
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Johannes Martinus Marie Engels | + 6636 word(s) | 6636 | 2020-08-10 08:58:28 | | | |
| 2 | Catherine Yang | Meta information modification | 6636 | 2020-08-19 04:21:08 | | | | |
| 3 | Catherine Yang | Meta information modification | 6636 | 2020-08-19 11:46:22 | | |
Video Upload Options
All our crops are the result from a domestication process that has been conducted by people from around the world. This domestication process of selected wild species in parts of the world that harbour a high level of species and genetic diversity was preceded by gathering plants or parts thereof (e.g. seeds; root and tubers) for their consumption 'at home', usually combined with hunting of wild animals. The selected and gradually domesticated plants, i.e. our today's crops, have 'nephews and cousins' that are closely related to a given crop species and that are wild plants, i.e. the crop wild relatives. These species sometimes naturally interbreed with their related crops or can be crossed with the crop by using specific techniques such as molecular genetic tools. These crop wild relatives possess important genes and traits that are critically important for the improvement of our crops, through research, pre-breeding and breeding of new varieties that are better adapted to our ever-changing production environment. Climate change is causing serious threats to a number of crops and crop varieties and their adaptation to the changed conditions is critical to maintain their production level.
Unfortunately, the drastic changes we have seen in our agricultural production systems during the past 70 - 80 years, the huge changes in our landscapes, the impacts of climate change and many other aspects cause a direct and severe threat to the survival of the crop wild relatives and concerted efforts are urgently required to ensure their survival.
This entry provides a general introduction to this often forgotten and little known subset of plant genetic resources for food and agriculture, it defines CWRs and provides information on classification of the species and presents an overview over the conservation status under in situ conditions (i.e. in nature), in ex situ conditions (i.e. in genebanks or botanic gardens) as well on how conservation approaches can be optimized by combining in situ and ex situ (the so-called complementary conservation) as well as on their use in breeding programmes. The final concluding session provides an overview of the problems encountered with the conservation (and use) and what should be done to improve the current situation to ensure a more effective and efficient conservation. The presented recommendations are based on an analysis of the threat status of the CWRs as well as on biological factors that hamper conservation as well as on other constraints encountered so far. the importance of CWRs. Details on these parameters are not included in the text below but are included in the related paper that has been recently published.
1. Introduction
Today’s cultivated crop plants have undergone more or less drastic changes since their first cultivation and domestication. The first signs of domesticating wild plant (and animal) species date back 10,500 years in Western Asia and domestication has since then been practiced in different parts of the world by different groups of people on new species [1]. The duration and intensity of this domestication process have been very variable from one crop to the other [2]. The one thing that all crops have in common is that they originated from (one or more) wild and naturally occurring species. For a number of crops, the domestication process is well known, based on archaeological finds and (experimental) research. In general, this process started with gathering in particular wild grasses and leguminous species, followed by their cultivation closer to the homestead under more controlled conditions and gradually undergoing transformation from wild into domesticated taxa [3][4][5]. In some instances, crops are the result of natural or man-made hybrids between two wild ancestor species (e.g., banana: Musa acuminata and M. balbisiana); in other cases, the wild relative is a 'subspecies' (sylvestris) of the cultivated crop (e.g., Vitis vinifera ssp. vinifera) or there is no difference between the wild and the domesticated species (e.g., the olive tree, Olea europea which has wild, weedy, and cultivated forms, and many forage crops), which are just two different forms of the same species. For other crops, the domestication process is much less known or even completely obscure, including which wild species might have been involved as ancestor(s) of the crop in question (e.g., Triticum spelta, spelt). For some crops, the domestication process is still ongoing, especially in local fruit trees [6]. Possibly the most important consequence of the domestication process is that the genetic diversity available in the crop genepool (in the narrow sense) is usually much smaller than that in the related wild species [7][8].
In this paper, we focus on the wild species that are related to our crops, i.e., the crop wild relatives (CWRs). They have in different ways contributed (genetically) to the domestication process and thus can be regarded as the ancestral species or progenitors of our present crops, and they are a valuable resource of genetic diversity and traits for plant breeding.
It has taken several years after the global initiation of systematic collecting and conserving threatened landraces of our crops, somewhere in the 1960/70’s, until CWRs were systematically included, both at the national and international level. In 1975, a global collecting program of threatened landraces and CWRs was initiated under the coordination of the International Board for Plant Genetic Resources (IBPGR) and approximately 220,000 samples were collected during more than 1000 collecting missions in more than 130 countries, largely before 1995. The collected materials were sent to and subsequently stored in selected national and regional/international genebanks around the world [9][10]. The inclusion of CWRs in collecting efforts was triggered by the observed genetic erosion, as well as by the apparent need to include more genetic diversity for the advancement of breeding programs of major crops (e.g., potato), triggered by the success of using CWRs in breeding programs, such as the tomato, for specific traits [11]. Due to breeding programs in need for more diversity, the first ‘push’ for CWR conservation came from the international CGIAR research centers, as well as some (international) breeding companies in the 1970/80’s [12].
Only during the past few decades, significant successes of transferring traits from CWRs into cultivated crops have been reported, mostly to overcome biotic stresses, such as pests and diseases, as well as abiotic stresses, such as drought tolerance [8][13]. More recently, adaptability to changing environmental conditions, in particular those caused by climate change, has also become important. Only gradually, CWRs became a priority for the more advanced national plant genetic resources centers for food and agriculture (PGRFA), such as in the USA, UK, Germany, The Netherlands, and Australia. Possibly the biggest ‘push’ for the conservation of CWRs was the advancement of molecular biology and genetic tools and techniques that greatly facilitate the transfer of traits, genes, and alleles from one species to another, almost independent of how closely they are related to each other.
The above-mentioned developments certainly had an important impact on the increasing (political) conservation priorities accorded to CWRs since the late 1980’s/early 1990’s. This has been reflected by the inclusion of CWRs in the text of the Convention on Biological Diversity (CBD) [14] and, in 2010, in the AICHI Biodiversity Targets, in particular Target 13, as well as in target 9, of its Global Strategy for Plant Conservation, where CWRs and wild food plants were accorded a high priority for conservation [15]. In almost half of the 18 priority activities of the Second Global Plan of Action (GPA II), adopted in 2011 by the Food and Agricultural Organization of the United Nations (FAO) Member Countries, it makes (again, like in the first GPA agreed upon in 1996) a special reference to CWRs and wild food plants, highlighting the need to strengthen their conservation and sustainable use [16]. More recently, CWRs have been included in the United Nations’ Sustainable Development Goals (SDG) [17]. The recent Global Assessment Report on Biodiversity and Ecosystem Services, published in 2019 by the United Nations’ Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) [18]], mentions CWRs explicitly as species that are important for long-term food security, helping render ecosystems more resilient to stressors including climate change, pests and pathogens, and that lack effective protection. The report highlights the decreasing number of CWRs and mentions that many hotspots of agrobiodiversity and CWRs are under threat or not formally protected.
In response to this increasing visibility and importance of CWRs in global and international political agendas since the early 1990’s, numerous projects, tools, and guidelines have been initiated and developed at local/national, regional, and global levels. Examples for the latter are the voluntary guidelines for the conservation of CWRs and wild food plants at the national level [19] or the interactive toolkit for CWR conservation planning [20].
Besides the more political framework facilitating conservation, technical and managerial considerations are also important in order to effectively include CWR species in routine conservation programs. As treated in the following sections, a number of specific requirements can be identified that determine the ability of genebanks, in particular, to cope more effectively with CWR conservation. Especially, the availability of adequate knowledge and experience to manage this very variable and sometimes extremely difficult category of genetic resources is one of the main hurdles to overcome.
It has been a long and is yet a continuous struggle to get CWRs as a high priority on, in particular, local and national conservation agendas [21][22]. Reasons for this are limited financial resources available to many conservation and use programs; the lack of technological resources to effectively exploit these resources; an increasing debate on access to and availability of PGRFA; the sometimes severe technical challenges, which the conservation of CWRs’ can cause to genebanks, due to biological peculiarities of CWRs; as well as the relatively low priorities these resources have for local people. Against this backdrop, the paper investigates the reasons for these constraints, focusing on difficulties, opportunities and synergies that characterize the conservation and use of CWRs. Furthermore, due to the biological peculiarities of CWRs, there is a need for a strong collaboration between actors operating at different levels, especially between local/national and international, as well as between different sectors, such as agriculture and environment.
2. Definition and Classification of CWRs
A ‘simple’ and broad definition of a CWR is that all wild species belonging to the same genus (and that coincides in most cases with the same genepool) of a given crop are treated as a crop wild relative [23]. A narrower definition refers to the genepool concept developed by Harlan and de Wet [24]. They used the easiness of crossing a given wild relative with the crop species in question as the basis for their classification. When a CWR species crosses easily with the related crop, the species is defined as a genepool I species (GP1a = cultivated form of the crop and GP1b = wild or weedy form of the crop). Wild relatives from whom genes can be transferred to the crop, but with difficulties using conventional breeding techniques, are included in genepool II. Those wild relatives that cannot be crossed with a given crop and where gene transfer is only possible using sophisticated techniques, such as embryo rescue, somatic fusion or genetic engineering, are defined as genepool III species. Although this classification is very ‘utility driven’ and from a plant breeding perspective, it makes good practical sense, as crossing barriers are a major limiting factor for the use of CWRs in conventional plant breeding.
However, for the majority of crop complexes, particularly those from tropical areas, too little information on crossability is available to use the genepool concept. Therefore, an alternative concept has been proposed by Maxted et al. [23], based on the existing taxonomic hierarchy to define to which of four recognized taxon groups a given species belongs. Taxon group TG1a corresponds to the crop, CWRs in TG1b correspond to the same species as the crop, CWRs of TG2 are in the same series or section as the crop, TG3 is the same subgenus as the crop, and CWRs of TG4 are those in the same genus. Thus, without detailed information on the reproductive isolation, this concept can be used to establish the degree of relationship between a CWR and a crop [23].
The number of CWR species account for about 21% of the world’s flora [19][25], assuming that any species belonging to the same genus as a given crop is a CWR. On that basis, it has been estimated that there are 50,000 to 60,000 CWR and wild food plant species worldwide [19]. For Europe, Kell et al. [26] found that 17,495 (8624 of them endemic), out of approximately 20,590 species, or 85% of the European flora, comprise crop and CWR species. Maxted et al. [23] argued that a more targeted list of globally important CWR species could be obtained by focusing on the crop genepool GP1b or on taxon groups TG1b and TG2, containing the closest CWR species. By applying this to genera that contain major and minor food crops, as defined by Groombridge and Jenkins [27], that the resulting 77 genera contain 10,739 CWR species that are congeneric to these genera, and of these 221 are very close wild relatives and 471 close wild relatives [25]. Thus, as a working estimate, there would be, globally, around 700 closely related CWR species (i.e., less than 0.26% of the world flora), which are of a high value in terms of their potential use in plant breeding programs and would deserve the highest priority to conserve the genetic diversity of major and minor food crops [21][28].
Vincent et al. [29] used the genepool and taxon group concepts to estimate CWR relatedness for 173 priority crops included in Groombridge and Jenkins [27] and the Annex 1 of the International Treaty for PGRFA. Additional taxa more remotely related to crops were added if they had useful traits for crop improvement. The inventory contains 1667 taxa, belonging to 1392 species in 108 genera and 37 families. It also includes ancillary data, such as their regional and national occurrence, seed storage behavior, and herbaria, housing major collections of CWRs. This inventory is available online as searchable resource, called the Harlan and de Wet inventory, and is actively maintained [30]. This list can be regarded as the most comprehensive one, based on clear criteria. A number of other global priority lists, typically developed in the context of specific projects, are less comprehensive, have less well defined or complex criteria, and have not been used as widely as the list by Vincent et al. [29]. Two African regional checklists [31][32] and several national checklists and inventories have also been developed and are available on the CWR global portal [33].
3. The Current CWR Conservation and Use Status
3.1. Facts and Figures on CWR Conservation
3.1.1. In Situ Conservation
Whereas the CBD [14], as well as the GPA [16], recognize the importance of CWR in situ conservation and regard ex situ conservation as a complementary conservation effort, the progress of CWR in situ conservation remains slow and difficult. In the second State of the World (SOW II) report, it is noted that in situ conservation is often envisaged to take place in protected areas or habitats and can be targeted at the species or at the ecosystem in which they occur [21]. However, the report also noted that in situ conservation of wild species of agricultural importance occurs mainly as an unplanned result of efforts to protect particular habitats or charismatic species. Furthermore, existing in situ protected areas do not always meet the required management standards to maintain CWR populations and their genetic diversity long-term [34]. Whereas the number of protected areas globally has increased considerably and the total area covered by protection expanded from 13 in 1996 to 20.3 million square kilometers in 2020, covering 15.2% of the terrestrial surface [35], it should also be mentioned that, in general, areas with the greatest diversity, for instance within centers of origin and/or diversity of our crops, have received significantly less protection than the global average [21].
Several countries informed as part of the SOW II report [21] the establishment of protected areas for CWRs, e.g., Armenia (CWRs of cereals), Ethiopia (wild populations of Coffea arabica), Mexico (Zea perennis and Z. diploperennis, CWR species of maize), China (86 in situ conservation sites for CWRs of different crops), Turkey (protected areas for CWRs of cereals and legumes), and Syria (protected areas for CWRs of cereals, legumes, and fruit trees). Hunter and Heywood [36] reported the establishment of a citrus wild relatives’ gene sanctuary in northeast India in 1981. A similar genetic reserve for wild relatives, including relatives of lychee, longan, and citrus, was established in Vietnam. They also mentioned that certain wild species of mangoes and other wild relatives are known to occur in biosphere reserves, national parks, and other reserves in India, Indonesia, Singapore, the Philippines, Thailand, and Sri Lanka, but little targeted in situ conservation has been undertaken. In Europe, the first CWR genetic reserves were designated in 2019, when, in Germany, a network of genetic reserves for four wild celery species was established [37][38][39]. As of February 2020, the network included 15 genetic reserves and more are in the process of being established.
The aforementioned summary assessment of GPA II [22] noted an increased attention to CWRs in the context of in situ conservation and management. Overall, 14.2% of the over 15,000 in situ conservation sites that were listed in 20 country reports had management plans addressing CWRs and wild food plants. A total of 78 activities on in situ conservation and management were implemented with institutional support in 19 countries. A total of 16 countries reported an estimated total of 2141 CWRs, including species from primary and secondary genepools, as well as species previously used for breeding but belonging to the tertiary genepools, and wild food plants, actively conserved in in situ areas. The average per country is amounting to 134 CWR species with a maximum of 840 species in one country. However, the overall developments, with respect to the implementation of the in situ conservation priority activities of GPA II, were limited in scope and the reporting countries rated their achievements with respect to this priority activity as the lowest across all the 18 priority areas that make up the Second GPA [22].
Vincent et al. [34] assessed 167 of the most important food crops for improving food security and income generation and identified 1425 priority CWR species related to these crops. They modeled the distributions of 791 of these priority CWRs as the basis for the identification of 150 sites for in situ conservation. Individual CWR species, in general, were found to be well represented in current protected areas; only 35 (2.5%) of the studied species, related to 28 crops, were distributed exclusively outside of protected areas. If a threshold of 50% or more of the potential genetic diversity of a CWR, based on ecogeographic land characterization diversity [40], occurring within protected areas, is considered adequate for genetic conservation, then 112 of the assessed CWR species are under-conserved, while 91% of CWRs are well represented within existing protected areas. Effectively conserving the top 10 CWR sites inside protected areas and the top 10 sites outside protected areas as defined in the pragmatic scenario, would only require active management of ~2000 km2 globally and would protect 475 CWR species, and 1257 unique CWR/adaptive scenario combinations. Vincent et al. [34] propose to manage these as a global in situ conservation network.
As any other wild species, most of the CWRs might not have any direct economic or nutritional relevance to local communities and, thus, might not be of interest to them. In fact, some of them might even be weedy and constitute a nuisance to local farmers. Therefore, CWRs might not be very attractive for inclusion in a local ‘on farm’ conservation program [15], and in case their distribution area is not part of a protected area setting, local communities will not be interested in participating in a conservation activity if no benefits/funding will be provided. Only in cases where the CWR species occur in a protected area (targeted or ‘by chance’: [15]), their conservation might be easier and more sustainable as long as some sort of a monitoring system does exist.
In some cases, however, CWRs play a known and appreciated role in local and, typically, traditional cropping systems and, thus, will be valued by local farmers or communities. Consequently, conservation approaches might be easier and could directly involve the local people, as long as benefits will be generated through such activities. Examples of such situations include the regular re-domestication of Dioscorea cayensis subsp. rotundata in Benin; the use of Dioscorea spp. in West African countries by facilitating the introgression between wild and domesticated yams, as this is an important improvement strategy; the use of Ensete ventricosum in Ethiopia for regular incorporation of ‘wild’ seedlings into the fields of the cultivated crop; or the selection of wild walnut genotypes for cultivation in Kyrgyzstan [21]. From a crop evolutionary perspective and more related to traditional agricultural production systems, tolerating CWR species which are weeds, especially in field-borders, as pollinators of the cultivated material and, thus, assumingly increasing the genetic diversity of the crop for subsequent selection, is another example. However, also the opposite can be true that CWR play a detrimental role in farmers’ field, for instance, as noxious weeds.
3.1.2. Ex Situ Conservation
Traditionally, ex situ conservation is the main approach that countries have taken to conserve CWRs. Genebanks play an important role in the overall conservation of CWR germplasm; in fact, they (should) provide a link between in situ conservation and the users’ communities at various levels. This role is essential as they typically are specialized in long-term conservation, distributing or exchanging requested materials, characterizing and evaluating the stored accessions, keeping detailed information records on the individual accessions and, in some instances, conducting pre-breeding activities to facilitate the use.
Genesys, the largest global database on ex situ conserved germplasm accessions, provided data (as of 11.01.2020) for 4,097,112 accessions, of which only 12% are classified as wild material, thus possibly also including some non-CWR species [41]. The European Search Catalogue for Plant Genetic Resources (EURISCO) [42] contains data for 2,023,530 accessions. Among those, 12.15% are reported as wild. According to Ford-Lloyd et al. [43], the 1095 CWR species reported in EURISCO, at the time the research was undertaken, only represented 6% of the 17,495 CWR species found in Europe. This means that 94% of European CWR species are not conserved in ex situ collections.
The SOW II report [21] provides an average percentage of wild species, predominantly CWRs, for each of the 11 major crop groups, varying from 4% (food legumes and fiber crops) to 35% (forages) and 46% (industrial and ornamental plants). The overall mean for the almost 7 million reported accessions of wild plants is 10%, most of them being CWRs.
For a number of reasons, many CWRs are represented by a small number of accessions per species in the collections, both in genebanks and in botanic gardens. As an example, of the 1076 global priority CWR taxa identified in a study about global CWR conservation priorities [44], ‘only’ 763 or 70.9% are included in genebanks; among those, 257 taxa are represented by less than 10 accessions each. Over 95% of the taxa examined were found to be insufficiently represented in genebank collections with respect to their full range of geographic and ecological variation in their native distribution area. In many instances one would find just few accessions per taxon, e.g., only 5.4% of the CWR taxa in EURISCO are represented by 10 or more accessions, whereas 90.5% of the CWR taxa have less than 5 accessions.
Due to the already mentioned difficulty to collect adequately sized numbers of seeds/plants per population, many of the accessions consist of (too) small quantities of seeds and are genetically poorly sampled [45]. In addition, the stored seed samples have frequently a low(er) viability due to the difficulties to grow them out for regeneration purposes [46]. Another aspect, related to lack of information/knowledge, concerns taxonomic identification of the CWR, including to which crop genepool they belong. This will directly impact on the priority-setting and possible subsequent conservation, both in situ and ex situ, as well as on their use.
In a study of ex situ holdings of 23 selected genepools of the major crops included in Annex I of the International Treaty, i.e., those materials that countries agreed to form the backbone of the multilateral system of the International Treaty, the authors calculated an average non-weighted percentage of CWR accessions in genebank collections (without the international collections held by the CGIAR genebanks) of the selected genepool worldwide of 9.6%, ranging from 0% for coconuts (there are no CWRs known) to 33% for grass pea (a little bred crop) (Figure 1). The total global holdings considered in the study of the selected genepools (without the collections held by the CGIAR genebanks) were 3,149,371 accessions [47].
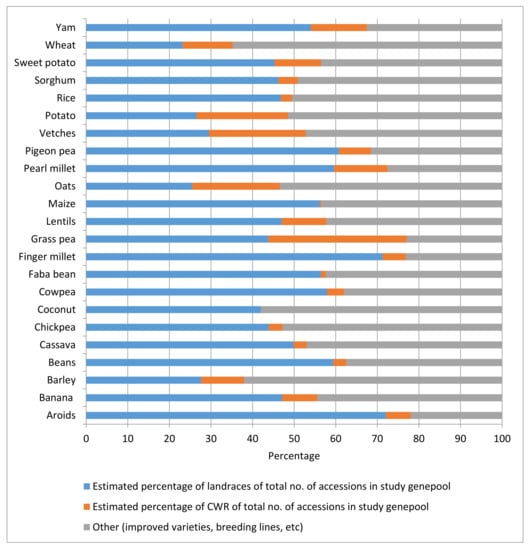
Figure 1. Percentages of CWR and landrace accessions in genebank collections of 23 selected crop genepools.
When looking at the primary genepool, 242 of the 1667 CWR taxa included in the Harlan and de Wet CWRs inventory were found to be under-represented in ex situ collections and the countries identified as the highest priority for further germplasm collecting are China, Mexico, and Brazil [29]. Khoury et al. [48] used gap analysis to assess the degree of representation of Cucurbita CWR taxa in conservation in situ, as well as ex situ in genebanks and botanic gardens. For the Cucurbita genus, including 16 CWR and six cultivated species, the authors established detailed taxon-related ex situ, as well as in situ (i.e., protected areas) conservation priorities and suggested further in situ protected areas that would cover the greatest amount of populations of the largest number of taxa. Khoury et al. [48] concluded that 68.8% of wild Cucurbita taxa were assessed as high or medium priority for further collecting for ex situ conservation and 81.3% had a high or medium priority for further protection in situ, including all of the progenitors of the cultivated species. Furthermore, four taxa were listed as having very few accessions and, thus, very limited diversity is available for crop breeding. Khoury et al. [48] suggested that these figures might be considered as ‘typical’ for the CWRs at large.
Besides their conservation in situ and in genebanks, botanic gardens have also been collecting and storing CWR materials in their collections, as demonstrated by the PlantSearch database, which is an information platform for 1155 botanic gardens that collectively maintain plant, seed, or tissue collections of 589,526 taxa [49]. The database reveals that botanic gardens maintain at least 30% of all known plant species in their own collections, including that more than 41% of species assessed are globally threatened. Many of these wild species are CWRs. Almost one-third (315, or 28.6%) of the 1076 aforementioned global priority CWR taxa are maintained by botanic gardens [50].
A recent major effort of collecting new CWR samples was made by the project “Adapting Agriculture to Climate Change” [51], which focuses on the wild relatives of 29 crops included in Annex 1 of the International Treaty; over 4500 new CWR samples were collected for ex situ storage, evaluated for useful traits, and enhanced or pre-bred for use in crop improvement programs.
3.1.3. Complementary Conservation
As already noted above, both the CBD [15] and GPA II [16] refer to the need to complement in situ conservation efforts with ex situ measures. Genebanks have recognized strengths in facilitating easy and targeted access to specific material (which is problematic for in situ conserved material) and to allow secure and long-term conservation as part of the conservation and use continuum. Especially when environmental change is too rapid for evolutionary change and adaptation, or migration, it can be easily understood how and why ex situ measures would complement or even replace in situ conservation and thus provide for the most effective approach [22][52]. Such a complementary approach requires that in situ and ex situ conservation measures have to be carefully planned and combined, thus securing a holistic combination of the two, which capitalizes on strengths and avoids weaknesses of one or the other. This will require a good understanding of the (seed) biology of the species, their threat status, priorities assigned to the individual CWR species, and other aspects; an assignment of clear responsibilities, including, for instance, to the agricultural and environmental sectors; if applicable, to link conservation and development; adequate and comprehensive information management; facilitation of adequate coordination with other stakeholders and countries; the verification of clear ownership rights over areas where the to-be-conserved CWRs occur; support of public awareness on the importance of CWR conservation; and, where necessary, to ensure the engagement of the broader public.
As an example, Hunter and Changtragoon [82] conclude, on the basis of regional project experiences, that for wild relatives of tropical fruit trees, any conservation strategy should contain elements of both in situ and ex situ conservation and should have a focus on conservation, both inside and outside protected areas. It should also ensure coordination of planning and implementation, institutionalize the practice of wild relative conservation, promote public awareness and understanding, create a suitable policy environment, and highlight the many benefits derived from their sustainable conservation and use. In situ approaches seem feasible for conserving wild relatives of tropical fruit trees, but experiences with targeted species and actions inside and outside protected areas appear to be relatively few. Consequently, wild relatives of tropical fruit trees remain a largely under-conserved natural resource, both ex situ and in situ, and are continuously under threat in their natural habitat from neglect and over-harvesting [53]. Vincent et al. [34] note the generally accepted requirement for complementary conservation, i.e., to also cover in situ conserved materials in genebanks, a process that has started recently. They further see a particular need to develop CWR in situ activities that enable the conservation of geographically partitioned genetic diversity which retains potential for local environmental-evolutionary adaptation.
3.2. Facts and Figures on CWR Use
The term ‘use’ needs to be applied in its widest sense for CWRs. The traditional understanding is the use of genetic diversity in plant breeding by crossing cultivated material, usually advanced varieties with CWRs and through a strong selection to obtain genotypes, with the traits that have been transferred from the CWR species. Furthermore, CWRs are an important target for research on crop evolution and are, indirectly, an important component of research on the origin and spread of agriculture. With the increasing focus of conserving CWR in situ (including on-farm), the ‘direct’ use of CWRs by local communities and farmers has now also received some more attention. Another dimension of ‘using’ CWRs is their not well understood and accepted role in and contributions to the evolution of crops and plants at large. Through the overall conservation efforts of the flora (and fauna) in natural habitats and protected areas, of which CWRs are an integral part, they contribute to a healthier environment, healthy ecosystems, and the provision of ecosystem services. However, this latter aspect is not part of the focus of this paper. Furthermore, the appreciation of the economic value of CWRs and their contribution to the global economy is an aspect that would fall under the term ‘use’.
In tropical zones, wild fruit harvested from forests contribute significantly to the total income and to sustainable nutritious diets of many rural households, apart from contributing substantially to important ecosystem services [29]. Wild relatives and wild-growing semi-domesticated species of tropical fruit trees also provide services to domesticated fruit trees in terms of resistance to extreme abiotic and biotic stresses through their high levels of genetic diversity [53].
More widely applied is the use of CWRs in pre-breeding and breeding programs and in research, in particular in countries with strong breeding companies, where facilities and technologies, as well as funding, are available to exploit these ‘difficult’ resources. Today, climate change is causing dramatic changes that are being experienced around the globe, especially global warming and the related increase of severe erratic weather conditions. These changes have a significant impact on agricultural production systems that need to be addressed as well. To allow crops to cope with and/or to adapt to more extreme weather conditions, including heat, drought, flooding, and increased salinity, there is a strong need for more genetic diversity than currently available for most crops from which plant breeders can select specific traits and resistance genes to ‘equip’ new varieties to cope with these changing conditions. In particular, the use of CWRs, as a known source of traits for introgression into the crops, has proven to offer such solutions, especially to overcome biotic stresses [8]]. As CWRs do possess a much wider array of traits and allelic diversity, as well as ‘new’ genetic variation compared to our modern crops, they are an important asset to be included in the breeding pools of our plant breeders and, thus, to be accorded a high priority in their conservation and research and management activities that facilitate their use by plant breeders, worldwide [44][54][55].
‘Historical’ examples of CWRs in plant breeding include the use of wild Aegilops, Secale, Haynaldia, and Agropyron species in wheat breeding [56], the introduction of resistance to late blight, which is caused by Phytophthora infestans and is found in the wild potato Solanum demissum [57], as well as other disease resistances and tolerances from different potato CWRs [58]. Resistance against stem rust caused by Puccinia graminis subsp. graminis derived from the wild wheat Aegilops tauschii [59], in another example. In the early 1970’s, resistance to the grassy stunt virus was found in wild Oryza nivara and now this gene can be found in almost all material bred by the International Rice Research Institute in the Philippines [43]. Maxted and Kell [25] reviewed the use of CWR in crop improvement in 291 papers reporting the identification and transfer of useful traits from 185 CWR taxa into 29 crop species. Wheat and rice accounted for almost 84% of the transfers and 56% of the inter-specific trait transfers related to pest and disease resistances.
The above historical examples demonstrate the past focus on trying to identify traits of interest through phenotypic characterization and evaluation [28]. Whereas the inclusion of genetic diversity from the wild genepool in breeding activities was difficult [21], the advancements in molecular genetics and the related tools allow a much more ‘targeted’ use of CWRs. Through the possibility of transferring specific parts of the genome, i.e., traits, genes, and/or alleles into the genetic background of improved breeding materials, the hesitation of using CWRs is fading and, thus, their importance for breeding is increasing. According to Ford-Lloyd et al. [43], genomic-based resources, map-based cloning, analysis of quantitative trait loci, gene isolation, and genetic modification are increasingly significant to exploit the potential of CWRs. Genomic databases containing information on genes associated with adaptive characters must increasingly be linked to web-enabled databases of ex situ conserved CWR germplasm, such as EURISCO [42]. Furthermore, predictive characterization, Focused Identification of Germplasm Strategy (FIGS) [28] and eco-geographical filtering method [60] are other promising approaches to facilitate the use of CWRs in breeding.
The number of CWR genomes sequenced has grown significantly over the past decade and in 2016 the number of crop genomes sequenced was ‘only’ about three times higher than that of sequenced CWR species, which were about 40 [61]. For example, Bertioli et al. [62] sequenced two wild peanut species (Arachis ipaensis and A. duranensis). Peanut is an important food source for many farmers in the developing world. The CWR genome sequences will provide breeders with new tools for enhancing the crop, and for developing new varieties more resistant to pests, diseases or with improved abiotic tolerance traits. It is hoped that this positive trend of more CWRs to be sequenced continues and thus, allows a better exploitation of the important traits that CWRs harbor, including quantitatively inherited traits.
A study carried out by PwC [63] assigned an indicative value of $42 billion to the CWRs of 29 major food crops, with a potential to reach a value of $120 billion in the future. All these 29 crops are included in Annex 1 of the International Treaty on PGRFA. Pimentel et al. [64] reported an estimated value of $115 billion that CWRs contributed toward increased crop yields per year worldwide. In addition to their economic value, CWRs are also being valued for their not so well-known contributions to ecosystem services [43]. Tyack and Dempewolf [65] have reviewed past economic values of CWRs, including the previously cited studies, and propose an improved conceptual model for understanding the economic value of CWRs under climate change, expanding it from the focus of gross production to including a series of other values and costs.
4. Conclusions
CWRs have been identified as threatened resources that are understudied, not properly conserved, and that possess a tremendous potential for the breeding of our crops. The latter is particularly important because of climate change, which calls for the urgent development of better adapted crops and varieties for the changing growing conditions in our vulnerable production systems. The protection of the environment is yet another important consideration that can be achieved, or at least important contributions can be made through the increase of crops and varieties that require less harmful inputs and provide still stable and high production levels.
In the above text, we distilled a number of actions that are recommended to be implemented at the various levels, whenever possible, in a timely and collaborative manner. Whereas a number of these recommendations can be implemented by individual countries, others will require agreement and coordination at the global level, where possible, using existing mechanisms and instruments.
4.1. Documentation
-
Collating, creating, and sharing more information and knowledge on CWR species, in particular, by stimulating and conducting more research.
-
Establishing national databases and inventories to enable better coordination and implementation of CWR conservation.
-
Developing a global data portal/platform for the exchange and provision of CWR data and information, including tools and guidelines that will facilitate a better coordinated and more efficient conservation, worldwide.
4.2. In Situ Conservation
-
Facilitating and encouraging the inclusion of CWRs in national and local conservation agendas and ensuring that they are being given an adequate priority supported possibly by a longer-term financial and organizational structure.
-
Complementing the management and monitoring of CWR in situ conservation sites and genetic reserves with ex situ conservation efforts of the priority species.
-
Identifying existing and novel mechanisms to finance and govern the proposed global coordination and facilitation of CWR in situ conservation should be of high priority. The proposed global network could play an important role in setting standards, sharing experiences, and providing the platform for monitoring and coordination and, thus, to provide a fundamental basis for ensuring our future food security.
-
Increasing the awareness and recognition among actors, especially within the environmental sector, about CWRs as important group of wild species that need to be conserved.
4.3. Ex Situ Conservation
-
Ensuring adequate ex situ conservation of threatened national priority CWRs.
-
Ensuring adequate ex situ conservation of a globally agreed list of priority CWRs (e.g., [29]) through national/regional/international genebanks, in particular those that already have global or regional conservation responsibilities for the corresponding crop genepools.
-
The identification and/or application of new methods to assess the viability of seeds, not requiring seed germination tests, could address current difficulties with viability tests and with small seed samples.
-
Large-scale research on CWR seed biology can lead to methods allowing for long-term storage of seeds of these species in genebanks. One such specific research area is the use of cryopreservation for long-term conservation.
4.4. Complementary Conservation and Collaboration
-
Development of a generic decision tree on complementary conservation approaches that can be applied to individual CWR species. Supporting guidelines should be developed to facilitate the application of the decision tree and the subsequent implementation of the conservation efforts, using gained experiences with individual species and cases as a basis.
-
Ensuring ready access to the genetic resources and related information, both from in situ as well as ex situ conservation within the framework of existing legal instruments.
-
Facilitating and coordinating phenotypic and molecular characterization of the priority CWRs to provide a basis for pre-breeding and breeding activities through the involvement of conservation, research, and breeding stakeholders.
-
Facilitating/strengthening the collaboration between stakeholders for more effective and efficient conservation, research and use of CWRs as well as to facilitate the transfer of technologies at the local, national, regional, and global levels.
4.5. Conservation System
-
Increasing awareness on the importance of and threat to CWRs, including through the active involvement of botanic gardens to ‘demonstrate’ this genetic wealth and the relationship between the CWRs and crop species.
-
Facilitating the training of staff on skills that strengthen the implementation of the above activity areas.
-
Providing a more stable organizational and financial basis for CWR conservation at national level.
References
- Willcox, G. The beginnings of cereal cultivation and domestication in Southwest Asia. In A Companion to the Archaeology of the Ancient Near East, 1st ed.; Potts, D.T., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 163–180.
- Evolutionary Insights into the Nature of Plant Domestication
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848.
- White, C.E. The Emergence and Intensification of Cultivation Practices at the Pre-Pottery Neolithic Site of El-Hemmeh, Jordan: An Archaeobotanical Study. Ph.D. Dissertation, Boston University, Boston, MA, USA, 2013; 240p. Available online: https://hdl.handle.net/2144/12888 (accessed on 18 June 2020).
- Weide, A.; Riehl, S.; Zeidi, M.; Conard, N.J. A systematic review of wild grass exploitation in relation to emerging cereal cultivation throughout the Epipalaeolithic and aceramic Neolithic of the Fertile Crescent. PLoS ONE 2018, 13, e0189811.
- Gepts, P. Domestication of Plants. In Encyclopedia of Agriculture and Food Systems; van Alfen, N.K., Ed.; Elsevier: San Diego, CA, USA, 2014; Volume 2, pp. 474–486.
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066.
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding. Crop Sci. 2017, 57, 1070–1082.
- Thormann, I.; Engels, J.M.M. Genetic diversity and erosion—A global perspective. In Genetic Diversity and Erosion in Plants—Indicators and Prevention; Ahuja, M.R., Jain, S.M., Eds.; Springer: Berlin, Germany, 2015; Chapter 10; Volume 1, pp. 263–294.
- Thormann, I.; Fiorino, E.; Halewood, M.; Engels, J.M.M. Plant genetic resources collections and associated information as baseline resource for genetic diversity studies—An assessment of the IBPGR supported collections. Genet. Resour. Crop Evol. 2015, 62, 1279–1293.
- Rick, C.M.; Chetelat, R. Utilization of related wild species for tomato improvement, First International Symposium on Solanaceae for Fresh Market. Acta Hortic. 1995, 412, 21–38.
- Hoyt, E. Conserving the Wild Relatives of Crops; IBPGR: Rome, Italy, 1988; 45p.
- Mammadov, J.; Buyyarapu, R.; Guttikonda, S.K.; Parliament, K.; Abdurakhmonov, I.Y.; Kumpatla, S.P. Wild Relatives of Maize, Rice, Cotton, and Soybean: Treasure Troves for Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2018, 9, 886.
- CBD. Global Strategy for Plant Conservation; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2002; 48p.
- CBD. Notification: Strengthening the In Situ Conservation of Plant Genetic Resources for Food and Agriculture through Incorporation of Crop Wild Relatives under Areas Important for Biodiversity in Protected Area Networks and Other Effective Area-Based Conservation Measures (Aichi Biodiversity Targets 7, 11, 12 and 13 and Global Strategy for Plant Conservation Targets 5, 6, 7 and 9); CBD Secretariat: Montreal, QC, Canada, 2015; 11p.
- FAO. Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture. Commission on Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; 96p, Available online: http://www.fao.org/3/i2624e/i2624e00.pdf (accessed on 18 June 2020).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; General Assembly, Seventieth Session; Agenda Items 15 and 116, A/RES/70/1; United Nations: New York, NY, USA, 2015; 35p.
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2019.
- FAO. Voluntary Guidelines for the Conservation and Sustainable Use of Crop Wild Relatives and Wild Food Plants; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; 106p.
- Brehm, J.M.; Kell, S.; Thormann, I.; Gaisberger, H.; Dulloo, M.E.; Maxted, N. New tools for crop wild relative conservation planning. Plant Genet. Resour. 2019, 17, 208–212.
- FAO. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; 399p.
- FAO. Assessment of the Implementation of the Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture 2012–2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; 76p.
- Maxted, N.; Ford-Lloyd, B.V.; Jury, S.L.; Kell, S.P.; Scholten, M.A. Towards a definition of a crop wild relative. Biodivers. Conserv. 2006, 15, 2673–2685.
- Harlan, J.R.; de Wet, J.M.J. Towards a rational classification of cultivated plants. Taxon 1971, 20, 509–517.
- Maxted, N.; Kell, S.P. Establishment of a Global Network for the In Situ Conservation of Crop Wild Relatives: Status and Needs Background Study Paper No. 39; FAO Commission on Genetic Resources for Food and Agriculture: Rome, Italy, 2009; 224p.
- Kell, S.P.; Knüpffer, H.; Jury, S.L.; Ford-Lloyd, B.V.; Maxted, N. Crops and wild relatives of the Euro-Mediterranean region: Making and using a conservation catalogue. In Crop Wild Relative Conservation and Use; Maxted, N., Ford-Lloyd, B.V., Kell, S.P., Iriondo, J.M., Dulloo, M.E., Turok, J., Eds.; CAB International: Wallingford, UK, 2008; pp. 69–109.
- Groombridge, B.; Jenkins, M.D. World Atlas of Biodiversity; Prepared by the UNEP World Conservation Monitoring Centre; University of California Press: Berkeley, CA, USA, 2002.
- Maxted, N.; Kell, S.P.; Toledo, A.; Dulloo, E.M.; Heywood, V.; Hodgkin, T.; Hunter, D.; Guarino, L.; Jarvis, A.; Ford-Lloyd, B.V. A Global Approach to Crop Wild Relative Conservation: Securing the Gene Pool for Food and Agriculture. Kew Bull. 2010, 65, 561–576.
- Vincent, H.; Wiersema, J.; Dobbie, S.; Kell, S.P.; Fielder, H.; Castañeda-Álvarez, N.P.; Eastwood, R.P.; Guarino, L.; Maxted, N. A prioritized crop wild relative inventory to help underpin global food security. Biol. Conserv. 2013, 167, 265–275.
- The Harlan and de Wet Crop Wild Relatives Inventory. Available online: https://www.cwrdiversity.org/checklist/ (accessed on 30 June 2020).
- Lala, S.; Amri, A.; Maxted, N. Towards the conservation of crop wild relative diversity in North Africa: Checklist, prioritization and inventory. Genet. Resour. Crop Evol. 2018, 65, 113–124.
- Allen, E.; Gaisberger, H.; Brehm, J.M.; Maxted, N.; Thormann, I.; Lupupa, T.; Dulloo, M.E.; Kell, S.P. A crop wild relative inventory for Southern Africa: A first step in linking conservation and use of valuable wild populations for enhancing food security. Plant Genet. Resour. 2019, 17, 128–139.
- Ford-Lloyd, B.V.; Schmidt, M.; Armstrong, S.J.; Barazani, O.; Engels, J.; Hadas, R.; Hammer, K.; Kell, S.P.; Kang, D.; Khoshbakht, K.; et al. Crop Wild Relatives—Undervalued, Underutilized and under Threat? BioScience 2011, 61, 559–565.
- Vincent, H.; Amri, A.; Castañeda-Álvarez, N.P.; Dempewolf, H.; Dulloo, E.M.; Guarino, L.; Hole, D.; Mba, C.; Toledo, A.; Maxted, N. Modeling of crop wild relative species identifies areas globally for in situ conservation. Commun. Biol. 2019, 2, 1–8.
- UNEP-WCMC, IUCN and NGS. Protected Planet Live Report 2020; UNEP-WCMC: Cambridge, UK; IUCN: Gland, Switzerland; NGS: Washington, DC, USA, 2020.
- Hunter, D.; Heywood, V. (Eds.) Crop Wild Relatives. A Manual of in situ Conservation; Routledge: London, UK, 2011; 414p.
- Frese, L.; Bönisch, M.; Herden, T.; Zander, M.; Friesen, N. In-situ-Erhaltung von Wildselleriearten. Nat. Landsch. 2018, 50, 155–163.
- Bönisch, M.; Frese, L. Designation of Genetic Reserves for Wild Celery Species in Germany. Crop Wild Relative Issue 12; ISSN 1742-3694 (Online). In Press. Available online: http://farmerspride.eu/.
- Thormann, I. The German Network of Genetic Reserves. Crop Wild Relative Issue 12; ISSN 1742-3694 (Online). In Press. Available online: http://farmerspride.eu/.
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Ecogeographical land characterization maps as a tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genet. Resour. Crop Evol. 2011, 59, 205–217.
- Genesys Is an Online Platform Where You Can Find Information about Plant Genetic Resources for Food and Agriculture (PGRFA) Conserved in Genebanks Worldwide. Available online: https://www.genesys-pgr.org/ (accessed on 1 July 2020).
- Eurisco. Finding Seeds for the Future. Available online: http://eurisco.ecpgr.org (accessed on 1 July 2020).
- Ford-Lloyd, B.V.; Schmidt, M.; Armstrong, S.J.; Barazani, O.; Engels, J.; Hadas, R.; Hammer, K.; Kell, S.P.; Kang, D.; Khoshbakht, K.; et al. Crop Wild Relatives—Undervalued, Underutilized and under Threat? BioScience 2011, 61, 559–565.
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16–22.
- Heywood, V.H. The role of botanic gardens in ex situ conservation of agrobiodiversity. In Implementation of the Global Plan of Action in Europe—Conservation and Sustainable Utilization of Plant Genetic Resources for Food and Agriculture, Proceedings of the European Symposium, Braunschweig, Germany, 30 June–3 July 1988; Gass, T., Frese, L., Begemann, F., Lipman, E., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 1999; pp. 102–107.
- Dempewolf, H.; (Global Crop Diversity Trust, Bonn, Germany). Personal communication, 2020.
- Engels, J.; Thormann, I. Final Report of a Consultancy “Increasing Climate Resilience for Poor Farmers: The role of National Plant Genetic Resource Collections”; Kreditanstalt für Wiederaufbau (KfW) and Global Crop Diversity Trust: Frankfurt/Bonn, Germany, 2017; 129p, Unpublished, manuscript in preparation.
- Khoury, C.K.; Carver, D.; Kates, H.R.; Achicanoy, H.A.; van Zonneveld, M.; Thomas, E.; Heinitz, C.; Jarret, R.; Labate, J.A.; Reitsma, K.; et al. Distributions, conservation status, and abiotic stress tolerance potential of wild cucurbits (Cucurbita L.). Plants People Planet 2019, 2, 269–283.
- Welcome to PlantSearch! Botanic Gardens Conservation. International. Available online: https://tools.bgci.org/plant_search.php (accessed on 30 June 2020).
- Meyer, A.; Barton, N. Botanic Gardens Are Important Contributors to Crop Wild Relative Preservation. Crop Sci. 2019, 59, 2404–2412.
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Muller, J.V.; Toll, J. Adapting agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377.
- Engels, J.M.M. Complementary strategies for improved conservation and use of plant genetic resources. In Towards Sustainable National Plant Genetic Resources Programmes—Policy, Planning and Conservation Issues; Engels, J.M.M., Vodouhe, R., Thompson, J., Zannou, A., Hehne, E., Grum, M., Eds.; IPGRI: Rome, Italy, 2000; pp. 69–77.
- Hunter, D.; Changtragoon, S. Good practices for conservation and sustainable use of crop wild relatives of tropical fruit tree diversity. In Tropical Fruit Tree Diversity. Good Practices for In Situ and On-Farm Conservation; Sthapit, B., Lamers, H., Rao, R.V., Bailey, A., Eds.; Earthscan from Routledge: London, UK, 2016; pp. 83–96.
- Jha, U.C.; Bohra, A.; Singh, N.P. Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed. 2014, 133, 679–701.
- Dulloo, M.E.; Fiorino, E.; Thormann, I. Research on Conservation and Use of Crop Wild Relatives. In Crop Wild Relatives and Climate Change; Chapter 7; Redden, R., Yadav, S.S., Maxted, N., Dulloo, M.E., Guarino, L., Smith, P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 108–129.
- Vavilov, N.I. The origin, variation, immunity and breeding of cultivated plants. Chron. Bot. 1951, 13, 1–54.
- Prescott-Allen, C.; Prescott-Allen, R. The First Resource: Wild Species in the North American Economy; Yale University Press: New Haven, CT, USA, 1986.
- Engels, J.M.M. Genetische bronnen, hun conservering en aanwending in de aardappelveredeling. In Genetic Resources, Conservation and Use in the Potato Breeding; Agricultural University Wageningen: Wageningen, The Netherlands, 1974; 83p.
- Kilian, B.; Martin, W.; Salamini, F. Genetic diversity, evolution and domestication of wheat and barley in the Fertile Crescent. In Evolution in Action; Glaubrecht, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 137–166.
- Thormann, I.; Parra-Quijano, M.; Endresen, D.T.F.; Rubio-Teso, M.L.; Iriondo, M.J.; Maxted, N. Predictive Characterization of Crop Wild Relatives and Landraces. Technical Guidelines, Version 1; Bioversity International: Rome, Italy, 2014; 40p.
- Willis, K.J. State of the World’s Plants Report—2017; Royal Botanic Gardens: Kew, UK, 2017; 96p.
- Bertioli, D.J.; Cannon, S.B.; Froenicke, L.; Huang, G.; Farmer, A.D.; Cannon, E.K.; Liu, X.; Gao, N.; Clevenger, J.; Dash, S.; et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 2016, 48, 438–446.
- PricewaterhouseCoopers PwC, 2013. Crop Wild Relatives: A Valuable Resource for Crop Development. www.pwc.co.uk/valuations. Available online: https://pwc.blogs.com/files/pwc-seed-bank-analysis-for-msb-0713.pdf (accessed on 15 January 2020).
- Pimentel, D.; Wilson, C.; McCullum, C.; Huang, R.; Dwen, P.; Flack, J.; Tran, Q.; Saltman, T.; Cliff, B. Economic and environmental benefits of biodiversity. Bioscience 1997, 47, 747–757.
- Tyack, N.; Dempewolf, H. The Economics of Crop Wild Relatives under Climate Change. In Crop Wild Relatives and Climate Change, 1st ed.; Redden, R., Yadav, S.S., Maxted, N., Dulloo, M.E., Guarino, L., Smith, P., Eds.; Wiley Online Library John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015.




