
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hanjie Guo | + 6968 word(s) | 6968 | 2020-08-12 06:25:54 | | | |
| 2 | Catherine Yang | Meta information modification | 6968 | 2020-08-19 10:37:02 | | |
Video Upload Options
The selective laser melting(SLM) technology has the characteristics of rapid solidification.Therefore, refined microstructures and high-performance products can be obtained.The microstructure of magnesium alloy varies with the cooling rate(processing).And the higher the cooling rate, the finer the microstructure of the magnesium alloy.Different cooling rates also affect the phase composition of magnesium alloys. The SLM process inhibits the formation of the second phase in the magnesium alloy due to the characteristics of rapid solidification.Post-treatment processes, such as heat treatment and hot isostatic pressing(HIP), can be applied to SLMed magnesium alloys. These processes help to close the pores , dissolve the second phase and reduce the source of cracks caused by the mismatch between the second phase and the α-Mg matrix, thereby improving the mechanical properties of the magnesium alloy, especially the elongation.However, SLMed magnesium alloys need further research in the application of post-processing, alloy design, base material purification, and thermodynamic and kinetic theoretical calculations of intermetallic compounds.
1. Introduction
Magnesium is one of the most abundant elements on Earth and represents approximately 2.5% of its composition. Magnesium and its alloys are lightweight metallic structural materials with certain advantages, such as low density, high specific strength and high stiffness [1][2]. Magnesium and its alloys are considered to have great application prospects in the aerospace, transportation, electronics, biomedicine, and energy fields due to their excellent physical and chemical properties, such as low density, good damping performance, biocompatibility, recyclability, large hydrogen storage capacity, and high theoretical specific capacity [3][4][5]. As the lightest structural material currently available, magnesium alloys have the potential to replace steel and aluminum in many structural applications [6]. Thus, magnesium alloys have already found considerable applications in various fields, including the aerospace, aircraft, automotive, and electronics fields; in particular, magnesium die castings have been widely used in the automotive industry [7]. However, despite the abovementioned advantages, magnesium and its alloys still face many difficulties in large-scale industrial applications. For instance, the poor room temperature plasticity and poor corrosion resistance of these materials still need to be addressed [8][9]. Currently, magnesium alloy development is focused on the production of complex structures with high efficiency and minimal environmental impact; accordingly, many new magnesium alloy preparation technologies have emerged.
Additive manufacturing (AM) is a promising new technology that can dramatically change the way components are manufactured in many different industries and greatly increase manufacturing efficiency. According to different technologies, AM can be divided into electron beam melting (EBM), direct laser forming (DLF) and selective laser melting (SLM). SLM is a technology widely used in the preparation of metal powders. Commonly used metal powders include Fe-based alloys, titanium-based alloys, Al-based alloys, Mg-based alloys, and nickel-based alloys. SLM uses a high-energy laser beam to completely melt metal powder in a protective atmosphere along a defined laser path, and this molten metal rapidly solidifies [10]. By repeating this step and overlapping subsequent layers, a three-dimensional component is eventually formed. SLM provides a means of manufacturing geometrically complex structures, eliminating the need to build molds, which would otherwise require a considerable amount of time and money to manufacture. The cooling rate of the molten pool reaches 103–108 K/s due to the rapid movement of the laser and the molten metal pool [11]. The characteristics of rapid solidification and layered manufacturing enable the SLM process to produce materials with a more uniform chemical composition, a more concentrated solid solution, a refined microstructure, and better mechanical properties [12][13]. However, due to the inherent heat treatment of the SLM process, wherein each layer is cyclically reheated by the deposition of subsequent layers, the microstructure of the material is also unique [14]. To date, the samples produced by SLM still have some defects, such as pores and cracks, which affect the final use of the produced parts. Therefore, how to control the material density, microstructure and performance by adjusting the SLM process conditions is a research focus.
Due to several characteristics of magnesium alloys, including a low melting point, easy oxidation, and dangerous production, research on the preparation of these materials is still in its infancy worldwide. Research institutions investigating these materials include the Fraunhofer Institute for Laser Technology, Huazhong University of Science and Technology, University of Science and Technology Beijing, Central South University, Université de Technologie de Belfort-Montbéliard, Chongqing University, Hong Kong Polytechnic University, Suzhou University, and Delft University of Technology. Current research is in the small-scale trial production stage in the laboratory, and research and experimental data on the forming characteristics and mechanical properties of selective laser melted (SLMed) magnesium alloys are scarce. On the one hand, owing to the inherent physical properties of magnesium alloys, the research progress of SLMed magnesium alloys is limited. On the other hand, during the production of SLMed magnesium alloys, the generation of defects and the characteristics of microstructures affect the performance of SLMed magnesium alloys. For instance, in the Mg-Al-Zn(AZ) series of magnesium alloys, the presence of the second phase Mg17Al12 limits the mechanical properties—especially the elongation—of the alloy, which restricts the further development of SLMed magnesium alloys. At present, many scholars have conducted research and comprehensive reviews on the spheroidization, defects, porosity, and alloying element loss in magnesium alloys and other metals prepared by SLM [15][16][17][18][19][20][21]. However, limited processable materials, immature process conditions and metallurgical defects are still problems that magnesium alloys need to face and solve in the SLM process. In the past two years, these three issues of SLMed magnesium alloys have been substantially improved by optimizing process parameters, introducing post treatment and adjusting different alloying elements. However, few review articles have been written in this regard. Therefore, it is necessary to summarize the development of SLMed magnesium alloys from the perspectives of process, element adjustment and post treatment. To improve the machinability of the material, this paper will review the research progress of the addition of alloying elements and the post-treatment to expand the processable magnesium alloy materials. Process conditions and new research progress on relative density, microstructure, mechanical properties and corrosion resistance of SLMed magnesium alloys will be reviewed. In addition, the formation mechanism of metallurgical defects, especially oxidation and cracks, will be discussed and analyzed to provide a reference for the application of SLMed magnesium alloys.
2. Microstructure of SLMed Magnesium Alloy
Because SLM has the characteristics of rapid solidification, the cooling rate can reach 104–105 K∙s−1. The microstructure varied with respect to variations in the cooling rate. The microstructures in the as-cast, sub-rapidly solidified, and rapidly solidified (i.e., SLMed) samples can be compared under different cooling rates.
Figure 1a,b are optical microscopy and SEM images of the as-cast AZ61 alloy [22]. The as-cast alloy exhibited a typical dendritic eutectic network structure. The phase composition in the as-cast AZ61 was composed of an α-Mg matrix and a β-Mg17Al12 eutectic phase distributed in grain boundaries and grains. The average grain size of the as-cast AZ61 alloy was approximately 320 ± 5 μm. Under normal conditions, the grains were coarse and non-uniform. The microstructure of as-cast AZ91D is shown in Figure 2a,b. As-cast AZ91 also consisted of an α-Mg solid solution and a β-Mg17Al12 eutectic phase. As shown in the enlarged micrograph of area A (Figure 2b), some of the divorced eutectic β-Mg17Al12 was surrounded by lamellar eutectic. In the die-cast AZ91D ingot, β precipitates existed in the form of partially divorced eutectic structures. AZ61 and AZ91D are hypoeutectic magnesium aluminum alloys with low zinc content. In hypoeutectic Mg-Al alloys, the morphology of the eutectic phase depends on the cooling rate. A higher cooling rate resulted in a more discrete microstructure [23]. Therefore, the inherently high cooling rate in the SLM process caused a change in β-Mg17Al12 between the SLMed part and the as-cast part of Mg alloy. For die-cast samples, most of the Al and Zn were concentrated in the β-Mg17Al12 phase [24]. The reduction in the Al content in the α-Mg solid solution not only reduced the effect of solid solution strengthening but also deteriorated the corrosion behavior.
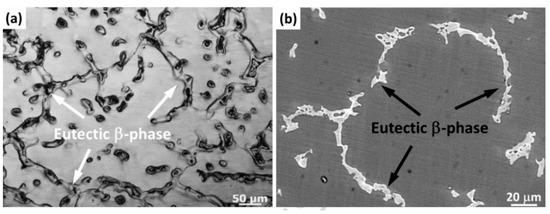
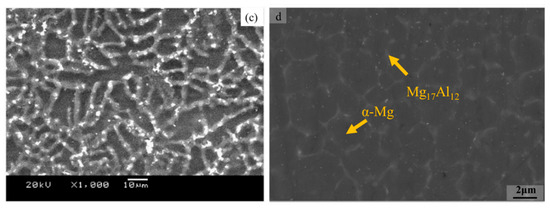
Figure 1. Microstructure of AZ61: (a,b) as-cast AZ61. Reproduction from [22], with permission fromElsevier, 2020. (c) Sub-rapidly solidified AZ61. Reproduction from [25], with permission from Elsevier, 2020. (d) SLMed AZ61. Reproduction from [26], with permission from Elsevier, 2020.
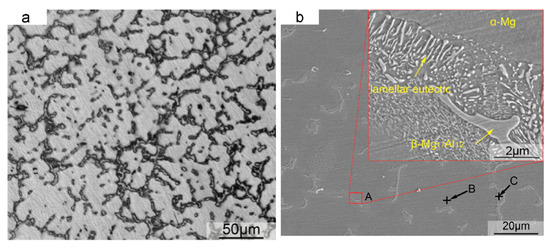
Figure 2. Microstructure of as-cast AZ91. (a) microscope picture; (b) SEM picture. Reproduction from [27], with permission from Elsevier, 2020.
Sub-rapid solidification is a nonequilibrium solidification process with a solidification rate of 103 K/s [28]. The sub-rapidly solidified structure changed considerably from that of the as-cast sample (Figure 1c). The β-Mg17Al12 phase continuously distributed on the grain boundaries disappeared. The microstructure was obviously refined and was mainly composed of small equiaxed grains. The literature noted that as the thickness of the sheet decreased (i.e., the faster the solidification process), the grain size decreased [25], and a large number of petal-like dendrites appeared in the microstructure. Owing to the high cooling rate, the dendrite structure was very small, so it was difficult to distinguish the dendrite arm spacing in the low-magnification metallographic microstructure. Under sub-rapid solidification, the grain size of the AZ61 magnesium alloy was 13.5 μm, which was much smaller than that of the as-cast AZ61 magnesium alloy.
The solidification rate of SLM was faster than that of sub-rapid solidification. As shown in Figure 1d, the SLMed microstructure was more uniform, the α-Mg grains were refined and equiaxed, and the β-Mg17Al12 phase precipitated at the grain boundaries. The α-Mg crystal grains and β-Mg17Al12 in the SLMed microstructure were more refined than those in the sub-rapidly solidified microstructures. The grain size in Figure 1d was 2.46 μm (at an energy density of 208 J/mm3). Other studies have also found the same microstructural characteristics of SLMed magnesium alloys [27][29][30]. Wei et al. [27] investigated the distribution of Mg and Al in SLMed AZ91D samples. Comparing the SLMed samples with as-cast AZ91D, the distribution of Mg and Al in the SLMed AZ91D was more uniform, and the content of Al in the matrix varied with respect to the energy density. This finding demonstrates that the chemical composition distribution was more uniform under rapid solidification, which is beneficial to reduce the segregation of the components, and the energy input has an effect on the solid solution [31].
The rapid solidification of SLM mainly affects the generation of the second phase in the alloy. Cai et al. [32] compared the microstructure and morphology of the second phase β-Mg17Al12 in the conventional as-cast part and in the part produced under rapid solidification. Compared with conventional casting, β-Mg17Al12 in the rapidly solidified AZ91 magnesium alloy had smaller and fewer micropores. The dispersive microporosity will act as crack initiation sites and promote crack propagation under the application of an external force, causing significant deterioration in tensile properties. These micropores were mainly formed by solidification shrinkage and dissolved gas. Liu et al. [26] compared the microstructures under different energy densities under SLM and found that as the energy density increased, the microstructure changed. When the energy density was low, the precipitated second phase was dispersed, and the grain size was small (1.61 μm). As the energy density increased, the second phase gradually precipitated along the grain boundaries, and the grain size slightly increased. Other studies noted that the solid solution was related to the energy input. As the energy input increased, the solid solution of the elements exhibited different monotonic or nonmonotonic changes [26][29][30]. The solid solution of the elements and the precipitation of the second phase were both related to the energy input and solute capture. Therefore, analysis and discussion need to be combined with the solidification path.
In Figure 3, the left diagram is the phase diagram of the Mg-Al binary system [33], whereas the right diagram is the phase diagram of the AZ61 Mg alloy [26]. The addition of Zn obviously had a greater impact on the Mg-Al binary system phase diagram. Analysis of the equilibrium cooling process of AZ61 shows that the solidification path of the red line in the figure was L → L + α-Mg → α-Mg → α-Mg + β-Mg17Al12 → α-Mg + β-Mg17Al12 + T.
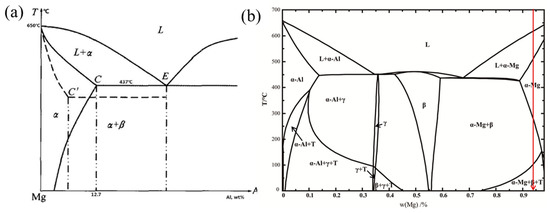
Figure 3. Mg-Al alloy equilibrium phase diagram: (a) Mg-Al binary system. Reproduction from [33], with permission from Elsevier, 2020. (b) AZ61 (1.27% Zn). Reproduction from [28], with permission from Elsevier, 2020.
Taking the AZ series Mg alloy as an example, the maximum solubility of Al in Mg was 12.7 wt.% at 437 °C during equilibrium solidification, whereas the solubility of Al was only 2 wt.% at room temperature. The reaction L → α-Mg occurred first under slow cooling. The Al atoms diffused sufficiently, and the alloy composition in α-Mg gradually became uniform. As the cooling process progressed, the reaction L → α-Mg ended when the melt temperature dropped to the solid phase line. Subsequently, the second phase β-Mg17Al12 began to precipitate in the α-Mg solid solution, and the final solidified products in the equilibrium state were α-Mg solid solution and the second phase β-Mg17Al12, and there was no eutectic phase. However, SLM is a nonequilibrium process under rapid solidification, so the final phase composition of the SLMed part was slightly different from the equilibrium phase diagram. The AZ61 Mg alloy was composed of two phases of α-Mg and β-Mg17Al12 at room temperature during a relatively slow cooling process. At a faster cooling rate (higher scanning speed and lower laser power), the atoms in the primary α-Mg in the liquid phase did not have time to diffuse sufficiently. From the principle of solute redistribution, it is known that the solid solution of Al was continuously enriched at the solidification front, and the residual liquid phase between the dendrites of the α-Mg reached the eutectic composition in the late solidification period. The two phases grew independently to form a divorced eutectic structure. As a result, the temperature of the molten pool was low, and the cooling rate as fast at low energy input (138.89 J/mm3), which is equivalent to being extremely cold in the α-Mg region. Due to the lack of sufficient diffusion time, Al dissolved into the matrix. The solid solution of Al in the matrix increased. Hence, the eutectic transformation process L → α-Mg + β-Mg17Al12 was suppressed. The precipitation of β-Mg17Al12 was less prominent at low energy input. In contrast, due to the accumulation of heat and the decrease in the solidification speed, the solute capture effect was weakened when the energy input was high (156.25~208.33 J/mm3). Al diffused more fully, the content of solid solution elements decreased with increasing Ev, the eutectic transformation L → α-Mg + β-Mg17Al12 occurred, and the crystallinity of β-Mg17Al12 increased.
The change of microstructure (grain shape, size, second phase distribution) is related to the cooling rate. As expected, various laser parameters resulted in different solidification rates of the molten pool and thermal cycles, leading to variation in microstructures of the melted zone. In the SLM process, the combination of scanning speed and laser power is the key to controlling the cooling rate. In addition, the faster the cooling rate, the finer the microstructure; the lower the cooling rate during solidification, the longer the time available for grain coarsening. Generally speaking, as the scanning speed decreases and the laser power increases (that is, the line energy density increases), the accumulation of heat in the molten pool causes the temperature of the molten pool to rise, the cooling rate slows down, and the grain size gradually becomes coarser [34]. The increase of the laser power or decrease of the laser scanning speed led to the coarsening of the grains in the melted zone because the higher laser power or slower laser scanning speed provided more driving force for grain boundary movement, which then promoted the growth of grains. At lower scanning speeds, the prolonged interaction time between the laser and the powder suppresses the heat dissipation in the molten pool. Therefore, due to the larger heat accumulation, the epitaxial growth kinetic conditions of the grains are enhanced [16]. Similar results were obtained for pulsed laser samples. When the laser power was low or the scanning speed was high, the average gain size became small, since decreasing the laser power in effect acts in the same way as reducing the preheat temperature of the powder when the next pulse hits the base. The relatively high cooling rate at low laser power or high laser scanning speed restrained the growth of α-Mg grain during solidification.
As the line energy density increases (low scanning speed and high laser power), the microstructure follows the evolution of clustered finer dendrites–refined equiaxed grains–coarsened equiaxed grains [27][28]. The grain size of AZ61 magnesium alloy can be refined to 1.61 μm (Ev = 138.89 J/mm3). Then, the grains changed to an equiaxed shaped (1.79 μm) with an increase in laser energy input to 156 J/mm3. Further increase in the laser energy input to 178 J/mm3 and 208 J/mm3, equiaxed grains of 2.12 μm and 2.46 μm, respectively [28]. This is due to the decrease in cooling rate caused by changes in scanning speed and laser power and accumulation of heat in the molten pool. This same reason can explain the change of the grain size far from the center of the molten pool. The α-Mg grains inside the molten pool show equiaxed crystal morphologies. On the contrary, with the farther away from the molten pool, the α-Mg grains show a transformation from equiaxed crystal to columnar crystal and the grain size increases [35]. The ratio G/R of the temperature gradient (G) and the solidification rate (R: the propulsion rate of the solidification interface in the normal direction) in the crystal direction of the pulsed light spot determines the microstructure morphology after solidification. Inside the molten pool, G is very high, but R tends to 0, so that the value of G/R is high, which causes the solidification structure to be equiaxed crystal. Further away from the molten pool, G decreases but R gradually increases, so that the value of G/R gradually decreases. The influence of scanning speed and laser power on the precipitation of the second phase has been described in detail above.
Consequently, the grain size, the second phase precipitation, and the element solid solution can be achieved by controlling the process parameters, i.e., controlling the scanning speed and laser power (energy density). In terms of grain size, by comparing the microstructure of magnesium alloys at different cooling rates, it can be seen that faster cooling rates are beneficial for refining the microstructure. From the as-cast state to sub-rapid solidification and rapid solidification (SLM), the grain size of the magnesium alloy drops from ~320 μm to ~2 μm, which is a change of two orders of magnitude. Properly increasing the scanning speed and lowering the laser power (i.e., lowering the energy density) is beneficial for the improvement of the cooling rate and the reduction of heat accumulation in the molten pool, thereby refining the microstructure. In terms of second phase precipitation, the increase of the cooling rate is beneficial to suppress the occurrence of the phase transition L → α-Mg + β-Mg17Al12 and the precipitation of the second phase is reduced. In addition, the energy density must be controlled within a suitable range. Too high an energy input will lead to coarse grains, an increase in element segregation and a decrease in the solid solution. However, if the laser power is reduced or the scanning speed is increased blindly (too low an energy density), the powder cannot be fully melted and combined, thus causing serious pores and affecting the sample quality. Therefore, further research must be performed to more accurately control the solidification structure by controlling scanning speed or laser power and the effect of the solidification structure on the defects and mechanical properties of SLMed magnesium alloys.
3. Effect of Heat Treatment on SLMed Magnesium Alloy
3.1. HIP
Some performance breakthroughs have been achieved in SLMed magnesium alloy, but there are still some problems, such as porosity and low elongation. Defects in the sample can be improved by adjusting the process parameters to improve the fusion of the powder. On the other hand, the pores caused by thermal convection in the molten pool are difficult to remove, requiring the introduction of post-treatment processes, such as HIP. The mechanism of HIP is to apply temperature and pressure to the sample at the same time and hold it for a certain time so that the sample is densified during the pressurization process after heating to eliminate the internal pores. There are some studies on collapsing the pores of SLMed parts by HIP [36][37][38].
According to the literature [39], the strength properties of SLMed samples are considerably inferior to those of the alloy consolidated using HIP technology, suggesting that it is reasonable to expose the complex-shaped SLMed workpieces to additional HIP treatment. Liu et al. [40] believed that the plasticity of the SLMed AZ61 magnesium alloy was greatly improved after 3 h of HIP at 450 °C and 103 MPa. The results showed that the elongation reached 8.2%, the UTS of the material remained unchanged, the elongation increased (160% greater than that of the SLMed part without HIP), and the plasticity was greatly improved. The improvement in plasticity mainly came from the following two parts: the reduction in the original defects (pores) in the SLMed magnesium alloy and the solid solution of the second phase β-Mg17Al12. The temperature of the SLMed AZ61 part increased below the melting point during HIP, the magnesium alloy softened and then gradually densified under pressure, eventually eliminating the internal pores, and the density was close to 100%. The elongation was related to the distribution of the second phase. The temperature during HIP was slightly higher than the solidus temperature. As a result, the second phase dissolved into the matrix, which greatly reduced the source of cracks at the interface between the β-Mg17Al12 and α-Mg phases. It can be seen that densification and solution treatment are beneficial to improving the plastic elongation of SLMed magnesium alloys.
However, HIP can also adversely affect certain mechanical properties of magnesium alloys. The YS of SLMed magnesium alloys decreased as the grain size increased [40]. Moreover, for SLMed aluminum alloys, HIP post-treatment can have an adverse effect on grain size [41]. HIP postprocessing can lead to grain growth in certain coarser grained areas, probably due to a local imbalance between driving and dragging forces, which corresponds to higher defect density and fewer pinning precipitates. This is a problem that can be solved by appropriately reducing the temperature or shortening the holding time. HIP experiments can verify that the second phase solid solution plays a positive role in improving the plasticity of magnesium alloys. Moreover, the second phase had an impact on the corrosion resistance [42][43]. HIP is a comprehensive heat treatment process that includes closing pores and providing internal heat treatment. Magnesium alloys are affected by the combined effects of temperature, pressure and holding time during HIP, which affect the pores and microstructure. Therefore, different magnesium alloys require different processing parameters, and there is still a lack of experimental data to support the selection of appropriate processing parameters. Hence, more exploration will be needed in order to accurately select the HIP parameters.
3.2. Heat Treatment
Reducing or eliminating the effect of the second phase on the properties of magnesium alloys can also be achieved by heat treatment and other methods. Common magnesium alloy heat treatment methods include solid solution heat treatment (T4), aging heat treatment (T5), and solid solution + aging heat treatment (T6). SLMed magnesium alloy has the problem of poor plasticity, which can be improved by introducing an appropriate heat treatment process.
In AZ series Mg alloys, the presence of the β-phase influences the corrosion behavior to a great extent. The machinability of Mg alloys is also influenced by the amount and distribution of the β-phase. In materials engineering, heat treatment is a promising technique to alter the microstructure to achieve the desired phases and corresponding volume fractions in order to alter the bulk behavior. Chowdary et al. [44] noted that heat treatment of AZ91 Mg alloy greatly affected the amount and distribution of the secondary phase at the grain boundaries and asserted that the machinability of AZ91 Mg alloys can be improved by developing supersaturated grains and reducing the amount of the secondary phase. The corrosion resistance was greatly affected after the heat treatment due to the supersaturated grains, which promoted a higher corrosion rate [44]. The literature [45] showed that the elongation increased from 7.4 to 11.2% in an as-cast magnesium alloy after T4 heat treatment. T4 treatment (solid solution) significantly improved ductility but reduced YS. T6 (solution + aging) increased the UTS but reduces the YS and ductility.
Wang et al. [45] proposed a new heat treatment idea: solution treatment at 413 °C caused the β phase to dissolve into the matrix. In contrast, the design purpose of Tx is to break the β-Mg17Al12 phase network, provide better ductility in exchange for a slight decrease in strength, and comprehensively improve strength and elongation. Treatment at a temperature close to the solvus, typically at 365 °C for just 2 h according to the equilibrium phase diagram, caused the β-Mg17Al12 phase to dissolve partially, and the β-Mg17Al12 network was effectively broken up. This resulted in a composite structure that, with the exception of a few primary α-Mg globules, consisted of fine α-grains and the fine remaining β-Mg17Al12-grains located along the grain boundaries, mainly at the triple grain boundary junctions. If the heat treatment temperature was set to Tx, rather than T4, T5 or T6, both strength and elongation can be improved.
In the literature [46], the solution treatment dissolved the β-Mg17Al12 phase and coarsened the grains. During the aging process, the β-Mg17Al12 phase preferentially and discontinuously precipitated at the grain boundaries, and then a continuous precipitated phase appeared inside the grains. Both the strength and the elongation were improved after solution treatment and aging treatment. Annealing dissolved β-Mg17Al12 and increased the concentration of Al solute in the matrix, which effectively reduced the damping capacity. Mg17Al12 distributed on the grain boundaries also caused local intergranular cracking.
Jia et al. [47] performed a solution heat treatment on Mg-Zn alloys. The enhancement of UTS was due to the solution strength effects of the alloying elements. In addition, Zn dissolving into the Mg matrix decreased the stacking fault energy of the matrix, which led to a change in the plastic deformation mechanism. Thus, the solution treatment enhanced the UTS and elongation simultaneously. The corrosion resistance of the solution-treated samples was superior to that of the as-cast samples.
It can be seen that heat treatment plays a positive role in improving the strength, plasticity, and corrosion resistance of magnesium alloys. However, there is still a lack of attention in the post-treatment of SLMed magnesium alloys. For the inherent defects in SLMed samples, such as pores, it is necessary to introduce postprocessing steps to eliminate them. Comprehensive improvements in the mechanical properties and corrosion resistance of SLMed magnesium alloys can be considered by adding heat treatment. However, it is necessary to further explore the optimal postprocessing parameters to control the adverse effects of grain size growth on the properties of magnesium alloys to balance the effects of grain size and microstructure.
4. Outlook
The development of SLM of magnesium alloys has been reviewed, and an extensive analysis of the available literature on the preparation of metals via SLM has led to the recognition of the influential process parameters and material properties. At present, the limited processable materials, immature process conditions and metallurgical defects restrict the development and application of SLMed magnesium alloys. Some efforts have been made to solve the above problems, such as adding alloy elements and applying postprocessing. However, the breakthroughs of SLMed magnesium alloys in these two areas have not been reviewed. Therefore, this article gives an overview of these three issues. In this paper, the process parameters, alloying elements, microstructure, properties, and postprocessing steps were systematically summarized. For the purpose of high efficiency, high quality, low cost, and more stable use of SLM to prepare high-performance magnesium alloys, scholars have carried out research on process parameters, alloying elements, and post-treatment steps. Although some progress has been made in terms of the relative density, mechanical properties, and corrosion resistance of magnesium alloys, the literature is still limited to unsystematic performance studies of a few types of magnesium alloys. Hence, current SLMed parts are produced under suboptimal process conditions, wherein it is difficult to control metallurgical defects.
In the future, there are still several issues that need attention in the manufacturing of magnesium alloys via SLM.
First of all, in view of the lack of processable materials, in order to expand more processable SLMed magnesium alloy materials and improve the problem of limited processable materials, we reviewed the progress of SLMed magnesium alloys in the addition of alloying elements, but there are still two problems in this area: the types of added alloying elements are limited, and the research of the addition of alloying elements needs to be in-depth. At present, most studies on SLM fabrication of magnesium alloys focus on the influence of process parameters and simple alloying elements on the microstructure and properties of binary alloys. Aside from research on Mg-Al-Zn alloys, Mg-Zn-Zr alloys, and Mg-Zn alloys, few studies have been reported on other as-cast Mg alloys and wrought Mg alloys, especially the high-strength and highly corrosion-resistant Mg alloys that are in great demand in the aerospace field (e.g., Mg-Mn alloys). We also combined the relevant research on the mature alloy element addition route in the traditional process to provide a reference for the alloy design of SLMed magnesium alloys, such as Mn and Ca, and the addition of these elements in SLMed magnesium alloys has rarely been reported. Therefore, there is a need to broaden the scope of the applicable magnesium alloys for SLM and design new materials whose compositions are suitable for the process characteristics of laser AM. In addition, post-treatment can also be applied to improve the machinability of magnesium alloys in the SLM process. We reviewed the current research progress in the postprocessing part, and the results showed that the post-processing can effectively improve the properties of SLM magnesium alloys, especially to improve the plasticity, but there are few studies at present, which is an area that needs to be further studied in the future. Traditional preparation techniques of magnesium alloys are also evaluated and related to the SLM process with a view to gaining useful insights especially with respect to postprocessing.
Second, in view of the immature process conditions, we comprehensively reviewed the new progress of different magnesium alloy materials in SLM process conditions and forming, and combined process conditions with relative density, grain size, microstructure, mechanical properties, and corrosion resistance. In the review of microstructure, the microstructure under different preparation processes (different cooling rates) was compared longitudinally, and the microstructure under different process conditions in the SLM process was also compared horizontally. In addition, it is different from the previous reviews of SLMed magnesium alloy.
Third, in terms of metallurgical defects, defects such as oxidation and cracks are reviewed. Research on cracks has made progress recently, but there is lack of review of the latest research on cracks. Cracks are a serious defect that limits the application of magnesium alloys and leads to premature failure of materials. Therefore, it is necessary to review the research progress of SLMed magnesium alloy cracks. It is well known that the application of SLMed Al alloy has been limited by cracks, so the paper combines the research progress of SLMed Al alloys on cracks to provide more references for the research of SLMed magnesium alloy cracks. In terms of oxidation, inspired by the purity of molten steel, this review not only looks forward to the development of SLMed magnesium alloys from the perspective of materials and processing, but also considers the future improvement direction of SLMed magnesium alloys from a new perspective—a metallurgical perspective. In addition, this was mentioned in the previous review of SLMed magnesium alloys. In research on steel materials, the harmful effect of nonmetallic inclusions on steel properties has been widely recognized [48][49]. For bearing steel, the deoxidation and purification of liquid steel has always been the focus of attention. Research has found that when the total oxygen content in molten steel is reduced from 0.0026 to 0.001%, the fatigue life of bearing steel is increased by a factor of 10, and when the total oxygen content is reduced from 0.001 to 0.0004%, the fatigue life is increased by an additional factor of 10. Therefore, to improve the performance of steel materials, it is important is to minimize both the dissolved oxygen in liquid steel and the content of oxide inclusions generated during the solidification process [50][51]. There have been many studies on the harmful effects of high oxygen content on material properties in powder metallurgy, such as 718 alloy [52], Al alloy [53], and titanium alloy [54]. Due to the highly oxyphilic nature of magnesium, the oxygen inevitably enters the magnesium alloy base metal and the magnesium alloy powder during the preparation process. However, the amount of oxygen entering the alloy system at various stages, the size and number of oxide inclusions formed in the alloy, and the mechanism of the influence of inclusions on the alloy—especially the influence of inclusions in the performance of magnesium alloys—have rarely been studied. Currently available research is limited to the understanding of the oxide film in the SLM process. It was found that the oxide film is usually located at the top of the molten pool, and the oxide film on the surface will affect the wettability, thereby weakening the interlayer bonding and resulting in the formation of defects. Improving the oxidation also means reducing the defects in the material. It was found that the use of a high-power laser could break the oxide film on the surface, but this increased the burning loss of alloying elements, and balancing the contradiction between these two aspects is a new topic. From the perspective of the preparation process of magnesium alloys, the oxygen that can enter into the alloy mainly comes from the mold cavity and the powder preparation process as well as the smelting of the magnesium base material. Future studies should focus on methods to inhibit the entry of oxygen in these processes and the mechanism of the influence of a small amount of oxygen on SLMed magnesium alloy materials.
In the future, the physical and chemical reactions in the SLMed magnesium alloy process need to be studied theoretically, especially the thermodynamics and kinetics of the formation of intermetallic compounds. For example, the thermodynamic calculation of the enthalpy of fusion of SLMed magnesium alloys with different compositions can theoretically determine the energy required for the magnesium alloy powder of a specific composition, which provides a theoretical basis for setting the laser power for actual experimental production (i.e., this approach can help ensure that the powder is melted under the optimal energy setting). In terms of dynamics research, it is necessary to establish a model of the relationship between the expansion and solidification of magnesium melt to seek the optimal energy input, control the melt temperature, and obtain the relationship between magnesium melt spreading and solidification rate to reduce the occurrence of defects, such as pores and spheroidization.
Furthermore, the influence of intermetallic compounds on SLMed magnesium alloys cannot be ignored. Intermetallic compounds have a dual effect on SLMed magnesium alloys. On the one hand, refining and dispersing intermetallic compounds can play a role in precipitation strengthening; on the other hand, intermetallic compounds can also have an adverse effect on corrosion resistance and mechanical properties. Therefore, controlling the size, shape and content of intermetallic compounds should be studied in further detail in the future. It is necessary to proceed from the theory of metallurgical physical chemistry and seek the conditions and mechanisms for the formation or decomposition of intermetallic compounds from the liquid state to the normal temperature state and finally obtain the best process parameters for controlling the intermetallic compounds in the SLM process, including an appropriate heat treatment method. According to the theoretical basis of postprocessing SLMed magnesium alloys presented above, there is still much research space in this aspect.
5. Conclusions
This review of the published literature on SLMed Mg offers insight into the current knowledge surrounding the influence of processing conditions, alloying elements, and post-treatment on the microstructure, properties, and fracture mechanisms of the produced parts. However, limited processable materials, immature process conditions and metallurgical defects are still problems that magnesium alloys need to face and solve in the SLM process. In the past two years, these three issues of SLMed magnesium alloys have been substantially improved by optimizing process parameters, introducing post treatment and adjusting different alloying elements. Due to the paucity of publications on postprocessing and alloy design of SLMed magnesium alloy powders, we review the current state of research and progress. Moreover, traditional preparation techniques of magnesium alloys are evaluated and related to the SLM process with a view to gaining useful insights especially with respect to postprocessing and alloy design of magnesium alloys. This article also discusses and summarizes the current factors that affect the formability, compactness, and mechanical properties of SLMed magnesium alloys. This article provides a reference for further investigating or controlling the microstructure of SLMed magnesium alloys and improving the densification, mechanical properties, and corrosion resistance of the produced materials. In addition, with respect to materials and metallurgy, new challenges and prospects in the SLM processing of magnesium alloy powders are proposed with respect to alloy design, base material purification, inclusion control and theoretical calculation, and the role of intermetallic compounds.
-
With respect to immature process conditions and metallurgical defects, the influence of processing parameters (scanning speed and laser power) on the forming and performance of magnesium alloys cannot be ignored. The relative density of magnesium alloys is closely related to the processing parameters. If the scanning speed is too high or laser power is to low (energy density is too low), the powder cannot be fully melted, and the system is in a state of solid–liquid coexistence. In this state, the surface tension and viscosity of the liquid increase, which inhibits the liquid from flowing smoothly and causing the liquid to agglomerate into spheres and pores, thereby preventing the sample from becoming dense. If the energy density is too high, it will lead to the loss of alloying elements due to the evaporation of elements in the powder. On the other hand, under these conditions, the solute capture effect will be weakened, and the decrease in the solid solution content will cause the relative density of the SLMed sample to decrease. Therefore, the preparation of dense SLMed magnesium alloy requires the energy density to be controlled in a suitable range to enhance the solid solution strengthening and reduce the liquid surface tension, thereby eliminating the pores and spherical particles between the tracks. However, in the SLM process of magnesium alloys, it is difficult to remove the pores by only adjusting the process conditions. Post-treatment methods such as HIP are required to remove these defects. Microcracks are commonly attributed to solidification cracking and liquation cracking. The cause of solidification cracking is a residual thin film of liquid phase between the primary crystallized grains, and liquation cracking is caused by cyclic heat input, which occurs in multilayer welding and layered fabrication.
-
The microhardness of the SLMed magnesium alloy is affected by the rapid solidification characteristics in the SLM process, which mainly affect the microstructure and the solid solution of the elements. On the one hand, the rapid solidification characteristics remarkably refine the microstructure of magnesium alloys, and the microhardness of SLMed magnesium alloys is notably higher than that of traditional as-cast magnesium alloys. On the other hand, the solid solution content of the alloying elements is different due to the differences in the solute trapping effects at different energy densities during the SLM process; thus, the strengthening effects are different. Moreover, the distribution and content of the second phase and the presence of defects will also affect the microhardness. Microhardness does not change monotonically with respect to the energy density. It is necessary to comprehensively consider the interaction between the solute capture effect at different molten pool temperatures and the solid solution of the elements under rapid solidification. Hence, while the microstructure is refined, the optimal solid solution is achieved, and the microhardness of the magnesium alloy is improved.
-
The grain size of magnesium alloys is increased by increasing the energy input during the SLM process, and the mechanical properties are affected by the grain size and microstructure. In SLMed magnesium alloys, attention should be paid to the dual influence of the second phase on the material properties. The content of the second phase needs to be controlled to balance the strengthening of the second phase and its limitation on plasticity. The mechanical properties and corrosion resistance of SLMed magnesium alloy can be improved by introducing alloying elements. At present, there are several alloy systems under investigation, and more alloying elements, such as rare earth elements and Mn, need to be introduced to develop different SLMed magnesium alloy systems. The micropores and the second phase generated during the SLM process have certain restrictions on the application of the material. Adjusting the process conditions can reduce the harm of the pores and the second phase to a certain extent, but it cannot be completely avoided. Post-treatments, such as HIP and heat treatment, can be applied to help eliminate inherent porosity and improve the precipitation of the second phase. However, much remains to be done in this area.
-
In view of the lack of processable materials, adding alloying elements and post-treatment is an effective way to improve SLMed magnesium alloy. These two methods have played an important role in improving the mechanical properties and corrosion resistance of SLMed magnesium alloy, especially the problem of poor plasticity of SLMed magnesium alloy. In the future, these two aspects need to be further studied to design magnesium alloy materials that are more suitable for SLM process applications.
References
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of advancement and development trend on magnesium alloy. J. Magnes. Alloys 2019, 7, 536–544.
- Liu, W.; Zhou, B.; Wu, G.; Zhang, L.; Peng, X.; Cao, L. High temperature mechanical behavior of low-pressure sand-cast Mg–Gd–Y–Zr magnesium alloy. J. Magnes. Alloys 2019, 7, 597–604.
- Ali, Y.; Qiu, D.; Jiang, B.; Pan, F.; Zhang, M.-X. Current research progress in grain refinement of cast magnesium alloys: A review article. J. Alloys Compd. 2015, 619, 639–651.
- Luo, K.; Zhang, L.; Wu, G.; Liu, W.; Ding, W. Effect of Y and Gd content on the microstructure and mechanical properties of Mg–Y–RE alloys. J. Magnes. Alloys 2019, 7, 345–354.
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloys 2018, 6, 59–70.
- Sanders, P.G.; Keske, J.S.; Leong, K.H.; Kornecki, G. High power Nd:YAG and CO2 laser welding of magnesium. J. Laser Appl. 1999, 11, 96–103.
- Zhou, Y.; Gui, Q.; Yu, W.; Liao, S.; He, Y.; Tao, X.; Yu, Y.; Wang, Y. Interfacial diffusion printing: An efficient manufacturing technique for artificial tubular grafts. ACS Biomater. Sci. Eng. 2019, 5, 6311–6318.
- Du, J.; Lan, Z.; Zhang, H.; Lü, S.; Liu, H.; Guo, J. Catalytic enhanced hydrogen storage properties of Mg-based alloy by the addition of reduced graphene oxide supported V2O3 nanocomposite. J. Alloys Compd. 2019, 802, 660–667.
- Ning, H.; Zhou, X.; Zhang, Z.; Zhou, W.; Guo, J. Ni catalytic effects for the enhanced hydrogenation properties of Mg17Al12(1 1 0) surface. Appl. Surf. Sci. 2019, 464, 644–650.
- Liu, Y.; Yang, Y.; Mai, S.; Wang, D.; Song, C. Investigation into spatter behavior during selective laser melting of AISI 316L stainless steel powder. Mater. Des. 2015, 87, 797–806.
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2015, 34, 369–385.
- Zhang, Y.; Zhang, J.; Yan, Q.; Zhang, L.; Wang, M.; Song, B.; Shi, Y. Amorphous alloy strengthened stainless steel manufactured by selective laser melting: Enhanced strength and improved corrosion resistance. Scr. Mater. 2018, 148, 20–23.
- Gokuldoss, P.K.; Eckert, J.; Gokuldoss, P.K. Formation of metastable cellular microstructures in selective laser melted alloys. J. Alloys Compd. 2017, 707, 27–34.
- Kürnsteiner, P.; Wilms, M.B.; Weisheit, A.; Barriobero-Vila, P.; Jägle, E.; Raabe, D. Massive nanoprecipitation in an Fe-19Ni- X Al maraging steel triggered by the intrinsic heat treatment during laser metal deposition. Acta Mater. 2017, 129, 52–60.
- Olakanmi, E.O.; Cochrane, R.; Dalgarno, K. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477.
- Manakari, V.; Parande, G.; Gupta, M. Selective laser melting of magnesium and magnesium alloy powders: A review. Metals 2016, 7, 2.
- Cao, X.; Jahazi, M.; Immarigeon, J.; Wallace, W. A review of laser welding techniques for magnesium alloys. J. Mater. Process. Technol. 2006, 171, 188–204.
- Jahangir, N.; Mamun, M.A.H.; Sealy, M.P. A review of additive manufacturing of magnesium alloys. In Proceedings of the 3rd International Conference on Mechanical Engineering (ICOME 2017), Birmingham, UK, 13–15 October 2017.
- Makarov, D.; Melzer, M.; Karnaushenko, D.; Schmidt, O.G. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2016, 3, 011101.
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284.
- Zhang, W.-N.; Wang, L.-Z.; Feng, Z.-X.; Chen, Y.-M. Research progress on selective laser melting (SLM) of magnesium alloys: A review. Optik 2020, 207, 163842.
- Jiang, M.; Yan, H.; Chen, R. Microstructure, texture and mechanical properties in an As-Cast AZ61 Mg alloy during multi-directional impact forging and subsequent heat treatment. Mater. Des. 2015, 87, 891–900.
- Dahle, A.K.; Lee, Y.C.; Nave, M.D.; Schaffer, P.L.; StJohn, D. Development of the As-cast microstructure in magnesium–aluminium alloys. J. Light Met. 2001, 1, 61–72.
- Wen, Z.; Wu, C.; Dai, C.; Yang, F. Corrosion behaviors of Mg and its alloys with different Al contents in a modified simulated body fluid. J. Alloys Compd. 2009, 488, 392–399.
- Teng, H.; Zhang, X.; Zhang, Z.; Li, T.; Cockcroft, S. Research on microstructures of sub-rapidly solidified AZ61 magnesium alloy. Mater. Charact. 2009, 60, 482–486.
- Liu, S.; Yang, W.; Shi, X.; Li, B.; Duan, S.; Guo, H.-J.; Guo, J. Influence of laser process parameters on the densification, microstructure, and mechanical properties of a selective Laser melted AZ61 magnesium alloy. J. Alloys Compd. 2019, 808, 151160.
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222.
- Teng, H.-T.; Li, T.-J.; Zhang, X.-L.; Zhang, Z.-T. Influence of sub-rapid solidification on microstructure and mechanical properties of AZ61A magnesium alloy. Trans. Nonferrous Met. Soc. China 2008, 18, s86–s90.
- He, C.; Bin, S.; Wu, P.; Gao, C.; Feng, P.; Shuai, C.; Liu, L.; Zhou, Y.; Zhao, M.; Yang, S.; et al. Microstructure evolution and biodegradation behavior of laser rapid solidified Mg–Al–Zn alloy. Metals 2017, 7, 105.
- Shuai, C.; Shuai, C.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser rapid solidification improves corrosion behavior of Mg-Zn-Zr Alloy. J. Alloys Compd. 2017, 691, 961–969.
- Chen, J.; Wei, J.; Yan, H.; Su, B.; Pan, X. Effects of cooling rate and pressure on microstructure and mechanical properties of sub-rapidly solidified Mg–Zn–Sn–Al–Ca alloy. Mater. Des. 2013, 45, 300–307.
- Cai, J.; Ma, G.; Liu, Z.; Zhang, H.; Xu, M. Influence of rapid solidification on the microstructure of AZ91HP alloy. J. Alloys Compd. 2006, 422, 92–96.
- Zhao, Y.-C.; Zhao, M.-C.; Xu, R.; Liu, L.; Tao, J.-X.; Gao, C.; Shuai, C.; Atrens, A. Formation and characteristic corrosion behavior of alternately lamellar arranged α and β in As-cast AZ91 Mg alloy. J. Alloys Compd. 2019, 770, 549–558.
- Ng, C.C.; Savalani, M.; Lau, M.; Man, H. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454.
- Liu, C.; Zhang, M.; Chen, C. Effect of laser processing parameters on porosity, microstructure and mechanical properties of porous Mg-Ca alloys produced by laser additive manufacturing. Mater. Sci. Eng. A 2017, 703, 359–371.
- Tradowsky, U.; White, J.; Ward, R.; Read, N.; Reimers, W.; Attallah, M.M. Selective laser melting of AlSi10Mg: Influence of post-processing on the microstructural and tensile properties development. Mater. Des. 2016, 105, 212–222.
- Zhao, X.; Li, S.; Zhang, M.; Liu, Y.; Sercombe, T.B.; Wang, S.; Hao, Y.; Yang, R.; Murr, L.E. Comparison of the microstructures and mechanical properties of Ti–6Al–4V fabricated by selective laser melting and electron beam melting. Mater. Des. 2016, 95, 21–31.
- Yan, X.; Lupoi, R.; Wu, H.; Ma, W.; Liu, M.; O’Donnell, G.; Yin, S. Effect of hot isostatic pressing (HIP) treatment on the compressive properties of Ti6Al4V lattice structure fabricated by selective laser melting. Mater. Lett. 2019, 255, 126537.
- Kaplanskii, Y.; Sentyurina, Z.A.; Loginov, P.; Levashov, E.; Korotitskiy, A.; Travyanov, A.Y.; Petrovskii, P. Microstructure and mechanical properties of the (Fe,Ni)Al-based alloy produced by SLM and HIP of spherical composite powder. Mater. Sci. Eng. A 2019, 743, 567–580.
- Liu, S.; Guo, H.-J. Influence of hot isostatic pressing (HIP) on mechanical properties of magnesium alloy produced by selective laser melting (SLM). Mater. Lett. 2020, 265, 127463.
- Spierings, A.; Dawson, K.; Dumitraschkewitz, P.; Pogatscher, S.; Wegener, K. Microstructure characterization of SLM-processed Al-Mg-Sc-Zr alloy in the heat treated and HIPed condition. Addit. Manuf. 2018, 20, 173–181.
- Feng, H.; Liu, S.; Du, Y.; Lei, T.; Zeng, R.; Yuan, T. Effect of the second phases on corrosion behavior of the Mg-Al-Zn alloys. J. Alloys Compd. 2017, 695, 2330–2338.
- Song, G.-L.; Bowles, A.L.; StJohn, D.H. Corrosion resistance of aged die cast magnesium alloy AZ91D. Mater. Sci. Eng. A 2004, 366, 74–86.
- Dumpala, S.C. Influence of heat treatment on the machinability and corrosion behavior of AZ91 Mg alloy. J. Magnes. Alloy 2018, 6, 52–58.
- Wang, Y.; Liu, G.; Fan, Z. Microstructural Evolution of Rheo-Diecast AZ91D Magnesium Alloy During Heat Treatment. Acta Mater. 2006, 54, 689–699.
- Zhao, D.; Wang, Z.; Zuo, M.; Geng, H. Effects of heat treatment on microstructure and mechanical properties of extruded AZ80 magnesium alloy. Mater. Des. 2014, 56, 589–593.
- Jia, H.; Feng, X.; Yang, Y. Influence of solution treatment on microstructure, mechanical and corrosion properties of Mg-4Zn alloy. J. Magnes. Alloys 2015, 3, 247–252.
- Zhang, L. State of the art in the control of inclusions in tire cord steels—A review. Steel Res. Int. 2006, 77, 158–169.
- Park, J.H.; Kang, Y. Inclusions in stainless steels—A review. Steel Res. Int. 2017, 88, 1700130.
- Shi, C.; Chen, X.-C.; Guo, H.-J.; Zhu, Z.-J.; Sun, X.-L. Control of MgO·Al2O3 spinel inclusions during protective gas electroslag remelting of die steel. Met. Mater. Trans. A 2012, 44, 378–389.
- Shi, C.; Zheng, D.; Guo, B.; Li, J.; Jiang, F. Evolution of oxide–sulfide complex inclusions and its correlation with steel cleanliness during electroslag rapid remelting (ESRR) of tool steel. Met. Mater. Trans. A 2018, 49, 3390–3402.
- Rao, G.A.; Srinivas, M.; Sarma, D. Effect of oxygen content of powder on microstructure and mechanical properties of hot isostatically pressed superalloy inconel 718. Mater. Sci. Eng. A 2006, 435, 84–99.
- Cao, L.; Zeng, W.; Xie, Y.; Liang, J.; Zhang, D. Effect of powder oxidation on interparticle boundaries and mechanical properties of bulk Al prepared by spark plasma sintering of Al powder. Mater. Sci. Eng. A 2018, 742, 305–308.
- Yan, M.; Xu, W.; Dargusch, M.; Tang, H.P.; Brandt, M.; Qian, M. Review of effect of oxygen on room temperature ductility of titanium and titanium alloys. Powder Met. 2014, 57, 251–257.




