LDL-c | [18] |
| Iyengaria stellata | Oral administration of ethanol extracts suspended in distilled water at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c |
[53] |
| Colpomenia sinuosa | Oral administration of ethanol extracts suspended in water at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c |
[53] |
| Spatoglossum asperum | Oral administration of ethanol extracts suspended in distilled water at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c |
[53] |
| Spatoglossum asperum | Diet supplemented with oily fractions of seaweed at 10 mg/200 g of body weight | ↓ Tc, TGs, LDL-c | [61] |
| Heterochordaria abietina | Diet supplemented with 5% seaweed powder | ↓ Tc, LDL-c | [62] |
| Sargassum micracanthum | Diet supplemented with 5% seaweed powder | ↓ Tc, TGs | [62] |
| Sargassum patens | Diet supplemented with 5% seaweed powder | ↓ Tc, LDL-c | [62] |
| Cystoseira sisymbrioides | Diet supplemented with 5% seaweed powder | ↓ Tc, free cholesterol, LDL-c | [62] |
| Laminaria diabolica | Diet supplemented with 5% seaweed powder | ↑ HDL-c | [62] |
| Sargassum ringgoldianum | Diet supplemented with 5% seaweed powder | ↑ HDL-c | [62] |
| Padina arborescens | Diet supplemented with 5% seaweed powder | ↑ HDL-c | [62] |
| Sargassum polycystum | Diet supplemented with 5% seaweed powder | ↓ Tc, TGs, LDL-c ↑ HDL-c |
[63] |
| Undaria pinnatifida | Diet supplemented with 100 g of seaweed powder/kg of body weight | ↓ Tc, TGs, LDL-c ↑ HDL-c |
[64] |
References
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982.
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831.
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The Global Satus of Seaweed Production, Trade and Utilization; FAO: Rome, Italy, 2018; Volume 124.
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25.
- Xia, B.; Abbott, I.A. Edible Seaweeds of China and Their Place in the Chinese Diet. Econ. Bot. 1987, 41, 341–353.
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390.
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547.
- FAO. The State of the World Fisheries and Aquaculture 2014; FAO: Rome, Italy, 2014.
- Lorenzo, J.; Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360.
- Edible seaweed and microalgae—Regulatory status in France and Europe—2019 update. Available online: https://www.ceva-algues.com/en/document/edible-algae-regulatory-update/ (accessed on 28 July 2020).
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263.
- Catarino, M.; Silva, A.; Cardoso, S. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249.
- Yoon, N.Y.; Kim, H.R.; Chung, H.Y.; Choi, J.S. Anti-hyperlipidemic effect of an edible brown algae, Ecklonia stolonifera, and its constituents on poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Arch. Pharm. Res. 2008, 31, 1564–1571.
- Neto, R.; Marçal, C.; Queirós, A.; Abreu, H.; Silva, A.; Cardoso, S. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987.
- Yamamoto, H.; Yamanashi, Y.; Takada, T.; Mu, S.; Tanaka, Y.; Komine, T.; Suzuki, H. Hepatic expression of Niemann-Pick C1-like 1, a cholesterol reabsorber from bile, exacerbates western diet-induced atherosclerosis in LDL receptor mutant mice S. Mol. Pharmacol. 2019, 96, 47–55.
- Li, R.; Liu, Y.; Shi, J.; Yu, Y.; Lu, H.; Yu, L.; Liu, Y.; Zhang, F. Diosgenin regulates cholesterol metabolism in hypercholesterolemic rats by inhibiting NPC1L1 and enhancing ABCG5 and ABCG8. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 1124–1133.
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Circulation 2014, 129, S1–S45.
- Choi, E.K.; Park, S.H.; Ha, K.C.; Noh, S.O.; Jung, S.J.; Chae, H.J.; Chae, S.W.; Park, T.S. Clinical trial of the hypolipidemic effects of a brown alga Ecklonia cava extract in patients with hypercholesterolemia. Int. J. Pharmacol. 2015, 11, 798–805.
- Sahoo, D.; Seckbach, J. (Eds.) The Algae World, 1st ed.; Springer: Dordrecht, The Netherlands, 2015; ISBN 9789401773201.
- Ge, L.; Wang, J.; Qi, W.; Miao, H.-H.; Cao, J.; Qu, Y.-X.; Li, B.-L.; Song, B.-L. The Cholesterol Absorption Inhibitor Ezetimibe Acts by Blocking the Sterol-Induced Internalization of NPC1L1. Cell Metab. 2008, 7, 508–519.
- Axmann, M.; Strobl, W.M.; Plochberger, B.; Stangl, H. Cholesterol transfer at the plasma membrane. Atherosclerosis 2019, 290, 111–117.
- Altmann, S.W.; Davis, H.R.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.N.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science 2004, 303, 1201–1204.
- Espinheira, M.C.; Vasconcelos, C.; Medeiros, A.M.; Alves, A.C.; Bourbon, M.; Guerra, A. Hipercolesterolemia—Uma patologia com expressão desde a idade pediátrica. Rev. Port. Cardiol. 2013, 32, 379–386.
- White, C.R.; Anantharamaiah, G.M.; Datta, G. HDL mimetic peptides: Novel therapeutic strategies for the treatment of inflammatory vascular disease. In The HDL Handbook; Elsevier Inc.: Philadelphia, PA, USA, 2010; pp. 179–197. ISBN 9780123821713.
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.C.; Lira, F.S.; Gonçalves, D.C. Reverse cholesterol transport: Molecular mechanisms and the non-medical approach to enhance HDL cholesterol. Front. Physiol. 2018, 9, 526.
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28.
- Yin, J.; Wang, J.; Li, F.; Yang, Z.; Yang, X.; Sun, W.; Xia, B.; Li, T.; Song, W.; Guo, S. The fucoidan from the brown seaweed: Ascophyllum nodosum ameliorates atherosclerosis in apolipoprotein E-deficient mice. Food Funct. 2019, 10, 5124–5139.
- Song, G.; Zong, C.; Liu, Q.; Si, Y.; Liu, J.; Li, W.; Zhu, P.; Qin, S. SR-BI associates with ABCG1 and inhibits ABCG1-mediated cholesterol efflux from cells to high-density lipoprotein 3. Lipids Health Dis. 2012, 11, 118.
- Dikkers, A.; Tietge, U.J.F. Biliary cholesterol secretion: More than a simple ABC. World J. Gastroenterol. 2010, 16, 5936–5945.
- Post, S.M.; De Crom, R.; Van Haperen, R.; Van Tol, A.; Princen, H.M.G. Increased fecal bile acid excretion in transgenic mice with elevated expression of human phospholipid transfer protein. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 892–897.
- Bosner, M.S.; Lange, L.G.; Stenson, W.F.; Ostlund, R.E. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 1999, 40, 302–308.
- Betters, J.L.; Yu, L. NPC1L1 and cholesterol transport. FEBS Lett. 2010, 584, 2740–2747.
- Jia, L.; Betters, J.L.; Yu, L. Niemann-Pick C1-Like 1 (NPC1L1) Protein in Intestinal and Hepatic Cholesterol Transport. Annu. Rev. Physiol. 2011, 73, 239–259.
- Yassine, H.N.; Belopolskaya, A.; Schall, C.; Stump, C.S.; Lau, S.S.; Reaven, P.D. Enhanced cholesterol efflux to HDL through the ABCA1 transporter in hypertriglyceridemia of type 2 diabetes. Metabolism 2014, 63, 727–734.
- Hui, D.Y.; Howles, P.N. Molecular mechanisms of cholesterol absorption and transport in the intestine. Semin. Cell Dev. Biol. 2005, 16, 183–192.
- Istvan, E. Statin inhibition of HMG-CoA reductase: A 3-dimensional view. Atheroscler. Suppl. 2003, 4, 3–8.
- Feingold, K.R. Cholesterol Lowering Drugs. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020.
- Ono, K. Current concept of reverse cholesterol transport and novel strategy for atheroprotection. J. Cardiol. 2012, 60, 339–343.
- Chapman, M. Are the effects of statins on HDL-cholesterol clinically relevant? Eur. Hear. J. Suppl. 2004, 6, C58–C63.
- Maejima, T.; Yamazaki, H.; Aoki, T.; Tamaki, T.; Sato, F.; Kitahara, M.; Saito, Y. Effect of pitavastatin on apolipoprotein A-I production in HepG2 cell. Biochem. Biophys. Res. Commun. 2004, 324, 835–839.
- Xie, P.; Zhu, H.; Jia, L.; Ma, Y.; Tang, W.; Wang, Y.; Xue, B.; Shi, H.; Yu, L. Genetic demonstration of intestinal NPC1L1 as a major determinant of hepatic cholesterol and blood atherogenic lipoprotein levels. Atherosclerosis 2014, 237, 609–617.
- Zhan, S.; Xia, P.; Tang, M.; Liu, F.; Shu, M.; Wu, X. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst. Rev. 2017, 2017.
- Jaiganesh, R.; Sampath Kumar, N.S. Marine Bacterial Sources of Bioactive Compounds. Adv. Food Nutr. Res. 2012, 65, 389–408.
- Ji, S.Q.; Wang, B.; Lu, M.; Li, F.L. Direct bioconversion of brown algae into ethanol by thermophilic bacterium Defluviitalea phaphyphila. Biotechnol. Biofuels 2016, 9, 1–10.
- Wehr, J.D. Chapter 19—Brown Algae. In Freshwater Algae of North America; Wehr, J.D., Sheath, R.G., Kociolek, J.P., Eds.; Elsevier: Boston, MA, USA, 2015; pp. 851–871. ISBN 978-0-12-385876-4.
- Petruzzello, M. Brown Algae. Available online: https://www.britannica.com/science/brown-algae (accessed on 28 July 2020).
- Sheath, R.G.; Wehr, J.D. Introduction to the Freshwater Algae. In Freshwater Algae of North America; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–11. ISBN 9780123858771.
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary fiber and antioxidant capacity in Fucus vesiculosus products. Int. J. Food Sci. Nutr. 2009, 60, 23–34.
- Scarpini, E.; Scheltens, P.; Feldman, H. Treatment of Alzheimer’s disease: Current status and new perspectives. Lancet Neurol. 2003, 2, 539–547.
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268.
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365.
- Lee, D.H.; Park, M.Y.; Shim, B.J.; Youn, H.J.; Hwang, H.J.; Shin, H.C.; Jeon, H.K. Effects of Ecklonia cava Polyphenol in Individuals with Hypercholesterolemia: A Pilot Study. J. Med. Food 2012, 15, 1038–1044.
- Ara, J.; Sultana, V.; Qasim, R.; Ahmad, V.U. Hypolipidaemic activity of seaweed from Karachi coast. Phyther. Res. 2002, 16, 479–483.
- Olivares-Bañuelos, T.; Gutiérrez-Rodríguez, A.; Méndez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juárez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.; Hernández-Kelly, L.; Ortega, A.; et al. Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules 2019, 24, 260.
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364.
- Lin, H.-T.; Tsou, Y.-C.; Chen, Y.-T.; Lu, W.-J.; Hwang, P.-A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113.
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae—A Mini Review. J. Diabetes Res. 2020, 2020, 1–13.
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224.
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374.
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, Antioxidant, and Anti-Inflammatory Activities. Mar. Drugs 2019, 17, 434.
- Ara, J.; Sultana, V.; Qasim, R.; Ehteshamul-Haque, S.; Ahmad, V.U. Biological activity of Spatoglossum asperum: A brown alga. Phyther. Res. 2005, 19, 618–623.
- Ren, D.; Noda, H.; Amano, H.; Nishino, T.; Nishizawa, K. Study on Antihypertensive and Antihyperlipidemic Effects of Marine Algae. Fish. Sci. 1994, 60, 83–88.
- Matanjun, P.; Mohamed, S.; Muhammad, K.; Mustapha, N.M. Comparison of Cardiovascular Protective Effects of Tropical Seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on High-Cholesterol/High-Fat Diet in Rats. J. Med. Food 2010, 13, 792–800.
- Wang, W.; Yoshie, Y.; Suzuki, T. Effect of small particle size of seaweeds on digestibility and lipid metabolism in rats. Nippon Suisan Gakkaishi 2002, 68, 172–179.




