1000/1000
Hot
Most Recent

Richter transformation (RT) is defined as the occurrence of an aggressive lymphoma in patients with a previous or concomitant diagnosis of chronic lymphocytic leukemia (CLL). It is characterized by a switch in the histopathology and biology of the original CLL.
Richter transformation (RT) is defined as the occurrence of an aggressive lymphoma in patients with a previous or concomitant diagnosis of chronic lymphocytic leukemia (CLL) [1]. It is characterized by a switch in the histopathology and biology of the original CLL.
In 95–99% of cases, such a switch is towards a diffuse large B cell lymphoma (DLBCL) (DLBCL-RT), cases of the Hodgkin’s variant of Richter transformation (HVRT) (0.5–5%) [2] have been described, and, less frequently, the transformation has been described in plasmablastic lymphomas [3].
Recently, the incidence of RT was evaluated using the Surveillance, Epidemiology, and End Results (SEER) database of CLL patients diagnosed between 2000 and 2016. In this large cohort of 74,116 patients with CLL, 530 cases with RT were identified, with a 0.7% incidence of transformation [4]. It was summarized that their pooled analysis of 2975 patients included in the frontline treatment trials and an RT incidence of 3% was observed, 92% of which with DLBCL-RT [5]. In the era of novel agents, one raised concern was whether there was an increased rate of this rare and aggressive transformation among patients treated with Bruton tyrosine kinase inhibitors (BTKis) or BCL2 inhibitors (BCL2is). Indeed, in the first clinical trials using novel agents, 2–15% incidence rates of RT have been described in relapsed/refractory (R/R) patients with CLL treated with ibrutinib [6][7][8][9][10][11], venetoclax [12][13][14], or idelalisib [15][16][17]. These alarming reports were probably related to the recruitment of patients with R/R disease or even already in the early stages of transformation.
In contrast, in clinical trials involving treatment-naïve patients with CLL treated with novel agents, the incidence of RT was reported to be 0–4% [9][18][19][20][21][22][23][24][25] , indicating that there is no increase in the number of cases of RT during therapy with these novel and effective biological agents.
It is of great interest to identify the patients with the highest risk to develop RT. Indeed, risk factors for the development of RT have been extensively studied and include clinical characteristics or molecular and genetic changes.
Immunoglobulin heavy-chain variable region gene (IGHV) mutational status indicate that patients with unmutated IGHV [26] or stereotyped B-cell receptor (BCR) [27][28][26][29][30][31] have increased risk of RT. Moreover, IGHV4–39 gene usage has been shown to carry a 24-fold increased risk of RT and when combined with stereotyped BCR (SUBSET 8) in the same patient, it showed a 5-year risk of RT of 68.7% [32]. Another recently noted point is that CLL patients with a complex karyotype at diagnosis seem to have the highest risk and shortest time to Richter transformation [33][34].
Murine models have shown that RT is characterized by constitutive active AKT, which seems to induce NOTCH1-signaling B cells via the NOTCH1 ligand expressed by T cells, and therefore apparently orchestrates RT [35].
It is understood that the microenvironment has a fundamental role in the supporting cancer genesis. CLL cells and their surrounding niche are closely related and constantly interact. From this point of view, microenvironment remodeling also seems to have a role in the development of RT. This observation is reflected by a high programmed death 1 (PD-1) expression by tumoral B lymphocytes [36][37], higher programmed death ligand 1 (PD-L1) expression in histiocytes and dendritic cells, the higher infiltration of FOXP3-positive T cells and CD163-positive macrophages, and lower peripheral blood T-cell receptor clonality compared to CLL without RT [37], suggesting changes in the immune signature of CLL after RT.
It is highly important to have a high index of suspicion of RT in a CLL patient with sudden clinical deterioration and to direct them to workout, performing a biopsy from the most accurate site for diagnosis as early as possible.
The diagnosis of RT is based on a biopsy and the histopathologic analysis of a suspected lesion (mainly lymph node) by an expert haemato-pathologist.
The cell of origin is generally of an activated-B-cell (ABC) type that expresses post-germinal center markers such as IRF-4, whereas only 5–10% display a germinal center B-cell (GCB) phenotype expressing CD10 and/or BCL6 [38]. Moreover, CLL markers such as CD5 and CD23 are generally lost during RT [39]. Due to the complexity of distinguishing DLBCL-RT from histologically aggressive CLL, criteria for the histological diagnosis of DLBCL-RT have been delineated [40].
As opposed to CLL, radiological evaluation is recommended for the workup diagnosis of RT. Conventional CT has been performed in the past, but it currently has limited use and is only recommended if other imaging modalities are not available [41]. 18-FDG-PET/CT is the recommended imaging technique, both for diagnosis and as a guide for the most adequate site of accurate biopsy. The probability of RT was shown to be significantly increased with higher standardized uptake values (SUVs) and maximal SUV (SUV max). This technique has the ability to distinguish between CLL (median SUV max : 3.7), accelerated CLL (median SUV max : 6.8), and RT (median SUV max : 17.6) [42]. Therefore, an 18-FDG-PET/CT showing low uptake (SUV of 5 and lower) seems to rule out RT, while an 18-FDG-PET/CT showing a high uptake (SUV of 10 and higher) may help guide biopsies for definite RT diagnosis [43][44][45] (Figure 1). Furthermore, some 18-FDG-PET/CT markers such as total metabolic tumor volume, SUV body weight, SUV lean body mass, SUV body surface area, lesion-to-liver SUV ratio, and lesion-to-blood-pool SUV ratio assessed at the time of RT into DLBCL seem to be correlated with overall survival (OS) [46][47]. However, in the novel therapy era, one should consider the limitations of 18-FDG-PET/CT, the results of which might be influenced by the use of biological agents as BTKi and anti-PD1 [48]. Other imaging techniques involving novel PET radiotracers, whole-body diffusion-weighted imaging, radiomics, and PET–MRI seem promising in this area [48].
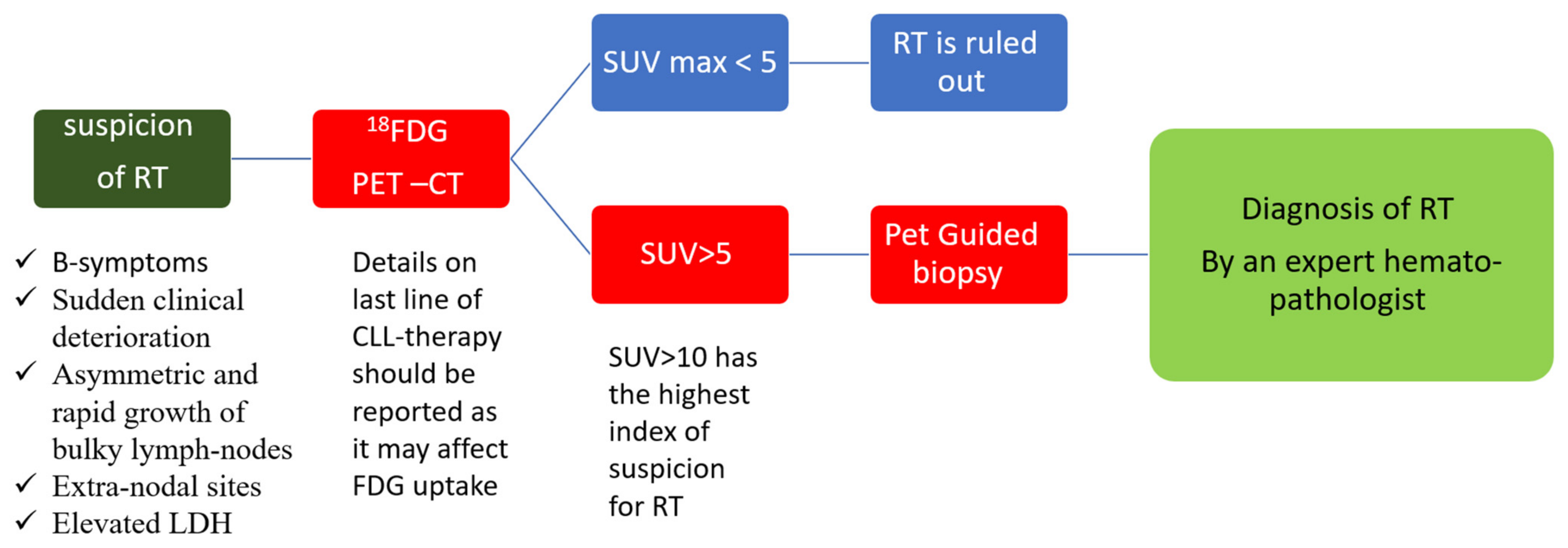
Figure 1. Diagnosis of Richter transformation. Legend: 18-FDG-PET–CT: positron emission tomography with 2-deoxy-fluorine-18-fluoro-D-glucose; CLL: chronic lymphocytic leukemia; LDH: lactate dehydrogenase; RT: Richter transformation; SUV: standardized uptake values.
Stem cell transplant (SCT) represents the only option for curing RT. The European bone marrow transplantation registry included 59 patients with RT from 1997 to 2007: 34 and 25 of them underwent autologous and allogeneic SCT, respectively, most of them with reduced intensity conditioning (RIC) [49]. The 3-year OS was estimated at 36% for allogeneic SCT and 59% for autologous SCT, with an age younger than 60 years, chemo-sensitive disease, and RIC being associated with a better prognosis after allogeneic SCT in RT [49]. The benefits of RIC preceding allogeneic SCT in RT were also underlined, including 58 CLL patients, 23 of them with RT with a median follow-up of 68 months that revealed a 5 year OS of 58% and a 5-year PFS of 40% [50]. Another was showed encouraging results in 10 patients with RT referred to allogeneic SCT after objective response to therapy, with a 4-year OS of 50%, a non-relapse mortality at both 1 and 4 years post-transplantation of 40%, and a 4 year incidence of relapse/progression of 10% [51]. It was included 27 patients with DLBCL-RT and one with HVRT, showing 4-year OS and PFS of 53% and 39%, respectively, and an acceptable 18% rate of grade III–IV graft-versus-host disease [52]. Finally, a systematic review and meta-analysis of four studies including 72 fit patients with RT that underwent allogeneic SCT identified an encouraging pooled overall response rate (ORR), complete remission (CR), OS, and PFS rates of 79%, 33%, 49%, and 30%, respectively [53].
Due to the relatively high expression of PD-1 and PD-L1 in DLBCL-RT compared to de novo DLBCL [54], therapy with PD-1 monoclonal antibodies (PDCD1) has been tested in patients with RT, all of them with prior BTKi therapy (Figure 2). PDCD1 was given as monotherapy or combined with ibrutinib with or without venetoclax. Only one patient responded, and the median OS was 2 months [55]. More recently, checkpoint inhibitors were tested in patients with DLBCL-RT. Nine patients with DLBCL-RT were treated with pembrolizumab monotherapy with a 44% ORR [56], and 23 patients received nivolumab combined with ibrutinib with an ORR of 43% [57]. It was recently evaluated that the effect of pembrolizumab on 23 patients with R/R RT, showing an ORR of 13% (3 patients), although two of them had Hodgkin’s lymphoma histology [58]. Following this potential clinical activity of checkpoint inhibitors in DLBCL-RT, some clinical trials were recently initiated. It was currently recruited RT patients to assess the efficacy and safety of the BTKi zanubrutinib combined with the PD-1 inhibitor tislelizumab for the CLL-RT1 (NCT04271956), and they have started recruiting for the Pembro-U2 phase I/II clinical trial to assess the safety and efficacy of U2 (both anti-CD20 ublituximab and anti-phosphoinositide 3 kinase (PI3K) umbralisib) combined with the anti-PD1 pembrolizumab in patients with R/R CLL and RT (NCT02535286).
Another approach that was recently adopted for the treatment of hematological malignancy with promising results is CD19-targeted chimeric antigen receptor T (CAR-T) cell therapy [72]. CAR-T therapy was evaluated in 24 patients with high-risk, heavily pretreated R/R CLL after ibrutinib failure, five of them with RT, and an ORR of 71% was reported at 4 weeks after CAR-T cell infusion [67]. They then tested this therapy among 19 patients concurrently treated with ibrutinib, four of them with RT; they found a 4 week ORR of 83%, a 61% minimal residual disease (MRD) negativity, and 1 year OS and PFS of 86% and 59%, respectively [68]. Due to these encouraging results, it was included eight patients with high-risk CLL with RT that were treated with CAR-T cell therapy in the 2019–2020 period; they reported a 71% (5/8) ORR, that all of them achieved CR on day 28, and a reasonable safety profile of seven patients with cytokine release syndrome—four of them were grade 1, three patients had neurotoxicity, and there were no CAR-T-cell-related fatalities [69]. Another recent group studied nine patients with RT treated with axicabtagene ciloleucel CAR-T cell therapy in a single center in Ohio; eight of them underwent formal response assessment and achieved an objective response (five cases of CR and three cases of PR as the best responses) [70] (Figure 2).
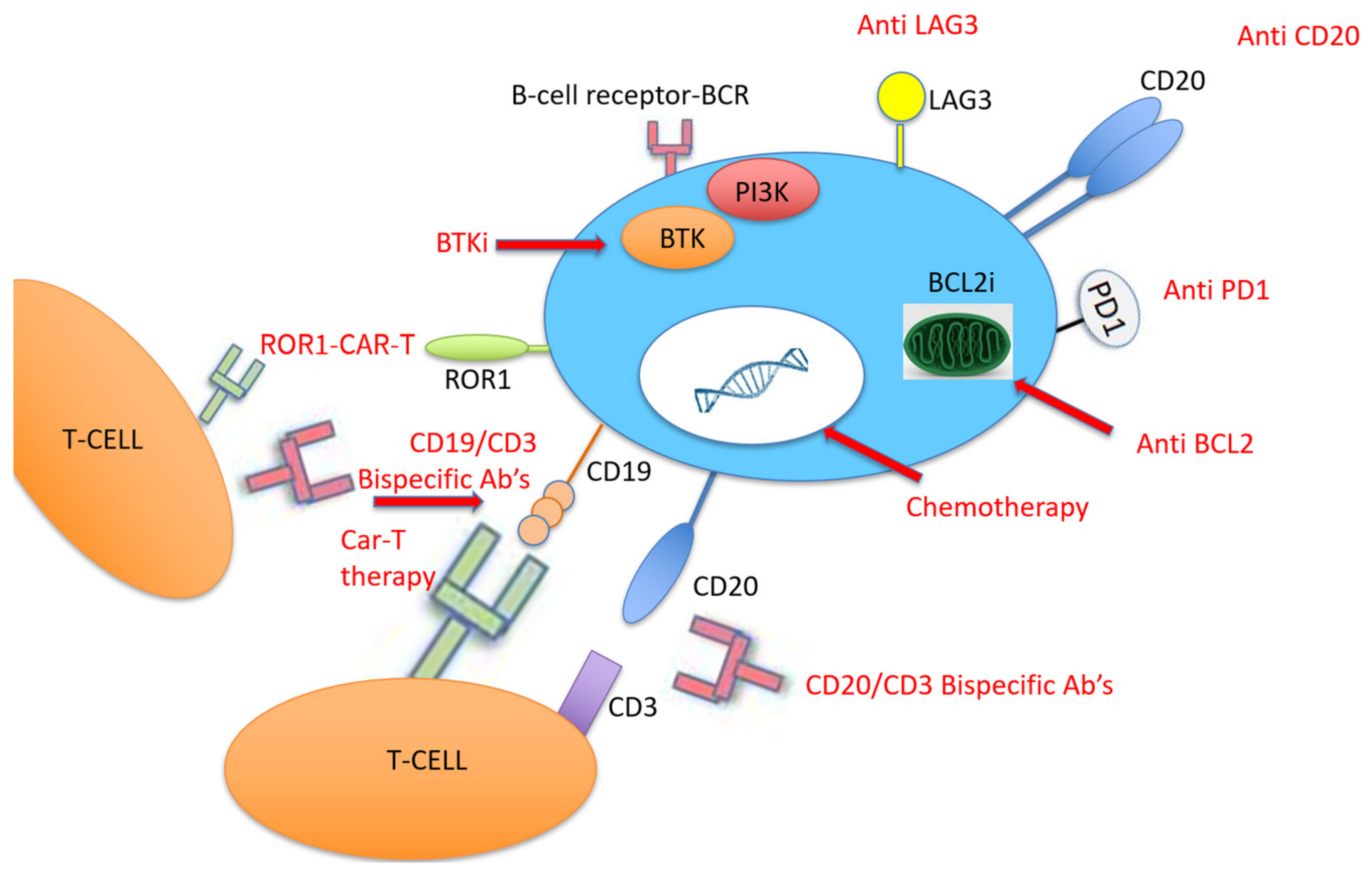
Combining the mechanisms of action of novel therapies represents the future for effective therapy in RT. One example is the “synthetic lethality” approach, which was recently investigated by Mato et al., who combined a triplet of a novel and clinically differentiated irreversible BTKi (DTRM-12) with the mechanistic target of rapamycin (mTOR) inhibitor everolimus and the immune-modulator pomalidomide to form an optimized, oral, once-daily DTRM-55. It was included 13 patients with RT-DLBCL and 11 with R/R DLBCL, and the 11 evaluable RT patient had an ORR of 45% and a median duration of response of 15 months [71] .