| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mirko Pesce | + 4069 word(s) | 4069 | 2020-10-22 05:07:03 | | | |
| 2 | Bruce Ren | Meta information modification | 4069 | 2020-10-29 02:48:32 | | |
Video Upload Options
Aging and sedentary life style are considered independent risk factors for many disorders. Under these conditions, accumulation of dysfunctional and damaged cellular proteins and organelles occurs, resulting in a cellular degeneration and cell death. Autophagy is a conserved recycling pathway responsible for the degradation, then turnover of cellular proteins and organelles. This process is a part of the molecular underpinnings by which exercise promotes healthy aging and mitigate age-related pathologies. Irisin is a myokine released during physical activity and acts as a link between muscles and other tissues and organs. Its main beneficial function is the change of subcutaneous and visceral adipose tissue into brown adipose tissue, with a consequential increase in thermogenesis. Irisin modulates metabolic processes, acting on glucose homeostasis, reduces systemic inflammation, maintains the balance between resorption and bone formation, and regulates the functioning of the nervous system. Recently, some of its pleiotropic and favorable properties have been attributed to autophagy induction, posing irisin as an important regulator of autophagy by exercise.
1. Introduction
The myofibers are cells multinucleated that composed the skeletal muscle (SkM). These cells are responsible of the force generation by sarcomeres’ contraction and release several soluble factors (e.g., cytokines) exerting paracrine and endocrine functions. These molecules are named “myokines” [1][2].
Myokines regulate a variety of metabolic processes in various tissues and organs, such as liver, bones, brain, or fat tissue, through several signaling pathways. The extent of the myokines release response after muscular contraction varies with the intensity, mode, and volume of exercise an individual performs. Some myokines may be anabolic and have direct growth-promoting effects. Still others generate signals that may mediate some of the health benefits of physical exercise [3][4][5]. Since their discovery in 2000 [6], myokines have continued to generate increasing interest.
In 2012, a new myokine expressed through the transcription factor peroxisome proliferator-activated receptor gamma co-activator-1a (PGC-1α) activation by exercise-induced effects, was discovered. The newly identified molecule has been called “irisin,” derived from the name of the Greek goddess Iris. It is released as a hormone-like myokine as protein cleaved from transmembrane protein precursor fibronectin type III domain-containing protein 5 (FNDC5) encoded by the FNDC5 gene. This hormone induces changes in the subcutaneous adipose tissue stimulating browning. In general, irisin causes a significant increase in total body energy expenditure and resistance to obesity-linked insulin-resistance (IR). Irisin function summarizes some of the most important benefits of exercise and muscle activity. It was proposed to act as a link between the muscles and other tissues of the body [7].
Autophagy is a catabolic process that has an essential role in cellular homeostasis, facilitating lysosomal degradation and recycling of intracellular misfolded proteins and injured organelles. It is involved in the maintenance of various physiological responses and plays dual roles in inducing cytoprotection and cell death [8]. Regulation of autophagy formation is tightly controlled to maintain nutrient and energy homeostasis. The process is well conserved in organisms, and in response to nutritional deprivation or intracellular pathogens, it acts as a protective mechanism that allows cells to survive under stressful conditions. The process begins with an expanding membrane structure which leads to formation of a double-membrane vesicle, called autophagosome. Autophagosomes are subsequently fused with lysosomes, in which the resulting molecules are degraded and used in the recycling process. Abnormal regulation of autophagy may be an attribute to various pathologic conditions [9] and has been widely implicated in cancer, metabolic disorders, and neurodegenerative diseases, as well as muscle dystrophy. It also plays an important role in aging [10].
Regular physical activity is considered as a nondrug therapy for the treatment of several diseases, such as metabolic diseases [11]. Its beneficial effects on adults’ health has been related also to the induction of autophagy. Recent findings indeed suggested that exercise can induce autophagy in several tissues (e.g., adipose tissue and SkM) and organs (e.g., pancreas, liver, heart, and brain), supporting the evidence that exercise-mediated induction of autophagy could occur at systemic level [12][13][14].
Since then, irisin has been the subject of extensive research, which enabled the gaining of insight into its pleiotropic properties. The role of irisin on autophagy has been studied only recently, particularly during the last 3 years. However, these recent studies have not yet been reviewed. Given the emerging importance of irisin and autophagy in mediating the beneficial effects of exercise, in this review, we perform a first update on studies focused on the relation within FNDC5/irisin system and autophagy process. As irisin is a myokine induced after physical exercise, relation between exercise and autophagy as well as myokines and autophagy have been also reviewed.
2. Molecular Mechanism of Autophagy
Autophagy is recognized as a vital process in the cells that plays important role in biological evolution, immune system, and cell death and is involved in fatal disorders such as nervous system defects, autoimmune diseases, and a variety of cancers. Autophagy has a dual function, on one hand, it increases the duration and survival of the cells and on the other hand, in the advanced stages, it causes cell death [15].
In detail, autophagy is a degradative process including lysosomes, mainly involved in degradation of damaged cells organelles to recycle the basic cellular components, in that way providing the energy for cell recovery. The autophagy impairment is characterized by the insufficiency to remove damaged organelles or debris. The process activation was originally identified in response to starvation in eukaryotic systems. The process is evolutionary, highly conserved, and play a housekeeping role in maintaining the integrity of intracellular proteins and organelles in many cell types. Recent studies have shown that the key role of autophagy is to keep cells alive under stressful conditions, including endoplasmic reticulum stress, infection, and hypoxia [16]. Activation of autophagy pathway in SkM during exercise training as a protective mechanism can also be effective in adaptation and improvement of physical performance [17].
Exercise is indeed a new defined stimulus that can induce autophagy in cells, and its relationship with autophagy is treated with more details in Section 5. Additionally, studies have shown that autophagy increases in a variety of liver injuries, e.g., it can increase cell survival during liver ischemia reperfusion, acute liver injury and liver surgery, improvement of hepatocellular carcinoma by its antitumor activity and improvement of fatty liver disease by lipid degradation to fatty acid and modulation of insulin signaling pathway [18]. In another study that evaluates the role of autophagy in renal physiology states that autophagy is involved in improvement of kidney defects such as diabetic nephropathy, tubular injuries, kidney development, and kidney cancers [19].
There are three defined types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy, all of which promote proteolytic degradation of cytosolic components at the lysosome [16]. Here, macroautophagy is referred to as autophagy.
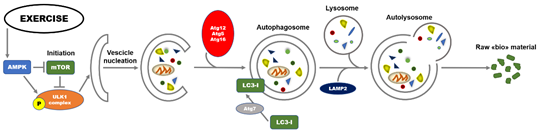
Diverse molecular signals are involved in the regulation of autophagy. At transcriptional level, regulation includes the transcription factors nuclear factor kappa B (NFkB), hypoxia-inducible factor-1 (HIF-1), and Forkhead Box O (FOXOs). The process is regulated by a highly conserved family of proteins referred to as autophagy-related genes (Atgs) and is mainly divided into different and coordinated stages: initiation, vesicle nucleation, vesicle elongation, vesicle retraction, and finally, fusion and degradation [20]. In the initiation stage of autophagy, there is the activation of the Unc-51-like autophagy activating kinase 1 (ULK1) complex and two protein factors, including focal adhesion kinase family interacting protein of 200 kDa (FIP200) and Atg13 responsible for maintaining the stability of ULK1 protein, and its correct localization to autophagy precursors [21]. When nutrients are deficient, AMP-activated protein kinase (AMPK, a sensor for detecting nutrition and energy efficiency in tissues) can directly phosphorylate the Raptor protein at Ser722 and Ser792 sites, inhibit mammalian target of rapamycin (mTOR) signaling, and deactivate the phosphorylation of ULK1, thereby activating autophagy [22]. The activity of ULK1 is enhanced by direct phosphorylation of AMPK. As a consequence, the formation of autophagic bilayer membrane is hasten following the formation of the complex including ULK1-Atg13-FIP200 [23]. On the other hand, under nutrient-rich conditions, mammalian target of rapamycin complex 1 (mTORC1) phosphorylates ULK1 at Ser757, to inhibit autophagy initiation. During the second stage, the complex Beclin1-class III phosphoinositide 3-kinase (PI3K) plays a significant role in the induction of autophagy acting on the recruitment of lipids and proteins from the cytoplasm for the synthesis of autophagosome membranes [24]. To follow, the extension of autophagosome membrane is mainly controlled by Atg7 and Atg10. Atg7 is responsible (and marker) of autophagic vasculogenesis and elongation regulating the formation of autophagic bubbles. Under this stage, Atg7 can catalyze the conversion of critical protein LC3I to LC3II that is considered the “hallmark” biomarker for evaluating the activation of autophagy. Through vesicle retraction stage, the mature autophagosome is finally formed recruiting Atg12-Atg5-Atg16L complex. Ultimately, the autophagolysosomes were formed by the fusion of lysosomes and autophagosomes, also through the involvement of the lysosomal-associated membrane protein 2 (LAMP2). The autophagolysosomes are responsible of degradation of damaged macromolecules and dysfunctional organelles in cells for the recycling; the contents are broken down into constituent macromolecular precursors that can be reused as raw “bio”-material or, alternatively, metabolized (fusion and degradation stage) [25]. A summary of the molecular events involved in the autophagy process is illustrated in Figure 1.
Nevertheless, some studies approve the inhibition of binding process between autophagosome and lysosome to result in the decrease in lysosomal degradation rate of autophagosomes and upstream the proportional expression of LC3II and LC3II/LC3I ratio. This indicates that the augmented expression of LC3II does not completely represent the occurrence of autophagic degradation process [26].
The autophagic adaptor p62/SQSTM1 permits to recycle nutrients and ATP by the recognition, division, and transfer of p62 or the degradation of its substrate. In this stage, p62 is negatively correlated to the autophagic flux downstream the lysosome activity of autophagolysosomes [27]. In summary, it is broadly accepted that the combination of expression level of p62 and the conversion rate of LC3 could be considered the best biomarkers for evaluating the functional status of autophagy.
Figure 1. Overview of basic molecular mechanisms involved in initiation of exercise-induced autophagy. Autophagy is a lysosomal-mediated degradation process for primarily degrading damaged cells and dysfunctional organelles to recycle damaged or dysfunctional cellular contents, thereby providing the energy for nascent cells. Exercise, as a newly defined stimulus, can induce autophagy in cells. mTOR and AMPK are currently recognized as the sensors for detecting nutrition and energy efficiency in skeletal muscle, especially in exercise responses. mTOR and AMPK in the initial steps of the autophagy process through phosphorylation interaction with the ULK1 complex, respectively, whereas AMPK inhibits mTOR activity. AMPK: AMP activated protein kinase; Atg: autophagy related gene; LC3: microtubule-associated protein 1A/1B-light chain 3; mTOR: mammalian target of rapamycin; P: Phosphate; ULK1: Unc-51 like autophagy activating kinase 1.
3. Myokines
The SkM has the function of maintaining body temperature, generating force, and maintaining posture interacting with bone and facilitating various movements. This tissue possesses the ability to adapt to various physiologic conditions such as chronic hypokinesia or exercise [28]. Nevertheless, during the last years, a great deal of data highlighted that the SkM tissue is the main protein reserve of the body [29], a regulator of health and survival, acts on metabolic balance, and finally, determines the quality of life [30]. The plasticity of SkM confers the tissue the property to constantly adapt to various settings such as hypokinesia, leading to maintenance of muscular atrophy (reduction in muscle mass), or mechanical stimulation, leading to the condition of maintenance of muscular hypertrophy (increase in muscle mass). SkM has also an elevated capacity to modify its phenotype in relation to the mechanical load applied to it [31]. Pedersen et al. (2007) first identified that SkM is additionally an organ that release a great amount of molecules such as cytokines and other peptides, which have been given the name of myokines. After the discovery that muscle contraction produces these molecules, in 2013, Pedersen defined a new paradigm, i.e., SkM is a secretory organ, synthesizing and secreting myokines in response to muscle contraction. The molecules secreted can influence the metabolism and function of SkM, in addition to other tissues and organs [32].
The interest on the molecules released by SkM became relevant during the second half of the last century. Physical exercise was proposed as a modulator of glucose metabolism, however, with no definition about the mechanisms involved in this process [33]. The terms “exercise factor,” “work factor,” or “work stimulus” were used to define the capacity of the SkM tissue to release myokines at systemic level during or after muscle contraction. This concept was founded on the fact that the engagement of metabolic and physiological responses in other organs by muscle contractions are not mediated by the nervous system [34]. It was supported by previous study with electrical stimulation in paralyzed muscles, in which Kjaer and coworkers verified that the ability of muscle contractions in regulating other tissues occurs through an independent pathway of activation of the nervous system [35]. Therefore, a muscle memory could hypothesize independently, but in any case, related to the central nervous system (CNS). Muscle memory could organize and structure itself based on the secretion, role, and activation of the muscle myokines, which open up interesting perspectives [36]. From that moment, myokines began to be understood as a protective factor against disease, aging and the adverse effects of physical inactivity.
The level of the myokines’ plethora is promoted by both exercise at high-intensity and hypokinesia, regulating numerous physiological adaptations. Several studies have demonstrated that myokines can regulate physiological and metabolic pathways in other tissues. To date, the myokines that have been described are included of cytokines, peptides, growth factors, and small organic acids. These molecules exert an effect on muscle function itself as well as on overall metabolism and comprise myostatin (MSTN), the small organic acid b-aminoisobutyric acid (BAIBA), meteorin-like (METRNL), myonectin, decorin, various interleukins (IL-4, IL-6, IL-7, IL-8, and IL-15), irisin, fibroblast growth factor-21 (FGF-21), brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1), leukemia inhibitory factor (LIF), and follistatin like protein-1 (FSTL-1) [36][37]. Their diverse effects can be positive or negative and provide the underpinning of the crosstalk between SkM and other organs, such as adipose tissue, brain, bone, liver, pancreas, kidney, and immune cells. The SkM is the most abundant tissue in healthy individuals, constituting approximately 40% of body mass in young adults, as a consequence, any endocrine activity would be expected to have a strong effect on physiology of the organism [33]. In general, muscle atrophy is related to aberration of metabolism associated with a variety of clinical conditions, including diabetes, obesity, renal failure, chronic obstructive pulmonary disease, cancer, and muscle hypokinesia [38]. For instance, individuals are highly predisposed to obesity, IR, and diabetes mellitus that are characterized by reduced basal metabolic rate and low physical activity [39].
GWAS and transcriptomics analyses suggested that more than 250 putative myokines exist, 100 of which have been also identified by proteomics approaches. Recent reviews have treated the important biological effects, on specific cells and tissues, of the most studied myokines (e.g., IL-6 and irisin), highlighting their biomedical relevance [40][41]. Several evidences supported the idea that autophagy acts as trait d’union in this process. In Section 6, the potential role of relevant myokines (IL-4, IL-6, IL-7, IL-15, decorin, LIF, metrnl, myonectin, and myostatin) on autophagy regulation has been discussed.
4. Irisin Structure and Function
In 2012, Boström et al. discovered a new molecule named “irisin” secreted by myocytes, which was proposed to act as a connection between the muscles and other tissues of the body, and in particular, was able to activate thermogenesis and induce changes in adipose tissue. Since then, irisin has been the subject matter of extensive research, which allowed the gaining of insight into its pleiotropic properties.
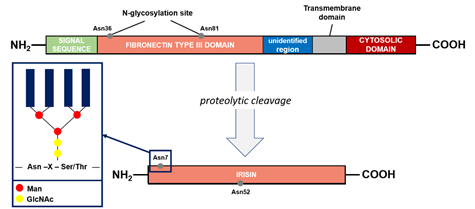
The precursor of irisin is FNDC5 (FRCP2/PeP), a cell membrane protein. This protein contains an N-terminal signal sequence of 29 amino acid (aa), a fibronectin type III domain with 94 aa, an unidentified region consisting of 28 aa, a transmembrane domain having 19 aa, and a C-terminal part with 39 aa (Figure 2), for a total of 209 aa residues. The extracellular N-terminal portion is proteolytically cleaved to produce irisin that is released into the circulation, whereas the C-terminal fragment of FNDC5 is located in the cytoplasm [42]. Irisin is composed of 112 aa. Its structure has been revealed by X-ray crystallography and biochemical studies: the protein occurs in the form of homodimers, where a b-sheet is created between the units. In addition, the interactions between the side chains of adjacent subunits occurs between Arg75 and Glu79, which in turn protect the dimer ends and Trp90/Trp90, stabilizing the structure [43].
During the post-translational process of N-glycosylation, a variable number and structure of oligosaccharides (glycans) were attached to the FNDC5 protein, resulting in a mass range from 20 to 32 kDa. In general, the glycosylation process is fundamental for the elicitation of the biological functions of the proteins. FNDC5 contains the sequence Asn-X-Ser/Thr in which oligosaccharides were attached to the asparagine residue by a N-acetylglucosamine residue (GlcNAc). The X indicates any amino acid apart from proline. A high-mannose/oligomannose type, complex type, and hybrid type are the main three groups that can be linked to Asn by N-glycosidic bonds [44]. The sequence of FNDC5 contains three potential N-glycosylation sites and two of them, Asn36 and Asn81, are occupied by N-glycans (Figure 2). However, the structure and the glycosylation process are still poorly characterized. The presence of glycans act significantly on the stability of the molecule. The glycosylated molecule is significantly more resistant against protein synthesis inhibitors compared to the de-N-glycosylated FNDC5. The half-life of the protein is significantly reduced, from 12 to 7 h, when one of the N-glycosylation sites is removed. In addition, the de-N-glycosylated protein is not incorporated in the plasmatic membrane, causing significant reduction in irisin release at systemic level. Irisin is also characterized by the presence of two N-glycosylation sites (Asn7 and Asn52), whereas deglycosylation lowers the molecular weight of irisin to 12 or 15 kDa. In alternative, the addition of one or two sugar chains increases its mass to 22 or 25 kDa, respectively. These modifications do not affect the formation of irisin dimers but are important to the primary function of irisin on the adipocytes [44][45].
Figure 2. Fibronectin type III domain-containing protein 5 (FNDC5) structure and formation of irisin. The potential N-glycosylation sites are marked as gray dots. Asn, asparagine; GlcNAc, N-acetylglucosamine; Man, mannose; Ser, serine; Thr, threonine; X, any amino acid except proline.
So far, a specific receptor for irisin has not been identified. Recent studies have showed that irisin could exerts its action via binding to integrins of av family in some tissues [46]. Integrins are transmembrane receptors ubiquitously expressed that permit to cell interactions with extracellular matrix ligands, intercellular interactions, and nevertheless recognize soluble ligands. In summary, these proteins are strictly involved in the adhesion, migration, and aggregation of cells. Twenty-four different integrin heterodimers exist, due to the interactions between 18 a-subunits and eight b-subunits [47]. In 2018, Kim and coworkers showed the binding of irisin with high affinity to aVb5 integrin present in the fat cells and osteocytes. The use of the integrin inhibitor RGD peptide (which binds to avb5) suppressed any signaling response triggered by irisin. Irisin also binds other integrins including a1b1. When the osteocytes were treated with irisin, the level of phosphorylation of focal adhesion kinase (FAK) increased significantly, whereas FAK is the main protein involved in the integrin signaling. The authors suggest that the main receptors for irisin could be the heterodimers belonging to the av integrin family in all the tissues [46].
Irisin is secreted primarily by SkM as well as subcutaneous and visceral adipose tissues. However, as showed by immunohistochemical studies, smaller amounts of irisin are also produced by brain, heart, liver, pancreas, spleen, stomach, and testes [48]. Its level increases in the blood after physical exercise. Bostrom et al. (2012) observed a 65% increase in irisin concentration in mice running regularly for 3 weeks. An increase in the level of this myokine by two times was also found after 10 weeks of supervised training in the blood of healthy people. Physical exercise is known to increase the level of irisin also in people with metabolic disorders [49]. Nevertheless, irisin level was significantly lower in the serum of urban inhabitants compared to rural subjects, so depending on the activities performed at residential place. In general, irisin concentration is lower in sedentary rather than in active individuals, where the concentration of irisin was found to be about 4.3 ng/mL in the blood of the active subjects and descend to 3.6 ng/mL in sedentary ones [50]. Moreover, some data suggested that the type of physical activity is important in determining irisin level, because irisin induction was noted after resistance training and high-intensity exercise, but not after endurance exercise [51][52].
The circulating level of irisin is related also to the phenotype of different disease. For example, modulation of irisin concentrations were described in obese subjects, in patients with type 2 diabetes (T2DM) [53], chronic renal disease (CKD) [54], and hypothyroidism [55].
The first discovered function of irisin is the ability to induce changes in adipose tissue. Irisin and PGC-1a regulate the expression of UCP-1 and thermogenesis in brown adipose tissue (BAT). As a consequence, the metabolism of glucose and lipids are driven through the increase in energy consumption [56]. Irisin also affects the functioning of white adipose tissue (WAT) by a process named “browning,” where irisin increased the expression of UCP-1 in mature fat cells allowing the conversion of the WAT phenotype in the BAT phenotype. The result is the formation of the beige/brite adipose tissue, the third type of adipose tissue. The treatment of WAT preadipocytes with irisin inhibited adipogenesis and effectively reduced the formation of new adipocytes [57].
Other effects of irisin involved glucose homeostasis and insulin sensitivity improvement in IR, acting on adipose tissue, SkM, liver, and pancreatic b cells [58]. Obesity is usually accompanied by IR, type 2 diabetes, and cardiovascular diseases [59]. Significant changes in the irisin levels have been described in the blood of people affected by obesity. Previous studies suggested that irisin circulating level is reduced in obese subjects [53], on the other hand, more recent studies showed opposite results [60][61]. A state of irisin resistance may be reported during the course of obesity development, which could explain the elevated levels of irisin in these subjects [60].
It was found that the decrease in irisin is associated with an increased risk of presenting metabolic syndrome and hyperglycemia in obese adults, considering it to be protective against IR because it shows negative associations with fasting insulin and glycosylated hemoglobin [62]. To this end, Al-Daghri et al. observed negative association between irisin and fasting glucose and HOMA-IR in school-age Saudi boys and girls [63]. Conversely, there are positive associations between irisin and insulin concentration, fasting glucose, and HOMA-IR [64][65][66][67]. Fukushima et al. analyzed a positive correlation on obese men and adult women [66], while Pardo et al. determined a correlation in women with anorexia nervosa, normal weight, and obesity [65]. Park et al., in a study with people with metabolic syndrome and healthy control, suggested that people with metabolic syndrome had higher concentrations of irisin, associating increased irisin with a greater amount of fat mass in obesity condition, in addition to the possible resistance to irisin or its compensatory role [67]. Nevertheless, it has been suggested that low level of irisin could be due to the reduction in sensitivity to insulin and lipid and glycolytic metabolism [64].
Irisin also exerts anti-inflammatory and antiapoptotic effects on cardiomyocytes and hepatocytes, protecting these cells from ischemia-reperfusion injury [68][69]. These effects are mediated by the induction of autophagy and have been described in more details in Section 7. Irisin has also favorable effect on brain, increasing the proliferation of mouse hippocampal neuronal cells [70], and reducing oxidative stress-induced neuronal damage by inhibiting the secretion of proinflammatory cytokines [71]. In mouse model, the FNDC5/irisin system is fundamental for long-term potentiation and memory in the hippocampal region, the expression of FNDC5/irisin is indeed necessary to establish synaptic plasticity and memory. In human, a study on adult volunteers at risk of dementia submitted to 10 weeks of physical training, showed a positive correlation between cognition/episodic memory and serum irisin [72]. In addition to serotonin, irisin may contribute to the antidepressant effect of exercise, most likely associated with the activation of the PGC-1a/BDNF pathway [73]. At bone level, irisin acts as a link between SkM and this tissue, increasing the mass and strength of the cortical bones and positively modifying their geometry by reducing the secretion of osteoblast inhibitors and causing the expression of bone-specific genes [74]. Nevertheless, irisin mediates the positive anti-inflammatory effect induced by regular and moderate physical activity on the functioning of the immune system reducing systemic inflammation [75] and protecting from the development of diseases associated with chronic inflammation.
References
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195.
- Pedersen, B.K.; Akerstrom, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098.
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25.
- Hoffmann, C.; Weigert, C. Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harb. Perspect. Med. 2017, 19, 270–275.
- Lee, J.H.; Jun, H.S. Role of myokines in regulating skeletal muscle mass and function. Front. Physiol. 2019, 10, 42.
- Steensberg, A.; Van Hall, G., Osada, T. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000, 529, 237–242.
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; A PGC1--dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468.
- Park, P.H. Autophagy induction: a critical event for the modulation of cell death/survival and inflammatory responses by adipokines. Arch. Pharmacal Res. 2018, 41, 1062–1073.
- Moulis, M.; Vindis, C. Autophagy in metabolic age-related human diseases. Cells 2018, 7, 149.
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. New Engl. J. Med. 2013, 368, 651–662.
- Pedersen, B.K., Saltin, B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport. 2015, 25, 1–72.
- He, C.; Sumpter, R.; Levine, B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 2012, 8, 1548–1551.
- Moreira, O.C.; Estebanez, B.; Martinez-Florez, S.; de Paz, J.A.; Cuevas, M.J.; Gonzalez-Gallego, J. Mitochondrial function and mitophagy in the elderly: effects of exercise. Oxidative Med. Cell. Longev. 2017, 2017, 2012798.
- Zhang, Y.; Chen, N. Autophagy is a promoter for aerobic exercise performance during high altitude training. Oxidative Med. Cell. Longev. 2018, 2018, 3617508.
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395.
- Lee, D.E.; Bareja, A.; Bartlett, D.B.; White, J.P. Autophagy as a Therapeutic Target to Enhance Aged Muscle Regeneration. Cells 2019, 20, 8.
- Lira, V.A.; Okutsu, M.; Zhang, M.; Greene, N.P.; Laker, R.C.; Breen, D.S. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013, 27, 4184–4193.
- Rautou, P.E.; Mansouri, A.; Lebrec, D.; Durand, F.; Valla, D.; Moreau, R. Autophagy in liver diseases. J. Hepatol. 2010, 53, 1123–1134.
- Wang, Z.; Choi, M.E. Autophagy in kidney health and disease. Antioxidants Redox Signal. 2014, 20, 519–537.
- Park, S.S.; Seo, Y.K.; Kwon, K.S. Sarcopenia targeting with autophagy mechanism by exercise. BMB Rep. 2019, 52, 64–69.
- Ganley, I.G.; Lam du, H.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 2009, 284, 12297–12305.
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. cell 2008, 30, 214–226.
- Sanchez, A.M.; Bernardi, H.; Py, G.; Candau, R.B. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R956–R969.
- Levine, B.; Sinha, S.C.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606.
- Morell, C.; Bort, A.; Vara-Ciruelos, D.; Ramos-Torres, A.; Altamirano-Dimas, M.; Diaz-Laviada, I.; Rodriguez-Henche, N. Up-Regulated Expression of LAMP2 and Autophagy Activity during Neuroendocrine Differentiation of Prostate Cancer LNCaP Cells. PLoS ONE 2016, 11, e0162977.
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. neurosci. 2010, 13, 805–811.
- Frankel, L.B.; Wen, J.; Lees, M.; Hoyer-Hansen, M.; Farkas, T.; Krogh, A.; Jaattela, M.; Lund, A.H. microRNA-101 is a potent inhibitor of autophagy. EMBO J. 2011, 30, 4628–4641.
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362.
- Bentzinger, C.F.; Lin, S.; Romanino, K.; Castets, P.; Guridi, M.; Summermatter, S. Differential response of skeletal muscles to mTORC1 signaling during atrophy and hypertrophy. Skelet. Muscle 2013, 3, 6.
- Salanova, M.; Gelfi, C.; Moriggi, M.; Vasso, M.; Vigano, A.; Minafra, L. Disuse deterioration of human skeletal muscle challenged by resistive exercise superimposed with vibration: evidence from structural and proteomic analysis. FASEB J. 2014, 28, 4748–4763.
- Williamson, D.L.; Gallagher, P.M.; Carroll, C.C.; Raue, U.; Trappe, S.W. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J. Appl. Physiol. 2001, 91, 1955–1961.
- Speranza, L.; Grilli, A.; Patruno, A. Plasmatic markers of muscular stress in isokinetic exercise. J. Biol. Regul. Homeost. Agents 2007, 21, 21–29.
- Goldstein, M.S. Humoral nature of the hypoglycemic factor of muscular work. Diabetes 1961, 10, 232–234.
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346.
- Kjaer, M.; Pollack, S.F.; Mohr, T.; Weiss, H.; Gleim, G.W.; Bach, F.W. Regulation of glucose turnover and hormonal responses during electrical cycling in tetraplegic humans. Am. J. Physiol. 1996, 271, R191–R199.
- Misra, A.; Bloomgarden, Z. Metabolic memory: Evolving concepts. J. Diabetes 2018, 10, 186–187.
- Cardozo, C.P.; Graham, Z.A. Muscle-bone interactions: Movement in the field of mechano–humoral coupling of muscle and bone. Ann. New York Acad. Sci. 2017, 1402, 10–17.
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2018, 41, 14–29.
- Bauman, W.A.; Cardozo, C.P. Spinal cord injury: Skeletal pathophysiology and clinical issues. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Eighth Edition; Rosen CJ, ed.; Wiley-Blackwell: Oxford, UK, 2013.
- Karstoft, K.; Pedersen, B.K. Skeletal muscle as a gene regulatory endocrine organ. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 270–275.
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307.
- Panati, K.; Narala, V.R.; Narasimha, V.R.; Derangula, M.; Arva Tatireddigari,V.R.R.; Yeguvapalli, S. Expression, purification and biological characterisation of recombinant human irisin (12.5 kDa). J. Genet. Eng. Biotechnol. 2018, 16, 459–466.
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. Biol. Chem. 2013, 288, 33738–33744.
- Nie, Y.; Liu, D. N-Glycosylation is required for FDNC5 stabilization and irisin secretion. Biochem. J. 2017, 474, 3167–3177.
- Tan, N.Y.; Bailey, U.M.; Jamaluddin, M.F.; Mahmud, S.H.; Raman, S.C.; Schulz, B.L. Sequence-based protein stabilization in the absence of glycosylation. Nat. Commun. 2014, 5, 3099.
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215.
- Martinez Munoz, I.Y.; Camarillo Romero, E.D.S.; Garduno Garcia, J.J. Irisin a Novel Metabolic Biomarker: Present Knowledge and Future Directions. Int. J. Endocrinol. 2018, 2018, 7816806.
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457.
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart,W.; Fernández-Real, J.M. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE. 2015, 10, e0124100.
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism 2015, 64, 1042–1050.
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health 2020, 17, 3589.
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–778.
- Liu, J.J.; Liu, S.; Wong, M.D.; Tan, C.S.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J. Diabetes Complicat. 2014, 28, 208–213.
- Zybek-Kocik, A.; Sawicka-Gutaj, N.; Wrotkowska, E.; Sowi’nski, J.; Ruchała, M. Time-dependent irisin concentration changes in patients affected by overt hypothyroidism. Endokrynol. Pol. 2016, 67, 476–480.
- Gouveia, M.C.; Vella, J.P.; Cafeo, F.R. Affonso Fonseca, F.L.; Bacci, M.R. Association between irisin and major chronic diseases: A review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4072–4077.
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541.
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The myokine irisin is released in response to saturated fatty acids and promotes pancreatic betacell survival and insulin secretion. Diabetes 2017, 66, 2849–2856.
- Meilianna, A.; Dewi, N.M.; Wijaya, A. Adipose tissue, inflammation (Meta-inflammation) and Obesity management. Indones. Biomed. J. 2015, 7, 129–146.
- Sahin-Efe, A.; Upadhyay, J.; Ko, B.J.; Dincer, F.; Park, K.H.; Migdal, A.; Vokonas, P.; Mantzoros, C. Irisin and leptin concentrations in relation to obesity, and developing type 2 diabetes: A cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism 2018, 79, 24–32.
- De Meneck, F.; de Souza, L.V.; Oliveira, V.; do Franco, M.C. High irisin levels in overweight/obese children and its positive correlation with metabolic profile, blood pressure, and endothelial progenitor cells. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 756–764.
- Yan, B.; Shi, X.; Zhang, H. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE 2014, 9, e94235.
- Al-Daghri, N.M.; Alkharfy, K.M.; Rahman, S. Irisin as a predictor of glucose metabolism in children: sexually dimorphic effects. Eur. J. Clin. Investig. 2014, 44, 119–124.
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738.
- Pardo, M.; Crujeiras, A.B.; Amil, M. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int. J. Endocrinol. 2014, 2014, 857270.
- Fukushima, Y.; Kurose, S.; Shinno, H. Relationships between serum irisin levels and metabolic parameters in Japanese patients with obesity. Obes. Sci. Pr. 2016, 2, 203–209.
- Park, K.H.; Zaichenko, L.; Brinkoetter, M. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907.
- Bi, J.; Yang, L.; Wang, T.; Zhang, J.; Li, T.; Ren, Y.; Wang, M.; Chen, X.; Lv, Y.; Wu, R. Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver Injury. Oxid. Med. Cell. Longev. 2020, 2020, 6946037.
- Xin, T.; Lu, C. Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction. Aging 2020, 12, 4474–4488.
- Moon, H.S.; Dincer, F.; Mantzoros, C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 2013, 62, 1131–1136.
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42.
- Küster, O.C.; Laptinskaya, D.; Fissler, P.; Schnack, C.; Zügel, M.; Nold, V.; Thurm, F.; Pleiner, S.; Karabatsiakis, A.; von Einem, B.; et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J. Alzheimers Dis. 2017, 59, 1097–1111.
- De Oliveira Bristot, V.J.; de Bem Alves, A.C.; Cardoso, L.R.; da Luz Scheffer, D.; Aguiar, A.S., Jr. The Role of PGC-1/UCP2 Signaling in the Beneficial Effects of Physical Exercise on the Brain. Front. Neurosci. 2019, 13, 292.
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162.
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469.