The peripheral nervous has important regenerative capacities that regulate and restore peripheral nerve homeostasis. Following peripheral nerve injury, the nerve undergoes a highly regulated degeneration and regeneration process, called Wallerian degeneration, where numerous cell populations interact to allow proper nerve regeneration. Recent studies have evidenced the prominent role of morphogenetic Hedgehog signaling pathway and its main effectors, Sonic Hedgehog (SHH) and Desert Hedgehog (DHH) in the regenerative drive following nerve injury. Furthermore, dysfunctional regeneration and/or dysfunctional Hedgehog signaling participate in the development of chronic neuropathic pain that sometimes accompanies nerve healing in the clinical context. Understanding the implications of this key signaling pathway could provide exciting new perspectives for future research on peripheral nerve healing.
- Hedgehog signaling
- Sonic Hedgehog
- Desert Hedgehog
- Peripheral nerve healing
- Peripheral nerve injury
1. Introduction
Contrary to its central counterpart, the peripheral nervous system exhibits a high regenerative capacity notably following peripheral nerve injury [1][2][1,2]. Such regenerative capacity is thought to result from the remarkable plasticity of Schwann cells, key effectors of peripheral nerve healing and homeostasis [3][4][3,4]. Following injury, Schwann cells transdifferentiate into repair Schwann cells that express a new genetic repair program to allow nerve healing and the restoration of peripheral nerve homeostasis [2]. This new repair program includes the activation of proregenerative genes that are repressed during development and in uninjured nerves such as the morphogen Sonic Hedgehog [2][5][6][2,5,6].
Hedgehog signaling plays an essential morphogenetic role in the development of the central [7][8][9][10][7,8,9,10] and peripheral nervous system [11], with major roles in patterning and cell fate specification [9]. The presence and/or reactivation of morphogens in adulthood has shed light on the implication of such morphogenetic pathways in central nervous system homeostasis [9][12] [9,12] and disease [13] [13] but also in physiological [14][15][16] [14,15,16] and pathological peripheral nerve healing [16][17] [16,17] and in neuropathic pain development [18][19][20][18,19,20].
2. Hedgehog Signaling in Peripheral Nerve Morphogenesis
The Hedgehog pathway, roughly comprised of three proteins Sonic Hedgehog (SHH), Desert Hedgehog (DHH), and Indian Hedgehog (IHH), three transmembrane receptors (Patched-1, Patched-2, and Smoothened), and three transcription factors (Gli-1, Gli-2, Gli-3), is essential for the ontogenic development of mammals [9] [9] with several time-dependent and concentration-dependent morphogenetic effects on numerous cell lines, including the central [12] [12] and peripheral nervous system [11].
Whereas the role of Hedgehog morphogens (and underlying signaling pathway) has been extensively studied in embryonic development and in the patterning/differentiation of the central nervous system (e.g., dorsal–ventral axis patterning of the neural tube and establishment of distinct ventral neuronal populations in a concentration-dependent manner) [9][9], the implication of the Hedgehog pathway is less clear in the peripheral nervous system. For instance, SHH is not expressed in Schwann cells at any stage of development [5][6] [5,6] (as it is constitutively repressed during embryonic and promyelinating states of development by the polycomb pathway [4]) whereas DHH plays key roles in peripheral nerve structure specification during development [11].
During peripheral nerve morphogenesis the concomitant and symbiotic development of both neuronal axons and Schwann cells drive the formation of the myelin sheath and specialization of axonal structures such as the nodes of Ranvier [21]. Such axo-glial interactions are under the dependence of Schwann cell development, originating from neural crest cells that differentiate into Schwann cell precursors, then into immature Schwann cells before specification into myelinating or nonmyelinating Schwann cells [21]. Myelinating Schwann cells form a 1:1 contact with large caliber axons, wrapping them in a myelin sheath whereas nonmyelinating Schwann cells (also called Remak Schwann cells) surround multiple small caliber axons without any myelination [2]. Schwann cells and axons are supported and insulated by a connective tissue sheath of fibroblasts scattered between the nerve fibers that form the epineurium, perineurium, and endoneurium of the peripheral nerve [22].
The appearance of immature Schwann cells during development appears to coincide with the generation of the peripheral nerve’s extracellular matrix and the development of endoneurial connective tissue and blood vessels [23]. DHH and vascular endothelial growth factor (VEGF)-mediated signaling, originating from such immature Schwann cells and related axons, drive the differentiation and organization of arterial and perineurial cells, modeling the peripheral nerve’s architecture [11].
Indeed, deletion of the Dhh gene in mice results in the disruption of the fascicular structure of peripheral nerves, with a thin and disorganized perineurial sheath and an increase in blood–nerve barrier permeability [11]. Interestingly, a similar structural anomaly was found in a case of homozygous missense mutation of the dhh gene in a human diagnosed with “minifascular neuropathy” [24].
3. Future Research and Clinical Perspectives
3.1. Could Hyperactive Nerve Regeneration Drive Neuropathic Pain Development?
Recent evidence has raised the possibility that neuropathic pain could indeed result from dysfunctional nerve healing, but actually from a hyperactive nerve regeneration that fails to properly reinnervate the injured nerve [25][94]. Following spinal nerve ligation (ligation of L5 spinal nerve and cut at 1 mm distal to the suture), a common temporarily painful neuropathy model, the nerve could regenerate into the sciatic nerve (with restoration of electrical conduction, mechanical responses, and proper tracer migration) and the regenerating nerve was in fact the source of pain in this model [25][94]. Interestingly, disrupting the nerve regeneration inhibited the pain. Comparatively, in a spared nerve injury model (where pain is thought to be permanent), the regeneration process resulted in tangled neuromas at the injury site without proper reinnervation (as observed in the spinal nerve ligation model). Blocking the regeneration process via perfusions of Semaphorin 3A (an inhibitory axonal guidance molecule) prevented or reversed the painful behavior (even without removing the tangled fibers). It is thus concluded that in this model, the long-lasting pain behavior could stem from the anatomical inability of regenerating nerves to successfully reinnervate their target tissues, resulting in a persistent, yet futile, regeneration process [25][94].
A recent clinical study supports this hypothesis. In a study on patients suffering from chronic inflammatory demyelinating polyneuropathy (CIDP), shh mRNA expression was significantly higher in skin biopsies of CIDP patients that those of control subjects (with similar intra-epithelial nerve fiber density), and this high expression of shh mRNA was decreased following treatment of CIDP [26][95]. This suggests that in the context of this immune-mediated neuropathy, the small depleted axons attempt to mount a regenerative response [26][95]. Whether this regenerative response takes part in the underlying pathophysiology of CIDP still remains to be answered.
3.2. Understanding Hedgehog Signaling Inhibition in the Chronic Constriction Injury (CCI) Model
Considering the essential role of Hedgehog signaling in molecular, vascular, and behavioral changes observed in CCI models [18][19][20], understanding the underlying mechanisms of such inhibition could be of interest and help explain the lack of pre/post-treatment effect of Hedgehog pathway activation on neuropathic pain in such experimental conditions [18][19].
It is well known that Hedgehog signaling requires a functional ciliary architecture [27][28] and that loss of primary cilia is associated with Hedgehog pathway inhibition [29][30]. Furthermore, it has been shown that the Smoothened receptor is necessary for the development and maintenance of ciliary architecture [31]. Interestingly, chronic constriction injury of the sciatic nerve in rats leads to a significant and persistent downregulation of
Smoothened
Figure 1), that could participate in the loss of primary ciliary architecture and subsequent Hedgehog pathway inhibition.
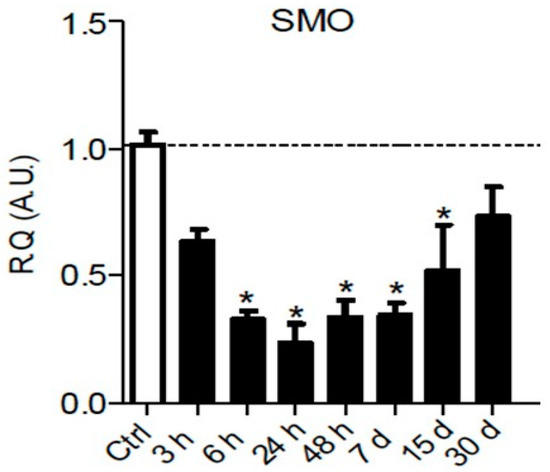
Figure 13.
p
Smoothened mRNA and protein [32]) (
Figure 2), possibly linked to a loss of Smoothened receptor [31].
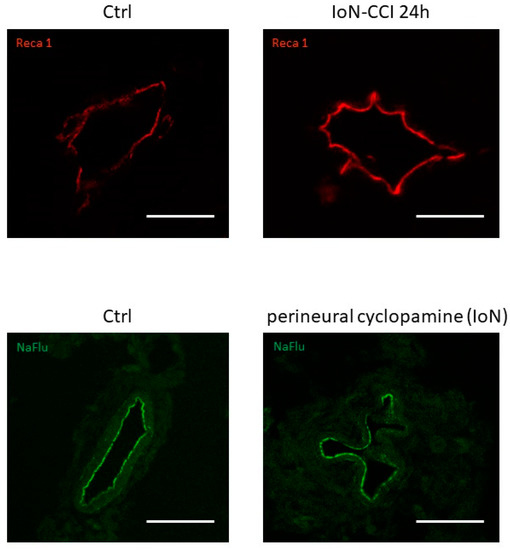
Figure 24.
upper left panel
upper right panel
lower left panel
lower right panel) using Reca-1 and sodium fluorescein (NaFlu) for vessel immunolabeling, respectively. Chronic constriction injury and perineural injections were performed according to previously described methodologies (see Moreau et al. 2016 [18], 2017 [19] for methodological details).
3.3. Investigating and Developing New Nerve Regeneration Strategies
Although adult peripheral nerves have an intrinsic ability to regenerate, the endogenous response is often limited and does not allow for full recovery of function. Manipulation of the nerve microenvironment to promote neuronal survival and repair could be key to improve regeneration strategies [33].
For instance, a few studies have invested the role of brief extracellular electrical stimulation in fostering nerve regeneration, as it has been shown that such electrical stimulation applied immediately after nerve injury could increase nerve regeneration in animal models and humans [34]. In a diabetic neuropathy model, an enhanced expression of
Shh mRNA was observed in ipsilateral DRGs following electrical stimulation, suggestion a potential role in electrical stimulation-mediated peripheral nerve regeneration. Interestingly, no rise in SHH protein was observed in the sciatic nerve [35], which suggests that
Shh
Finally, several studies have investigated new means of SHH protein delivery in a model of cavernous nerve crush injury, using a peptide amphiphile nanofiber hydrogel that allowed efficient local delivery of SHH [36][37][33][38][39][40]. Such delivery strategy could be applied to other models for future investigations.
4. Conclusions
The Hedgehog pathway that plays key morphogenetic roles during development also assumes critical roles in peripheral nerve healing following injury and in peripheral nerve homeostasis, such as preventing myelin degradation or maintaining blood–nerve barrier impermeability. Schwann cell, neuronal and endothelial-mediated Hedgehog signaling cooperate to promote physiological Wallerian degeneration and proper nerve regeneration.
Elucidating the numerous roles of Hedgehog signaling during normal and dysfunctional nerve healing could bring a better understanding of nerve regeneration processes and their still-ambiguous relation to neuropathic pain development.
As hyperactive regeneration could participate in neuropathic pain development, nerve regeneration can be construed as a physiological process that requires precise temporal and spatial controls, and thus morphogens such as Sonic Hedgehog or Desert Hedgehog would be ideal regulators of such peripheral nerve homeostasis.
