
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Annina Lyly | + 2307 word(s) | 2307 | 2020-12-17 04:59:32 | | | |
| 2 | Catherine Yang | -2 word(s) | 2305 | 2020-12-25 04:32:52 | | |
Video Upload Options
Monoclonal antibodies, biologics, are a relatively new treatment option for severe chronic airway diseases, asthma, allergic rhinitis, and chronic rhinosinusitis (CRS).
1. Introduction
Chronic inflammatory airway diseases include several overlapping morbidities, such as asthma and chronic obstructive pulmonary disease (COPD) in the lower airways; and allergic rhinitis (AR), nonallergic rhinitis (NAR), and chronic rhinosinusitis (CRS) in the upper airways. AR has a prevalence of 20–30%, NAR has a prevalence of 10%, and CRS has a prevalence of 10–20%, and these common diseases cause remarkable suffering and costs [1][2][3]. They can be subdivided based on such as age of onset, presence of allergy (skin prick test or systemic allergen specific immunoglobulin E (IgE)), with or without nasal polyps and/or T helper (Th) cell 2 prominent inflammation. Exposure to environmental irritants (such as smoking and occupational exposure), recurrent infections, lifestyle factors (such as obesity, stress), co-existing diseases, and genetic/epigenetic factors play a role in disease onset and progression [4][5]. The diagnostic methods include clinical examination, lung function tests, allergy tests, and paranasal sinus computed tomography scans [5][6][7]. Symptom control of mild cases can be well achieved by the basic treatment such as inhaled/intranasal corticosteroids, inhaled beta agonists, antihistamines, and nasal lavage [5][6]. Patients with moderate to severe forms often suffer from recurrent infective exacerbations and disease recurrence/progression despite maximal baseline therapy and surgeries. Hence, they require advanced diagnostic methods and therapeutics. Antibodies are an important part of humoral adaptive immunity and homeostasis. They also play a role in airway diseases such as IgE in allergy and CRS with nasal polyps (CRSwNP), antibody deficiency in CRS, and aberrant antiviral IgG responses in asthma exacerbations [5][8]. Since their introduction about five decades ago, a wide range of monoclonal antibodies are nowadays commercially available and have been largely used in basic and clinical science of airways.
2. Monoclonal Antibodies
Antibodies (immunoglobulin (Ig) A, IgD, IgE, IgG, IgM) are secreted by B-cells that are activated to plasma cells after antigen presentation in regional lymph nodes or secondary lymphoid organs (Figure 1) [9]. Monoclonal antibodies (mAbs) come from a single B-cell parent clone and recognize specifically a single epitope per antigen [10]. Antibodies are crucial to make leukocytes (such as T killer cells) to detect and destroy pathogens and infected host cells. MAbs are made for laboratory and therapeutic use by various techniques. The first technique described in 1975 was based on creating a hybridoma by combining an activated B-cell from an immunized animal spleen and immortalized myeloma cell, resulting in a stable hybrid cell line producing monoclonal antibody [11]. The first mAbs used in therapeutic purposes were of murine origin, which generated unwanted immunogenic reactions and human anti-mouse antibody formation [12]. The revolution of molecular biology techniques has enabled the production of humanized and fully human mAbs that have helped to tackle this problem, although anti-drug antibodies are still one of the outcomes of immunogenicity [12]. For research and laboratory use, there are exponential numbers of commercially available specific monoclonal antibodies for immunoassays such as immunohistochemistry, immunofluorescence and enzyme-linked immunosorbent assay (ELISA) [13]. Since their invention about 50 years ago, there has been a large interest to use monoclonal antibodies in experiments to discover relevant proteins and pathways behind airway pathologies [14][15].
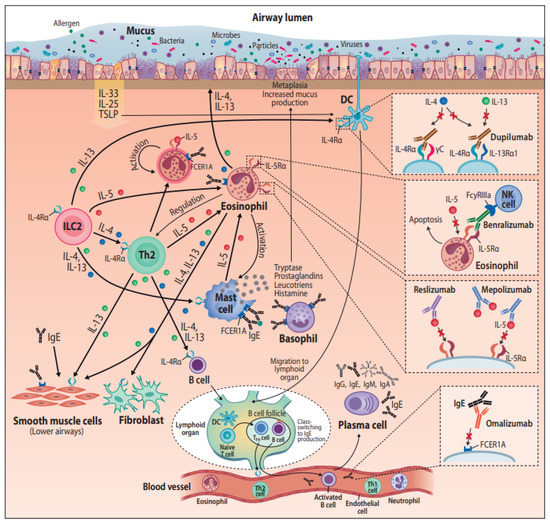
Figure 1. Monoclonal antibodies in the treatment of airway diseases, with their postulated pathways. Abbreviations: DC = dendritic cell, FCER1A = Fc fragment of Immunoglobulin E receptor 1A, FcyRIIIa = Fc fragment of IgG low affinity IIIa receptor, IgA = Immunoglobulin A, IgE = Immunoglobulin E, IgG = Immunoglobulin G, IgM = Immunoglobulin M, IL(-4, -4Rα, -5, -5Rα, -13, -13Rα, -25, -33) = Interleukin(-type), ILC2 = Group 2 innate lymphoid cells, NK cell = Natural killer cell, TFH cell = T follicular helper cell, Th1 = T helper type 1, Th2 = T helper type 2, TSLP = Thymic stromal lymphopoietin.
3. Monoclonal Antibodies and Treatment of Airway Diseases
Unraveling the pathogenesis of diseases has provided the basis for the pharmaceutical industry to develop protein drugs, or “biologics”, with higher specificity and mechanism of action than small molecule drugs. In 2015, monoclonal antibodies were the most important class of biologics approved by the United States Food and Drug Administration (FDA) [16], and their utilization in therapy has rapidly increased since. Personalized medicine is addressing the issue of providing targeted treatment for the right patient [17]. The endotype-driven treatment approach requires careful selection of the patient population who might benefit from a treatment by advanced therapies [18][19]. In the following chapter, mAbs used to treat asthma and CRSwNP are introduced; their main mechanisms of actions are illustrated in Figure 1.
3.1. Commercially Available Monoclonal Antibodies and Their Mechanisms of Action
In the fast phase of allergic reaction, allergen-specific IgE produced by B-cells binds to high affinity FcR (FceRI) expressed on immune cells such as basophils and mast cells. Then, allergen exposure can lead to antigen cross-linking IgE molecules on the same mast cell, receptor aggregation, and initiation of the intracellular signal cascade leading to degranulation and the release of histamine, prostaglandins, and cytokines that mediate the clinical manifestations of atopy [20]. Omalizumab, a humanized IgG1/k monoclonal antibody, targets the Fc region of IgE, and by binding to free IgE in blood and body fluids, it neutralizes the ability of IgE to bind to its receptor (FcεRI, high-affinity receptor and FcεRII, low-affinity receptor) [21]. On top of inhibiting the cross-linking on mast cells, this induces the down-regulation of IgE receptor expression on other immune cells such as basophils and dendritic cells [22][23]. Omalizumab was the first biological therapy developed for asthma, and it has now been used for 15 years. During these years, the functions of IgE in bronchial asthma have proven to be more complex than that of the classical role in allergy and anaphylaxis (reviewed in [89]). For example, smooth muscle cells in lung tissue have receptors for IgE, and it is involved in their proliferation, independent of the presence of allergens. IgE also plays a role in non-allergic diseases such as chronic idiopathic urticaria and CRSwNP and is involved in eosinophilic inflammation [24].
3.1.2. Mepolizumab and Reslizumab—Anti-IL-5
Type 2 inflammation present in asthma and CRSwNP is featured with airway eosinophilic infiltration, particularly in nasal polyps. Eosinophils are also frequently elevated in peripheral blood in type 2 asthma. High eosinophil levels are associated with exacerbations and bronchial obstruction [25]. The key mediator of eosinophils is interleukin-5 (IL5), being responsible for their differentiation, growth, activation, and survival as well as recruitment to airways [26][27]. Mepolizumab is a humanized IgG1/k monoclonal antibody toward IL-5, binding to it with high affinity and preventing its linkage to IL-5Rα [28][29]. Reslizumab is a humanized IgG4/κ monoclonal antibody specifically interacting with the epitope IL-5 uses to bind its receptor IL-5Ra, thereby blocking its bioactivity [30].
3.1.3. Benralizumab—Anti-IL-5Ralpha
Different from mepolizumab and reslizumab, benralizumab binds to IL-5-receptor instead of its ligand. Benralizumab is an afucosylated humanized IgG1/κ monoclonal antibody, selectively recognizing the IL-5Rα subunit [31]. The interaction of benralizumab with IL-5Rα prevents IL-5 binding to target cells and impedes the heterodimerization of IL-5Rα and βc subunits, thus inhibiting the activation of IL-5-dependent signaling cascades. In addition, benralizumab binds to the FcγRIIIa membrane receptor expressed by natural killer cells through the constant Fc region. FcγRIIIa activation induces the eosinophil apoptosis mechanism called antibody-dependent cell-mediated cytotoxicity, which is amplified by afucosylation [32], resulting in depletion of the blood eosinophils. A recent study describes also reduction in the number of basophiles after treatment with benralizumab [33].
3.1.4. Dupilumab—Anti IL-4Ralpha
Dupilumab is a fully human monoclonal antibody to the interleukin-4 receptor α subunit, IL-4Ralpha, which is utilized by two cytokines IL-4 and IL-13 [34]. IL-4 mediates its biological effects by binding to IL-4Rα, which is followed by the recruitment of either gamma c or IL-13 receptor alpha 1 (IL-13Rα1) to form a signaling complex [35]. IL-13 binds to IL-13Rα1 and then forms a signaling complex by recruiting IL-4Rα [35]. Altogether, IL-4Ralpha is involved in three different combinations of receptor complexes, and the intracellular response potencies are varied between the binding ligand, IL-4 vs. IL-13 [35][36].
Due to the shared receptor, IL-4 and IL-13 also have overlapping functions, and these sister cytokines act both cooperatively as well as independently in type 2 inflammation cascades. Both interleukins promote B-cell proliferation and class switch to IgG4 and IgE [37]. IL-13 is a cytokine secreted by activated Th2 cells, and it acts as an important mediator of allergic inflammation pathogenesis. Distinct functions for IL-13 include tissue remodeling, goblet cell mucus hypersecretion, subepithelial fibrosis, and emphysematous changes [38]. IL-4 and IL-13 can both induce Th2 cells and epithelial cells to produce eosinophil-promoting factors (i.e., IL-5 and eotaxins) and stimulate eosinophils to migrate to sites of inflammation from blood [39]. However, a recent murine model study shows that only dual IL-4/IL-13 blockade prevented type 2 inflammation broadly enough to prevent lung-function impairment—blocking only IL-4 or IL-13 alone was not enough to provide major clinical benefits [40]. This has been seen also in clinical experiments with IL-4 and IL-13 blockers for the treatment of type 2 diseases [41]. Dual blockade of IL-4/IL-13 with dupilumab halted eosinophil infiltration into lung tissue in mouse model without affecting circulating eosinophils, demonstrating that tissue, but not circulating eosinophils, contribute to disease pathology [40].
3.2. Monoclonal Antibodies in Asthma Treatment
Monoclonal antibodies are considered as a treatment option for severe asthma [42]. First, the patient’s symptoms are carefully assessed in order to estimate if the patient truly has asthma, if the current symptoms are associated with asthma, if the current asthma drug therapy is adequate, if the patient is adherent for the drug therapy, and that there are no environmental factors that should be considered [42][43]. Poor symptom control, frequent yearly exacerbations or serious exacerbations, and diminished lung function are signs of uncontrolled asthma and an indication for biologicals if the situation is not controlled with other maximal medication [42]. Controlled asthma that deteriorates if high-dose inhaled corticosteroids or systemic corticosteroids are tapered is another indication for biologicals [42]. The selection of a suitable drug is based both on allergy (whether the patient has allergic asthma to perennial allergens) but also on eosinophils (whether the patient has high or low blood eosinophils) [44]. Contradictory to biologicals in rheumatic diseases, the biologicals targeting IgE or Th2 cytokines have been well tolerated and safe to use [45][46].
3.3. Monoclonal Antibodies in CRS Treatment
Targeted monoclonal antibody therapies have shown encouraging results in the management of severe CRSwNP. As type 2 CRSwNP and asthma largely overlap, also therapeutics are in some cases targeted to both severe asthma and severe CRSwNP. According to the European Position Paper on Rhinosinusitis and Nasal polyps 2020 (EPOS 2020) guidelines, the indications for using biological treatment for CRSwNP include bilateral polyps and at least one previous endoscopic sinus surgery, together with at least three of the following criteria: evidence of type 2 inflammation, need for systemic corticosteroids (or contraindication for it), significantly impaired quality of life, significant loss of smell, or diagnosis of comorbid asthma. The effect of the treatment should be evaluated after 4 months and 1 year, and in case there is no response, treatment should be discontinued [5].
Anti-IgE therapy (omalizumab) is the second and latest biologic therapy approved for CRSwNP by the European Medicines Agency (EMA) in August 2020, and it is pending FDA approval for CRSwNP [47]. A study by Gevaert et al. has shown a decrease of symptom score for nasal congestion, anterior rhinorrhoea, loss of sense of smell, wheeze and dyspnea, and a significant reduction of endoscopic nasal polyp score, radiologic Lund–MacKay score, and asthma symptoms [48]. Another randomized controlled trial (RCT) by Pinto et al. showed improvement in symptoms, but no significant improvement in Lund–Mackay score or other endpoints [49]. In a recent study on patients with N-ERD, both nasal and lung symptoms improved significantly with omalizumab treatment [50]. However, these studies were small, with only around 20 patients in each group. Recent results from two bigger phase 3 RCTs of 265 patients has shown that omalizumab significantly reduced endoscopic nasal polyp score, nasal congestion score, and SNOT-22 score compared to placebo at week 24 [51]. Patients with comorbid asthma reported significant improvement in Asthma Quality of Life Questionnaire scores [51].
3.4. Future Monoclonal Antibody Treatments for Airway Diseases
3.4.1. Anti-TSLP
Thymic stromal lymphopoietin (TSLP) is produced by fibroblasts and epithelium and plays a role in T cell maturation. TSLP enhances IL type2 cytokine production in mast cells and activates ILC2s together with IL-33 or IL-25. TSLP has shown to associate with asthma and CRSwNP after virus challenge [52]. Tezepelumab (AMG-157/MEDI9929) is a human anti-TSLP antibody. A DBRCT of 31 mild asthmatics has shown that AMG-157 attenuated allergen-induced early and late asthmatic responses, and it decreased blood and sputum eosinophils [53]. Anti-OX40L promotes regulatory T (Treg) cells and suppresses T-cell mediated inflammation, and hence, it might be a potential therapeutic target for severe asthma [54]. Yet, in a study that used a combination of anti-OX40L and anti-TSLP, the expected effects on Treg-mediated inflammation was not observed [55]. Tezepelumab (anti-TSLP) decreases exacerbations and improves lung function measured by FEV1 (forced expiratory volume in one second) statistically significantly compared to placebo in patients with medium-to-high dose inhaled corticosteroids and long-acting beta-2-agonist [56]. The exacerbation rates were 61–71% lower than in the placebo group depending on the dose of the tezepelumab [56]. A reduction in asthma exacerbations was found irrespective of eosinophil level.
3.4.2. Anti-TNF
Type 2 low pathways might also comprise future targets for monoclonal antibody therapy [57]. Anti-TNF could have potential in patients with neutrophilic non-infectious COPD [58] and in severe asthma with mixed type 1/type2 [59][60]. ILC3s secrete IL-17, which leads to airway mucosal neutrophilia in some forms of asthma and CRS. A randomized, placebo-controlled double-blind trial was performed in 300 patients with moderate to severe asthma by using anti-IL-17, brodalumab, and it did not show a remarkable effect [61].
3.4.3. Anti-IL-8
Neutrophils have surface IL-8 receptors and are the main target cells for IL-8 responses. Anti-IL-8R, CXCR2, has been shown to reduce airway neutrophilia [62]. Two placebo-controlled studies with CXCR2 antagonists have been performed in severe (neutrophilic) asthma patients [63][64]. The results did not show clinical effectiveness; however, in one of the studies, a reduction in sputum and blood neutrophils was observed [63].
References
- Dierick, B.J.; Van Der Molen, T.; Flokstra-de-Blok, B.M.J.; Muraro, A.; Postma, M.J.; Kocks, J.W.; Van Boven, J.F. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharm. Outcomes Res. 2020, 1–17.
- Hastan, D.; Fokkens, W.; Bachert, C.; Newson, R.B.; Bislimovska, J.; Bockelbrink, A.; Bousquet, P.J.; Brozek, G.; Bruno, A.; Dahlén, S.E.; et al. Chronic rhinosinusitis in Europe—An Underestimated Disease. A GA2LEN study. Allergy 2011, 66, 1216–1223.
- Settipane, R.A.; Kaliner, M. Nonallergic Rhinitis. Am. J. Rhinol. Allergy 2013, 27, S48–S51.
- Toskala, E.; Kennedy, D.W. Asthma risk factors. Int. Forum Allergy Rhinol. 2015, 5, S11–S16.
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464.
- Global Strategy for Asthma Management and Prevention. Available online: www.ginasthma.org (accessed on 20 April 2020).
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Canonica, G.W.; Melen, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. 2020, 6, 1–17.
- Barnes, P.J. Intrinsic asthma: Not so different from allergic asthma but driven by superantigens? Clin. Exp. Allergy 2009, 39, 1145–1151.
- Panda, S.; Ding, J.L. Natural Antibodies Bridge Innate and Adaptive Immunity. J. Immunol. 2014, 194, 13–20.
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 1–10.
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497.
- Vaisman-Mentesh, A.; Gutierrez-Gonzalez, M.; DeKosky, B.J.; Wine, Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment with Monoclonal Antibodies. Front. Immunol. 2020, 11, 11.
- Wang, L.L.; Moshiri, A.S.; Novoa, R.; Simpson, C.L.; Takeshita, J.; Payne, A.S.; Chu, E.Y. Comparison of C3d immunohistochemical staining to enzyme-linked immunosorbent assay and immunofluorescence for diagnosis of bullous pemphigoid. J. Am. Acad. Dermatol. 2020, 83, 172–178.
- Black, C.A. A brief history of the discovery of the immunoglobulins and the origin of the modern immunoglobulin nomenclature. Immunol. Cell Biol. 1997, 75, 65–68.
- Platts-Mills, T.A.; Heymann, P.W.; Commins, S.P.; Woodfolk, J.A. The discovery of IgE 50 years later. Ann. Allergy Asthma Immunol. 2016, 116, 179–182.
- Kinch, M.S. An overview of FDA-approved biologics medicines. Drug Discov. Today 2015, 20, 393–398.
- De Greve, G.; Hellings, P.; Fokkens, W.; Pugin, B.; Callebaut, I.; Seys, S.F. Endotype-driven treatment in chronic upper airway diseases. Clin. Transl. Allergy 2017, 7, 1–14.
- Cardell, L.; Stjärne, P.; Jonstam, K.; Bachert, C. Endotypes of chronic rhinosinusitis: Impact on management. J. Allergy Clin. Immunol. 2020, 145, 752–756.
- Manka, L.A.; Wechsler, M.E. Selecting the right biologic for your patients with severe asthma. Ann. Allergy Asthma Immunol. 2018, 121, 406–413.
- Geha, R.S.; Jabara, H.H.; Brodeur, S.R. The regulation of immunoglobulin E class-switch recombination. Nat. Rev. Immunol. 2003, 3, 721–732.
- Pelaia, C.; Calabrese, C.; Terracciano, R.; De Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the first available antibody for biological treatment of severe asthma: More than a decade of real-life effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 12.
- Beck, L.A.; Marcotte, G.V.; MacGlashan, D.M., Jr.; Togias, A.; Saini, S.S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J. Allergy Clin. Immunol. 2004, 114, 527–530.
- MacGlashan, D.W.; Bochner, B.S.; Adelman, D.C.; Jardieu, P.M.; Togias, A.; McKenzie-White, J.; Sterbinsky, S.A.; Hamilton, R.G.; Lichtenstein, L.M. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J. Immunol. 1997, 158, 1438–1445.
- Novosad, J.; Krčmová, I. Evolution of our view on the IgE molecule role in bronchial asthma and the clinical effect of its modulation by omalizumab: Where do we stand today? Int. J. Immunopathol. Pharmacol. 2020, 34.
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barnéon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P.; et al. Eosinophilic Inflammation in Asthma. N. Engl. J. Med. 1990, 323, 1033–1039.
- Fulkerson, P.C.; Rothenberg, M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 117–129.
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514.
- Gnanakumaran, G.; Babu, K.S. Technology evaluation: Mepolizumab, GlaxoSmithKline. Curr. Opin. Mol. Ther. 2003, 5, 321–325.
- Fainardi, V.; Pisi, G.; Chetta, A. Mepolizumab in the treatment of severe eosinophilic asthma. Immunotherapy 2016, 8, 27–34.
- Zhang, J.; Kuvelkar, R.; Murgolo, N.J.; Taremi, S.S.; Chou, C.-C.; Wang, P.; Billah, M.M.; Egan, R.W. Mapping and characterization of the epitope(s) of Sch 55700, a humanized mAb, that inhibits human IL-5. Int. Immunol. 1999, 11, 1935–1944.
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’Acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a humanized anti–IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353.e2.
- Ghazi, A.; Trikha, A.; Calhoun, W.J. Benralizumab—A Humanized Mab to IL-5rα with Enhanced Antibody-Dependent Cell-Mediated Cytotoxicity—A Novel Approach for the Treatment of Asthma. Expert Opin. Biol. Ther. 2012, 12, 113–118.
- Lommatzsch, M.; Marchewski, H.; Schwefel, G.; Stoll, P.; Virchow, J.C.; Bratke, K. Benralizumab strongly reduces blood basophils in severe eosinophilic asthma. Clin. Exp. Allergy 2020, 50, 1267–1269.
- Wenzel, S.E.; Ford, L.; Pearlman, D.; Spector, S.; Sher, L.; Skobieranda, F.; Wang, L.; Kirkesseli, S.; Rocklin, R.; Bock, B.; et al. Dupilumab in Persistent Asthma with Elevated Eosinophil Levels. N. Engl. J. Med. 2013, 368, 2455–2466.
- Laporte, S.L.; Juo, Z.S.; Vaclavikova, J.; Colf, L.A.; Qi, X.; Heller, N.M.; Keegan, A.D.; Garcia, K.C. Molecular and Structural Basis of Cytokine Receptor Pleiotropy in the Interleukin-4/13 System. Cell 2008, 132, 259–272.
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50.
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456.
- Wynn, T.A. IL-13 effector functions. Annu. Rev. Immunol. 2003, 21, 425–456.
- Rosenberg, H.F.; Phipps, S.; Foster, P.S. Eosinophil trafficking in allergy and asthma. J. Allergy Clin. Immunol. 2007, 119, 1303–1310.
- Le Floc’H, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204.
- May, R.D.; Fung, M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine 2015, 75, 89–116.
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2013, 43, 343–373.
- Porsbjerg, C.; Ulrik, C.; Skjold, T.; Backer, V.; Laerum, B.; Lehman, S.; Janson, C.; Sandstrøm, T.; Bjermer, L.; Dahlen, B.; et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur. Clin. Respir. J. 2018, 5, 1440868.
- Bousquet, J.J.; Brusselle, G.; Buhl, R.; Busse, W.W.; Cruz, A.A.; Djukanovic, R.; Domingo, C.; Hanania, N.A.; Humbert, M.; Gow, A.M.; et al. Care pathways for the selection of a biologic in severe asthma. Eur. Respir. J. 2017, 50, 1701782.
- Boyman, O.; Kaegi, C.; Akdis, M.; Bavbek, S.; Bossios, A.; Chatzipetrou, A.; Eiwegger, T.; Firinu, D.; Harr, T.; Knol, E.F.; et al. EAACI IG Biologicals task force paper on the use of biologic agents in allergic disorders. Allergy 2015, 70, 727–754.
- Kotisalmi, E.; Hakulinen, A.; Mäkelä, M.; Toppila-Salmi, S.; Kauppi, P. A Comparison of Biologicals in the Treatment of Adults with Severe Asthma—Real-Life Experiences. Asthma Res. Pract. 2020, 6, 1–11.
- Kim, C.; Han, J.; Wu, T.; Bachert, C.; Fokkens, W.; Hellings, P.; Hopkins, C.; Lee, S.; Mullol, J.; Lee, J.T. Role of Biologics in Chronic Rhinosinusitis with Nasal Polyposis: State of the Art Review. Otolaryngol. Neck Surg. 2020.
- Gevaert, P.; Calus, L.; Van Zele, T.; Blomme, K.; De Ruyck, N.; Bauters, W.; Hellings, P.; Brusselle, G.; De Bacquer, D.; Van Cauwenberge, P.; et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J. Allergy Clin. Immunol. 2013, 131, 110–116.e1.
- Pinto, J.; Mehta, N.; DiTineo, M.; Wang, J.; Baroody, F.; Naclerio, R. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinol. J. 2010, 48, 318–324.
- Forster-Ruhrmann, U.; Stergioudi, D.; Pierchalla, G.; Fluhr, J.; Bergmann, K.-C.; Olze, H. Omalizumab in patients with NSAIDs-exacerbated respiratory disease. Rhinology 2020, 58, 226–232.
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605.
- Golebski, K.; Van Tongeren, J.; Van Egmond, D.; De Groot, E.J.; Fokkens, W.J.; Van Drunen, C.M. Specific Induction of TSLP by the Viral RNA Analogue Poly(I:C) in Primary Epithelial Cells Derived from Nasal Polyps. PLoS ONE 2016, 11, e0152808.
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.-P.; Wang, Y.; Cockcroft, D.; Bigler, J.; Fitzgerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an Anti-TSLP Antibody on Allergen-Induced Asthmatic Responses. N. Engl. J. Med. 2014, 370, 2102–2110.
- Catley, M.C.; Coote, J.; Bari, M.; Tomlinson, K.L. Monoclonal antibodies for the treatment of asthma. Pharmacol. Ther. 2011, 132, 333–351.
- Baatjes, A.J.; Smith, S.G.; Dua, B.; Watson, R.; Gauvreau, G.M.; O’Byrne, P.M. Treatment with anti-OX40L or anti-TSLP does not alter the frequency of T regulatory cells in allergic asthmatics. Allergy 2015, 70, 1505–1508.
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; Van Der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946.
- Michel, O.; Dinh, P.H.D.; Doyen, V.; Corazza, F. Anti-TNF inhibits the Airways neutrophilic inflammation induced by inhaled endotoxin in human. BMC Pharmacol. Toxicol. 2014, 15, 60.
- Antoniu, S.A.; Mihaltan, F.; Ulmeanu, R. Anti-TNF-α therapies in chronic obstructive pulmonary diseases. Expert Opin. Investig. Drugs 2008, 17, 1203–1211.
- Cazzola, M.; Polosa, R. Anti-TNF-α and Th1 cytokine-directed therapies for the treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 43–50.
- Antoniu, S.A. Infliximab for chronic obstructive pulmonary disease: Towards a more specific inflammation targeting? Expert Opin. Investig. Drugs 2006, 15, 181–184.
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.-L. Randomized, Double-Blind, Placebo-controlled Study of Brodalumab, a Human Anti–IL-17 Receptor Monoclonal Antibody, in Moderate to Severe Asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302.
- Nair, P.; Aziz-Ur-rehman, A.; Radford, K. Therapeutic implications of “neutrophilic asthma”. Curr. Opin. Pulm. Med. 2015, 21, 33–38.
- Nair, P.; Gaga, M.; Zervas, E.; Alagha, K.; Hargreave, F.E.; O’Byrne, P.M.; Stryszak, P.; Gann, L.; Sadeh, J.; Chanez, P.; et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: A randomized, placebo-controlled clinical trial. Clin. Exp. Allergy 2012, 42, 1097–1103.
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806.




