
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nathan MOREAU | + 1734 word(s) | 1734 | 2020-12-10 10:26:17 | | | |
| 2 | Catherine Yang | Meta information modification | 1734 | 2020-12-21 07:49:20 | | |
Video Upload Options
The peripheral nervous has important regenerative capacities that regulate and restore peripheral nerve homeostasis. Following peripheral nerve injury, the nerve undergoes a highly regulated degeneration and regeneration process, called Wallerian degeneration, where numerous cell populations interact to allow proper nerve regeneration. Recent studies have evidenced the prominent role of morphogenetic Hedgehog signaling pathway and its main effectors, Sonic Hedgehog (SHH) and Desert Hedgehog (DHH) in the regenerative drive following nerve injury. Furthermore, dysfunctional regeneration and/or dysfunctional Hedgehog signaling participate in the development of chronic neuropathic pain that sometimes accompanies nerve healing in the clinical context. Understanding the implications of this key signaling pathway could provide exciting new perspectives for future research on peripheral nerve healing.
1. Introduction
Contrary to its central counterpart, the peripheral nervous system exhibits a high regenerative capacity notably following peripheral nerve injury [1][2]. Such regenerative capacity is thought to result from the remarkable plasticity of Schwann cells, key effectors of peripheral nerve healing and homeostasis [3][4]. Following injury, Schwann cells transdifferentiate into repair Schwann cells that express a new genetic repair program to allow nerve healing and the restoration of peripheral nerve homeostasis [2]. This new repair program includes the activation of proregenerative genes that are repressed during development and in uninjured nerves such as the morphogen Sonic Hedgehog [2][5][6].
Hedgehog signaling plays an essential morphogenetic role in the development of the central [7][8][9][10] and peripheral nervous system [11], with major roles in patterning and cell fate specification [9]. The presence and/or reactivation of morphogens in adulthood has shed light on the implication of such morphogenetic pathways in central nervous system homeostasis [9][12] and disease [13] but also in physiological [14][15][16] and pathological peripheral nerve healing [16][17] and in neuropathic pain development [18][19][20].
2. Hedgehog Signaling in Peripheral Nerve Morphogenesis
The Hedgehog pathway, roughly comprised of three proteins Sonic Hedgehog (SHH), Desert Hedgehog (DHH), and Indian Hedgehog (IHH), three transmembrane receptors (Patched-1, Patched-2, and Smoothened), and three transcription factors (Gli-1, Gli-2, Gli-3), is essential for the ontogenic development of mammals [9] with several time-dependent and concentration-dependent morphogenetic effects on numerous cell lines, including the central [12] and peripheral nervous system [11].
Whereas the role of Hedgehog morphogens (and underlying signaling pathway) has been extensively studied in embryonic development and in the patterning/differentiation of the central nervous system (e.g., dorsal–ventral axis patterning of the neural tube and establishment of distinct ventral neuronal populations in a concentration-dependent manner) [9], the implication of the Hedgehog pathway is less clear in the peripheral nervous system. For instance, SHH is not expressed in Schwann cells at any stage of development [5][6] (as it is constitutively repressed during embryonic and promyelinating states of development by the polycomb pathway [4]) whereas DHH plays key roles in peripheral nerve structure specification during development [11].
During peripheral nerve morphogenesis the concomitant and symbiotic development of both neuronal axons and Schwann cells drive the formation of the myelin sheath and specialization of axonal structures such as the nodes of Ranvier [21]. Such axo-glial interactions are under the dependence of Schwann cell development, originating from neural crest cells that differentiate into Schwann cell precursors, then into immature Schwann cells before specification into myelinating or nonmyelinating Schwann cells [21]. Myelinating Schwann cells form a 1:1 contact with large caliber axons, wrapping them in a myelin sheath whereas nonmyelinating Schwann cells (also called Remak Schwann cells) surround multiple small caliber axons without any myelination [2]. Schwann cells and axons are supported and insulated by a connective tissue sheath of fibroblasts scattered between the nerve fibers that form the epineurium, perineurium, and endoneurium of the peripheral nerve [22].
The appearance of immature Schwann cells during development appears to coincide with the generation of the peripheral nerve’s extracellular matrix and the development of endoneurial connective tissue and blood vessels [23]. DHH and vascular endothelial growth factor (VEGF)-mediated signaling, originating from such immature Schwann cells and related axons, drive the differentiation and organization of arterial and perineurial cells, modeling the peripheral nerve’s architecture [11].
Indeed, deletion of the Dhh gene in mice results in the disruption of the fascicular structure of peripheral nerves, with a thin and disorganized perineurial sheath and an increase in blood–nerve barrier permeability [11]. Interestingly, a similar structural anomaly was found in a case of homozygous missense mutation of the dhh gene in a human diagnosed with “minifascular neuropathy” [24].
3. Future Research and Clinical Perspectives
3.1. Could Hyperactive Nerve Regeneration Drive Neuropathic Pain Development?
Recent evidence has raised the possibility that neuropathic pain could indeed result from dysfunctional nerve healing, but actually from a hyperactive nerve regeneration that fails to properly reinnervate the injured nerve [25]. Following spinal nerve ligation (ligation of L5 spinal nerve and cut at 1 mm distal to the suture), a common temporarily painful neuropathy model, the nerve could regenerate into the sciatic nerve (with restoration of electrical conduction, mechanical responses, and proper tracer migration) and the regenerating nerve was in fact the source of pain in this model [25]. Interestingly, disrupting the nerve regeneration inhibited the pain. Comparatively, in a spared nerve injury model (where pain is thought to be permanent), the regeneration process resulted in tangled neuromas at the injury site without proper reinnervation (as observed in the spinal nerve ligation model). Blocking the regeneration process via perfusions of Semaphorin 3A (an inhibitory axonal guidance molecule) prevented or reversed the painful behavior (even without removing the tangled fibers). It is thus concluded that in this model, the long-lasting pain behavior could stem from the anatomical inability of regenerating nerves to successfully reinnervate their target tissues, resulting in a persistent, yet futile, regeneration process [25].
A recent clinical study supports this hypothesis. In a study on patients suffering from chronic inflammatory demyelinating polyneuropathy (CIDP), shh mRNA expression was significantly higher in skin biopsies of CIDP patients that those of control subjects (with similar intra-epithelial nerve fiber density), and this high expression of shh mRNA was decreased following treatment of CIDP [26]. This suggests that in the context of this immune-mediated neuropathy, the small depleted axons attempt to mount a regenerative response [26]. Whether this regenerative response takes part in the underlying pathophysiology of CIDP still remains to be answered.
3.2. Understanding Hedgehog Signaling Inhibition in the Chronic Constriction Injury (CCI) Model
Considering the essential role of Hedgehog signaling in molecular, vascular, and behavioral changes observed in CCI models [18][19][20], understanding the underlying mechanisms of such inhibition could be of interest and help explain the lack of pre/post-treatment effect of Hedgehog pathway activation on neuropathic pain in such experimental conditions [18][19].
It is well known that Hedgehog signaling requires a functional ciliary architecture [27][28] and that loss of primary cilia is associated with Hedgehog pathway inhibition [29][30]. Furthermore, it has been shown that the Smoothened receptor is necessary for the development and maintenance of ciliary architecture [31]. Interestingly, chronic constriction injury of the sciatic nerve in rats leads to a significant and persistent downregulation of Smoothened mRNA in the sciatic nerve parenchyma from 6 h to 15 days post-injury (Figure 1), that could participate in the loss of primary ciliary architecture and subsequent Hedgehog pathway inhibition.
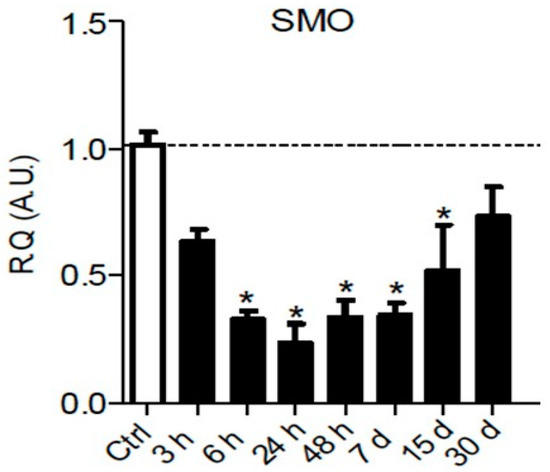
Figure 1. Chronic constriction injury (CCI) of the sciatic nerve induces early and prolonged downregulation of Smoothened receptor mRNA. Changes over time of Smoothened (SMO) mRNA levels were assessed in the sciatic nerve of sham or CCI-injured rats using semiquantitative reverse transcription polymerase chain reaction analyses. Data are presented as relative quantification (R.Q.) in arbitrary units (A.U.) corresponding to the ratio of specific mRNA over RPS18 mRNA. Each bar corresponds to the mean ±SEM of n = 6–8 animals for each time post-injury; * p < 0.05. One-way analysis of variance followed by Bonferroni post hoc test was used.
Furthermore, alterations in endoneurial vessel morphology have been serendipitously observed both in infra-orbital nerves of rats subjected to infra-orbital nerve chronic constriction injury and in uninjured infra-orbital nerves treated with cyclopamine (a Smoothened antagonist known to decrease production of both Smoothened mRNA and protein [32]) (Figure 2), possibly linked to a loss of Smoothened receptor [31].
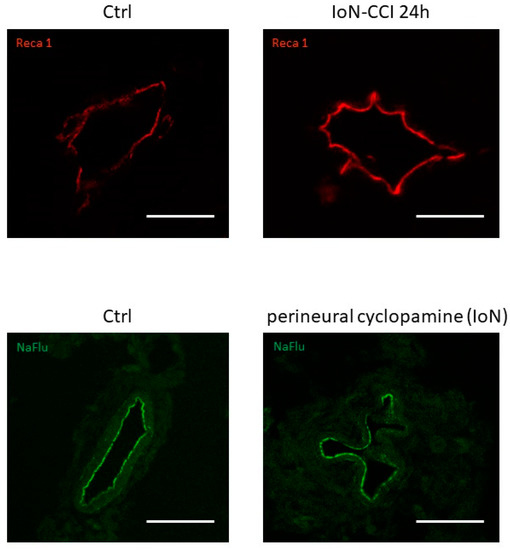
Figure 2. Vascular morphological changes observed using confocal microscopy in axial slices of infra-orbital nerves (IoN) subjected to chronic constriction injury (IoN-CCI) at 24 h post-injury (upper left panel) or sham surgery (upper right panel) or perineural injections of cyclopamine at 6 h post-injection (lower left panel) or vehicle (lower right panel) using Reca-1 and sodium fluorescein (NaFlu) for vessel immunolabeling, respectively. Chronic constriction injury and perineural injections were performed according to previously described methodologies (see Moreau et al. 2016 [18], 2017 [19] for methodological details).
Exploring the microvascular changes related to chronic constriction injury could thus be of interest to better understand the role of loss of Hedgehog signaling in neuropathic pain development.
3.3. Investigating and Developing New Nerve Regeneration Strategies
Although adult peripheral nerves have an intrinsic ability to regenerate, the endogenous response is often limited and does not allow for full recovery of function. Manipulation of the nerve microenvironment to promote neuronal survival and repair could be key to improve regeneration strategies [33].
For instance, a few studies have invested the role of brief extracellular electrical stimulation in fostering nerve regeneration, as it has been shown that such electrical stimulation applied immediately after nerve injury could increase nerve regeneration in animal models and humans [34]. In a diabetic neuropathy model, an enhanced expression of Shh mRNA was observed in ipsilateral DRGs following electrical stimulation, suggestion a potential role in electrical stimulation-mediated peripheral nerve regeneration. Interestingly, no rise in SHH protein was observed in the sciatic nerve [35], which suggests that Shh mRNA could function without protein transcription as seen in other experimental paradigms [8].
Finally, several studies have investigated new means of SHH protein delivery in a model of cavernous nerve crush injury, using a peptide amphiphile nanofiber hydrogel that allowed efficient local delivery of SHH [36][37][33][38][39][40]. Such delivery strategy could be applied to other models for future investigations.
4. Conclusions
The Hedgehog pathway that plays key morphogenetic roles during development also assumes critical roles in peripheral nerve healing following injury and in peripheral nerve homeostasis, such as preventing myelin degradation or maintaining blood–nerve barrier impermeability. Schwann cell, neuronal and endothelial-mediated Hedgehog signaling cooperate to promote physiological Wallerian degeneration and proper nerve regeneration.
Elucidating the numerous roles of Hedgehog signaling during normal and dysfunctional nerve healing could bring a better understanding of nerve regeneration processes and their still-ambiguous relation to neuropathic pain development.
As hyperactive regeneration could participate in neuropathic pain development, nerve regeneration can be construed as a physiological process that requires precise temporal and spatial controls, and thus morphogens such as Sonic Hedgehog or Desert Hedgehog would be ideal regulators of such peripheral nerve homeostasis.
References
- Richner, M.; Ulrichsen, M.; Elmegaard, S.L.; Dieu, R.; Pallesen, L.T.; Vaegter, C.B. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol. Neurobiol. 2014, 50, 945–970.
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989.
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531.
- Ma, K.H.; Hung, H.A.; Svaren, J. Epigenomic regulation of Schwann cell reprogramming in peripheral nerve injury. J. Neurosci. 2016, 36, 9135–9147.
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647.
- Lin, H.P.; Oksuz, I.; Hurley, E.; Wrabetz, L.; Awatramani, R. Microprocessor complex subunit DiGeorge syndrome critical region gene 8 (Dgcr8) is required for Schwann cell myelination and myelin maintenance. J. Biol. Chem. 2015, 290, 24294–24307.
- Charrier, J.B.; Lapointe, F.; Le Douarin, N.M.; Teillet, M.A. Anti-apoptotic role of Sonic Hedgehog protein at the early stages of nervous system organogenesis. Development 2001, 128, 4011–4020.
- Yam, P.T.; Langlois, S.D.; Morin, S.; Charron, F. Sonic Hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 2009, 62, 349–362.
- Petrova, R.; Joyner, A.L. Roles of Hedgehog signaling in adult organ homeostasis and repair. Development 2014, 141, 3445–3457.
- Peng, J.; Fabre, P.J.; Dolique, T.; Swikert, S.M.; Kermasson, L.; Shimogori, T.; Charron, F. Sonic Hedgehog is a remotely produced cue that controls axon guidance trans-axonally at a midline choice point. Neuron 2018, 97, 326–340.e4.
- Parmantier, E.; Lynn, B.; Lawson, D.; Turmaine, M.; Namini, S.S.; Chakrabarti, L.; McMahon, A.P.; Jessen, K.R.; Mirsky, R. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 1999, 23, 713–724.
- Vergara, M.N.; Arsenijevic, Y.; Del Rio-Tsonis, K. CNS regeneration: A morphogen’s tale. J. Neurobiol. 2005, 64, 491–507.
- Patel, S.S.; Tomar, S.; Sharma, D.; Mahindroo, N.; Udayabanu, M. Targeting Sonic Hedgehog signaling in neurological disorders. Neurosci Biobehav Rev. 2017, 74 Pt A, 76–97.
- Akazawa, C.; Tsuzuki, H.; Nakamura, Y.; Sasaki, Y.; Ohsaki, K.; Nakamura, S.; Arakawa, Y.; Kohsaka, S. The upregulated expression of Sonic Hedgehog in motor neurons after rat facial nerve axotomy. J. Neurosci. 2004, 24, 7923–7930.
- Hashimoto, M.; Ishii, K.; Nakamura, Y.; Watabe, K.; Kohsaka, S.; Akazawa, C. Neuroprotective effect of Sonic Hedgehog up-regulated in Schwann cells following sciatic nerve injury. J. Neurochem. 2008, 107, 918–927.
- Martinez, J.A.; Kobayashi, M.; Krishnan, A.; Webber, C.; Christie, K.; Guo, G.; Singh, V.; Zochodne, D.W. Intrinsic facilitation of adult peripheral nerve regeneration by the Sonic Hedgehog morphogen. Exp. Neurol. 2015, 271, 493–505.
- Yamada, Y.; Ohazama, A.; Maeda, T.; Seo, K. The Sonic Hedgehog signaling pathway regulates inferior alveolar nerve regeneration. Neurosci. Lett. 2018, 671, 114–119.
- Moreau, N.; Mauborgne, A.; Bourgoin, S.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain 2016, 157, 827–839.
- Moreau, N.; Dieb, W.; Mauborgne, A.; Bourgoin, S.; Villanueva, L.; Pohl, M.; Boucher, Y. Hedgehog pathway-mediated vascular alterations following trigeminal nerve injury. J. Dent. Res. 2017, 96, 450–457.
- Moreau, N.; Mauborgne, A.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Could an endoneurial endothelial crosstalk between Wnt/β-catenin and Sonic Hedgehog pathways underlie the early disruption of the infra-orbital blood-nerve barrier following chronic constriction injury? Mol. Pain. 2017, 13, 1744806917727625.
- Fledrich, R.; Kungl, T.; Nave, K.A.; Stassart, R.M. Axo-glial interdependence in peripheral nerve development. Development 2019, 146, dev151704.
- Richard, L.; Védrenne, N.; Vallat, J.M.; Funalot, B. Characterization of endoneurial fibroblast-like cells from human and rat peripheral nerves. J. Histochem. Cytochem. 2014, 62, 424–435.
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682.
- Umehara, F.; Tate, G.; Itoh, K.; Yamaguchi, N.; Douchi, T.; Mitsuya, T.; Osame, M. A novel mutation of Desert Hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Hum. Genet. 2000, 67, 1302–1305.
- Xie, W.; Strong, J.A.; Zhang, J.M. Active nerve regeneration with failed target reinnervation drives persistent neuropathic pain. eNeuro 2017, 4, e0008-17.
- Bautista, J.; Chandrasekhar, A.; Komirishetty, P.K.; Duraikannu, A.; Zochodne, D.W. Regenerative plasticity of intact human skin axons. J. Neurol Sci. 2020, 417, 117058.
- Sasai, N.; Briscoe, J. Primary cilia and graded Sonic Hedgehog signaling. Wiley Interdiscip Rev. Dev. Biol. 2012, 1, 753–772.
- Pan, J.; Seeger-Nukpezah, T.; Golemis, E.A. The role of the cilium in normal and abnormal cell cycles: Emphasis on renal cystic pathologies. Cell Mol. Life Sci. 2013, 70, 1849–1874.
- Das, R.M.; Storey, K.G. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 2014, 343, 200–204.
- Gazea, M.; Tasouri, E.; Tolve, M.; Bosch, V.; Kabanova, A.; Gojak, C.; Kurtulmus, B.; Novikov, O.; Spatz, J.; Pereira, G.; et al. Primary cilia are critical for Sonic Hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev. Biol. 2016, 409, 55–71.
- Huangfu, D.; Anderson, K.V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 2005, 102, 11325–11330.
- Zhao, L.; Yu, Y.; Deng, C. Protein and mRNA expression of Shh, Smo and Gli1 and inhibition by cyclopamine in hepatocytes of rats with chronic fluorosis. Toxicol. Lett. 2014, 225, 318+24.
- Bond, C.W.; Angeloni, N.; Harrington, D.; Stupp, S.; Podlasek, C.A. Sonic Hedgehog regulates brain-derived neurotrophic factor in normal and regenerating cavernous nerves. J. Sex. Med. 2013, 10, 730–737.
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202.
- Singh, B.; Krishnan, A.; Micu, I.; Koshy, K.; Singh, V.; Martinez, J.A.; Koshy, D.; Xu, F.; Chandrasekhar, A.; Dalton, C.; et al. Peripheral neuron plasticity is enhanced by brief electrical stimulation and overrides attenuated regrowth in experimental diabetes. Neurobiol. Dis. 2015, 83, 134–151.
- Angeloni, N.L.; Bond, C.W.; Tang, Y.; Harrington, D.A.; Zhang, S.; Stupp, S.I.; McKenna, K.E.; Podlasek, C.A. Regeneration of the cavernous nerve by Sonic Hedgehog using aligned peptide amphiphile nanofibers. Biomaterials 2011, 32, 1091–1101.
- Angeloni, N.; Bond, C.W.; Harrington, D.; Stupp, S.; Podlasek, C.A. Sonic Hedgehog is neuroprotective in the cavernous nerve with crush injury. J. Sex. Med. 2013, 10, 1240–1250.
- Dobbs, R.; Choe, S.; Kalmanek, E.; Harrington, D.A.; Stupp, S.I.; McVary, K.T.; Podlasek, C.A. Peptide amphiphile delivery of Sonic Hedgehog protein promotes neurite formation in penile projecting neurons. Nanomedicine 2018, 14, 2087–2094.
- Dobbs, R.; Kalmanek, E.; Choe, S.; Harrington, D.A.; Stupp, S.I.; McVary, K.T.; Podlasek, C.A. Sonic Hedgehog regulation of cavernous nerve regeneration and neurite formation in aged pelvic plexus. Exp. Neurol. 2019, 312, 10–19.
- Choe, S.; Bond, C.W.; Harrington, D.A.; Stupp, S.I.; McVary, K.T.; Podlasek, C.A. Peptide amphiphile nanofiber hydrogel delivery of Sonic Hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanomedicine 2017, 13, 95–101.




