Richter transformation (RT) is defined as the occurrence of an aggressive lymphoma in patients with a previous or concomitant diagnosis of chronic lymphocytic leukemia (CLL). It is characterized by a switch in the histopathology and biology of the original CLL.
- richter syndrome
- richter transformation
- chronic lymphocytic leukemia
- DLBCL
- novel agents
- BTKi
- BCL2
1. Definition, Epidemiology, and Clinical Presentation of Richter Transformation
Richter transformation (RT) is defined as the occurrence of an aggressive lymphoma in patients with a previous or concomitant diagnosis of chronic lymphocytic leukemia (CLL) [1]. It is characterized by a switch in the histopathology and biology of the original CLL.
In 95–99% of cases, such a switch is towards a diffuse large B cell lymphoma (DLBCL) (DLBCL-RT), cases of the Hodgkin’s variant of Richter transformation (HVRT) (0.5–5%) [2] have been described, and, less frequently, the transformation has been described in plasmablastic lymphomas [3].
Recently, the incidence of RT was evaluated using the Surveillance, Epidemiology, and End Results (SEER) database of CLL patients diagnosed between 2000 and 2016. In this large cohort of 74,116 patients with CLL, 530 cases with RT were identified, with a 0.7% incidence of transformation [4]. It was summarized that their pooled analysis of 2975 patients included in the frontline treatment trials and an RT incidence of 3% was observed, 92% of which with DLBCL-RT [5]. In the era of novel agents, one raised concern was whether there was an increased rate of this rare and aggressive transformation among patients treated with Bruton tyrosine kinase inhibitors (BTKis) or BCL2 inhibitors (BCL2is). Indeed, in the first clinical trials using novel agents, 2–15% incidence rates of RT have been described in relapsed/refractory (R/R) patients with CLL treated with ibrutinib [6][7][8][9][10][11], venetoclax [12][13][14], or idelalisib [15][16][17]. These alarming reports were probably related to the recruitment of patients with R/R disease or even already in the early stages of transformation.
In contrast, in clinical trials involving treatment-naïve patients with CLL treated with novel agents, the incidence of RT was reported to be 0–4% [9][18][19][20][21][22][23][24][25] , indicating that there is no increase in the number of cases of RT during therapy with these novel and effective biological agents.
2. Pathogenesis and Risk Factors for the Development of Richter Transformation
It is of great interest to identify the patients with the highest risk to develop RT. Indeed, risk factors for the development of RT have been extensively studied and include clinical characteristics or molecular and genetic changes.
Immunoglobulin heavy-chain variable region gene (IGHV) mutational status indicate that patients with unmutated IGHV [26] or stereotyped B-cell receptor (BCR) [27][28][26][29][30][31] have increased risk of RT. Moreover, IGHV4–39 gene usage has been shown to carry a 24-fold increased risk of RT and when combined with stereotyped BCR (SUBSET 8) in the same patient, it showed a 5-year risk of RT of 68.7% [32]. Another recently noted point is that CLL patients with a complex karyotype at diagnosis seem to have the highest risk and shortest time to Richter transformation [33][34].
Murine models have shown that RT is characterized by constitutive active AKT, which seems to induce NOTCH1-signaling B cells via the NOTCH1 ligand expressed by T cells, and therefore apparently orchestrates RT [35].
It is understood that the microenvironment has a fundamental role in the supporting cancer genesis. CLL cells and their surrounding niche are closely related and constantly interact. From this point of view, microenvironment remodeling also seems to have a role in the development of RT. This observation is reflected by a high programmed death 1 (PD-1) expression by tumoral B lymphocytes [36][37], higher programmed death ligand 1 (PD-L1) expression in histiocytes and dendritic cells, the higher infiltration of FOXP3-positive T cells and CD163-positive macrophages, and lower peripheral blood T-cell receptor clonality compared to CLL without RT [37], suggesting changes in the immune signature of CLL after RT.
3. Diagnosis of Richter Transformation
It is highly important to have a high index of suspicion of RT in a CLL patient with sudden clinical deterioration and to direct them to workout, performing a biopsy from the most accurate site for diagnosis as early as possible.
The diagnosis of RT is based on a biopsy and the histopathologic analysis of a suspected lesion (mainly lymph node) by an expert haemato-pathologist.
The cell of origin is generally of an activated-B-cell (ABC) type that expresses post-germinal center markers such as IRF-4, whereas only 5–10% display a germinal center B-cell (GCB) phenotype expressing CD10 and/or BCL6 [38]. Moreover, CLL markers such as CD5 and CD23 are generally lost during RT [39]. Due to the complexity of distinguishing DLBCL-RT from histologically aggressive CLL, criteria for the histological diagnosis of DLBCL-RT have been delineated [40].
As opposed to CLL, radiological evaluation is recommended for the workup diagnosis of RT. Conventional CT has been performed in the past, but it currently has limited use and is only recommended if other imaging modalities are not available [41]. 18-FDG-PET/CT is the recommended imaging technique, both for diagnosis and as a guide for the most adequate site of accurate biopsy. The probability of RT was shown to be significantly increased with higher standardized uptake values (SUVs) and maximal SUV (SUV max). This technique has the ability to distinguish between CLL (median SUV max : 3.7), accelerated CLL (median SUV max : 6.8), and RT (median SUV max : 17.6) [42]. Therefore, an 18-FDG-PET/CT showing low uptake (SUV of 5 and lower) seems to rule out RT, while an 18-FDG-PET/CT showing a high uptake (SUV of 10 and higher) may help guide biopsies for definite RT diagnosis [43][44][45] (Figure 1). Furthermore, some 18-FDG-PET/CT markers such as total metabolic tumor volume, SUV body weight, SUV lean body mass, SUV body surface area, lesion-to-liver SUV ratio, and lesion-to-blood-pool SUV ratio assessed at the time of RT into DLBCL seem to be correlated with overall survival (OS) [46][47]. However, in the novel therapy era, one should consider the limitations of 18-FDG-PET/CT, the results of which might be influenced by the use of biological agents as BTKi and anti-PD1 [48]. Other imaging techniques involving novel PET radiotracers, whole-body diffusion-weighted imaging, radiomics, and PET–MRI seem promising in this area [48].
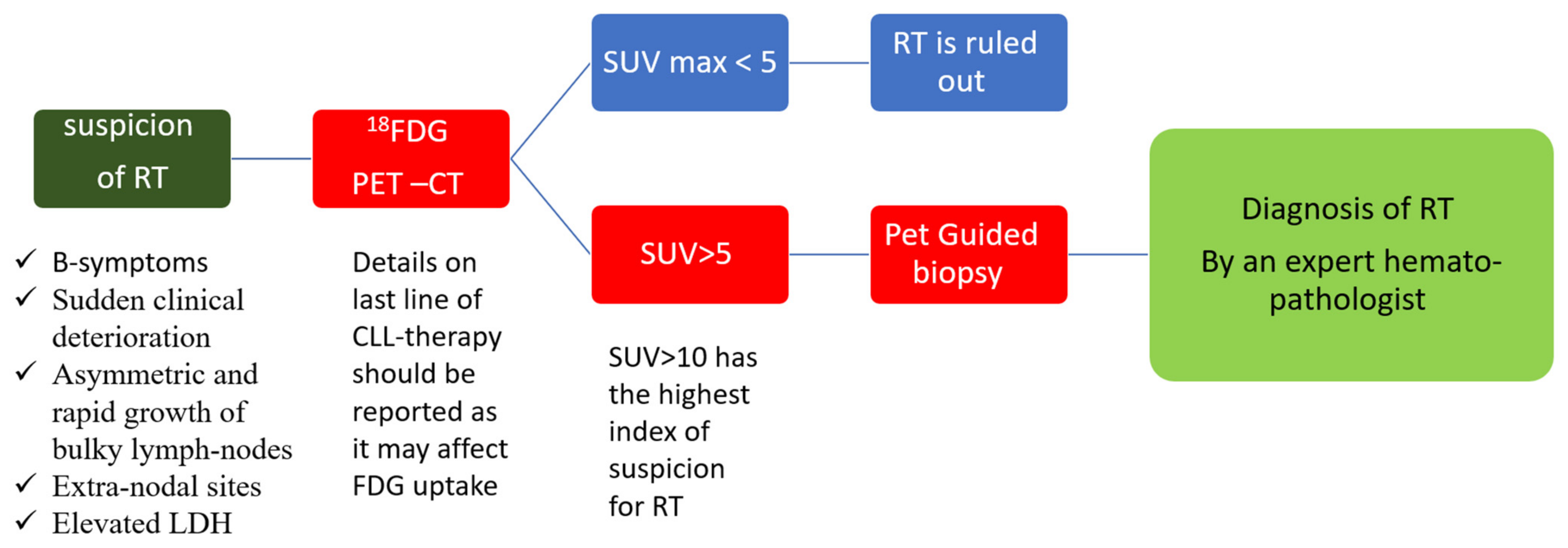
Figure 1. Diagnosis of Richter transformation. Legend: 18-FDG-PET–CT: positron emission tomography with 2-deoxy-fluorine-18-fluoro-D-glucose; CLL: chronic lymphocytic leukemia; LDH: lactate dehydrogenase; RT: Richter transformation; SUV: standardized uptake values.
4. Current Treatment Strategies of Richter Transformation
Stem cell transplant (SCT) represents the only option for curing RT. The European bone marrow transplantation registry included 59 patients with RT from 1997 to 2007: 34 and 25 of them underwent autologous and allogeneic SCT, respectively, most of them with reduced intensity conditioning (RIC) [49]. The 3-year OS was estimated at 36% for allogeneic SCT and 59% for autologous SCT, with an age younger than 60 years, chemo-sensitive disease, and RIC being associated with a better prognosis after allogeneic SCT in RT [49]. The benefits of RIC preceding allogeneic SCT in RT were also underlined, including 58 CLL patients, 23 of them with RT with a median follow-up of 68 months that revealed a 5 year OS of 58% and a 5-year PFS of 40% [50]. Another was showed encouraging results in 10 patients with RT referred to allogeneic SCT after objective response to therapy, with a 4-year OS of 50%, a non-relapse mortality at both 1 and 4 years post-transplantation of 40%, and a 4 year incidence of relapse/progression of 10% [51]. It was included 27 patients with DLBCL-RT and one with HVRT, showing 4-year OS and PFS of 53% and 39%, respectively, and an acceptable 18% rate of grade III–IV graft-versus-host disease [52]. Finally, a systematic review and meta-analysis of four studies including 72 fit patients with RT that underwent allogeneic SCT identified an encouraging pooled overall response rate (ORR), complete remission (CR), OS, and PFS rates of 79%, 33%, 49%, and 30%, respectively [53].
Due to the relatively high expression of PD-1 and PD-L1 in DLBCL-RT compared to de novo DLBCL [54], therapy with PD-1 monoclonal antibodies (PDCD1) has been tested in patients with RT, all of them with prior BTKi therapy (Figure 2). PDCD1 was given as monotherapy or combined with ibrutinib with or without venetoclax. Only one patient responded, and the median OS was 2 months [55]. More recently, checkpoint inhibitors were tested in patients with DLBCL-RT. Nine patients with DLBCL-RT were treated with pembrolizumab monotherapy with a 44% ORR [56], and 23 patients received nivolumab combined with ibrutinib with an ORR of 43% [57]. It was recently evaluated that the effect of pembrolizumab on 23 patients with R/R RT, showing an ORR of 13% (3 patients), although two of them had Hodgkin’s lymphoma histology [58]. Following this potential clinical activity of checkpoint inhibitors in DLBCL-RT, some clinical trials were recently initiated. It was currently recruited RT patients to assess the efficacy and safety of the BTKi zanubrutinib combined with the PD-1 inhibitor tislelizumab for the CLL-RT1 (NCT04271956), and they have started recruiting for the Pembro-U2 phase I/II clinical trial to assess the safety and efficacy of U2 (both anti-CD20 ublituximab and anti-phosphoinositide 3 kinase (PI3K) umbralisib) combined with the anti-PD1 pembrolizumab in patients with R/R CLL and RT (NCT02535286).
Another approach that was recently adopted for the treatment of hematological malignancy with promising results is CD19-targeted chimeric antigen receptor T (CAR-T) cell therapy [72]. CAR-T therapy was evaluated in 24 patients with high-risk, heavily pretreated R/R CLL after ibrutinib failure, five of them with RT, and an ORR of 71% was reported at 4 weeks after CAR-T cell infusion [67]. They then tested this therapy among 19 patients concurrently treated with ibrutinib, four of them with RT; they found a 4 week ORR of 83%, a 61% minimal residual disease (MRD) negativity, and 1 year OS and PFS of 86% and 59%, respectively [68]. Due to these encouraging results, it was included eight patients with high-risk CLL with RT that were treated with CAR-T cell therapy in the 2019–2020 period; they reported a 71% (5/8) ORR, that all of them achieved CR on day 28, and a reasonable safety profile of seven patients with cytokine release syndrome—four of them were grade 1, three patients had neurotoxicity, and there were no CAR-T-cell-related fatalities [69]. Another recent group studied nine patients with RT treated with axicabtagene ciloleucel CAR-T cell therapy in a single center in Ohio; eight of them underwent formal response assessment and achieved an objective response (five cases of CR and three cases of PR as the best responses) [70] (Figure 2).
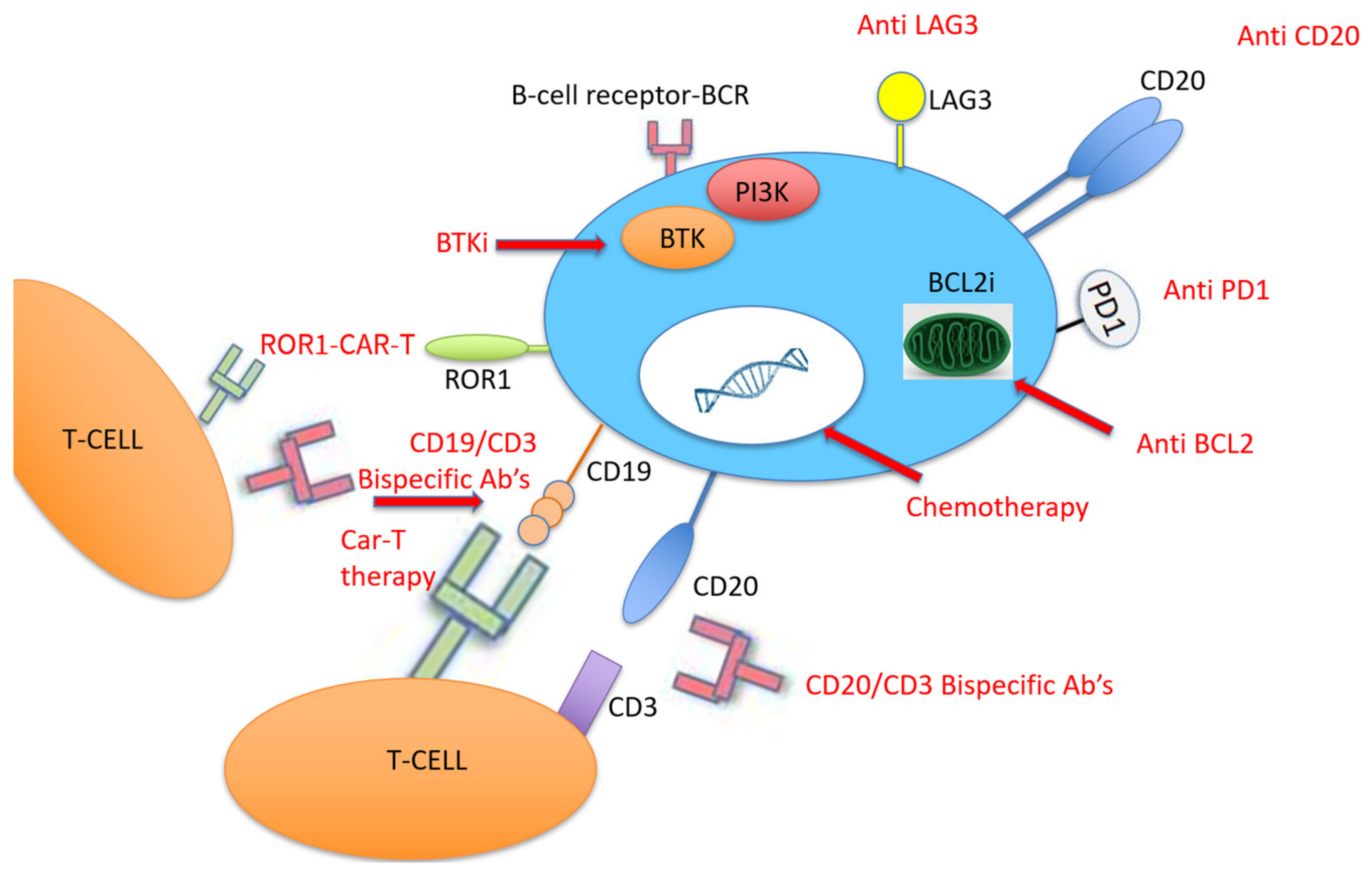
Combining the mechanisms of action of novel therapies represents the future for effective therapy in RT. One example is the “synthetic lethality” approach, which was recently investigated by Mato et al., who combined a triplet of a novel and clinically differentiated irreversible BTKi (DTRM-12) with the mechanistic target of rapamycin (mTOR) inhibitor everolimus and the immune-modulator pomalidomide to form an optimized, oral, once-daily DTRM-55. It was included 13 patients with RT-DLBCL and 11 with R/R DLBCL, and the 11 evaluable RT patient had an ORR of 45% and a median duration of response of 15 months [71] .
This entry is adapted from the peer-reviewed paper 10.3390/cancers13205141
References
- Jamroziak, K.; Tadmor, T.; Robak, T.; Polliack, A. Richter syndrome in chronic lymphocytic leukemia: Updates on biology, clinical features and therapy. Leuk. Lymphoma 2015, 56, 1949–1958.
- Allan, J.N.; Furman, R.R. Current trends in the management of Richter’s syndrome. Int. J. Hematol. Oncol. 2019, 7, IJH09.
- Marvyin, K.; Tjønnfjord, E.B.; Breland, U.M.; Tjønnfjord, G.E. Transformation to plasmablastic lymphoma in CLL upon ibrutinib treatment. BMJ Case Rep. 2020, 13, e235816.
- Elnair, R.; Ellithi, M.; Kallam, A.; Shostrom, V.; Bociek, R.G. Outcomes of Richter’s transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): An analysis of the SEER database. Ann. Hematol. 2021, 100, 2513–2519.
- Al-Sawaf, O.; Robrecht, S.; Bahlo, J.; Fink, A.M.; Cramer, P.; Tresckow, J.V.; Lange, E.; Kiehl, M.; Dreyling, M.; Ritgen, M.; et al. Richter transformation in chronic lymphocytic leukemia (CLL)—a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials. Leukemia 2021, 35, 169–176.
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363.
- O’Brien, S.; Jones, J.A.; Coutre, S.E.; Mato, A.R.; Hillmen, P.; Tam, C.; Österborg, A.; Siddiqi, T.; Thirman, M.J.; Furman, R.R.; et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): A phase 2, open-label, multicentre study. Lancet Oncol. 2016, 17, 1409–1418.
- Chanan-Khan, A.; Cramer, P.; Demirkan, F.; Fraser, G.; Silva, R.S.; Grosicki, S.; Pristupa, A.; Janssens, A.; Mayer, J.; Bartlett, N.L.; et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): A randomised, double-blind, phase 3 study. Lancet Oncol. 2016, 17, 200–211.
- Ahn, I.E.; Underbayev, C.; Albitar, A.; Herman, S.E.M.; Tian, X.; Maric, I.; Arthur, D.C.; Wake, L.; Pittaluga, S.; Yuan, C.M.; et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood 2017, 129, 1469–1479.
- Farooqui, M.Z.H.; Valdez, J.; Martyr, S.; Aue, G.; Saba, N.; Niemann, C.U.; Herman, S.E.M.; Tian, X.; Marti, G.; Soto, S.; et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 169–176.
- Brown, J.R.; Hillmen, P.; O’Brien, S.; Barrientos, J.C.; Reddy, N.M.; Coutre, S.E.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; Barr, P.M.; et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia 2018, 32, 83–91.
- Roberts, A.W.; Stilgenbauer, S.; Seymour, J.F.; Huang, D.C.S. Venetoclax in patients with previously treated chronic lymphocytic leukemia. Clin. Cancer Res. 2017, 23, 4527–4533.
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778.
- Seymour, J.F.; Ma, S.; Brander, D.M.; Choi, M.Y.; Barrientos, J.; Davids, M.S.; Anderson, M.A.; Beaven, A.W.; Rosen, S.T.; Tam, C.S.; et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: A phase 1b study. Lancet Oncol. 2017, 18, 230–240.
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 997–1007.
- Jones, J.A.; Robak, T.; Brown, J.R.; Awan, F.T.; Badoux, X.; Coutre, S.; Loscertales, J.; Taylor, K.; Vandenberghe, E.; Wach, M.; et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: An open-label, randomised phase 3 trial. Lancet Haematol. 2017, 4, e114–e126.
- Zelenetz, A.D.; Barrientos, J.C.; Brown, J.R.; Coiffier, B.; Delgado, J.; Egyed, M.; Ghia, P.; Illés, Á.; Jurczak, W.; Marlton, P.; et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: Interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017, 18, 297–311.
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437.
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.L.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528.
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56.
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib–Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443.
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291.
- O’Brien, S.M.; Lamanna, N.; Kipps, T.J.; Flinn, I.; Zelenetz, A.D.; Burger, J.A.; Keating, M.; Mitra, S.; Holes, L.; Yu, A.S.; et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood 2015, 126, 2686–2694.
- Lampson, B.L.; Kim, H.T.; Davids, M.S.; Abramson, J.S.; Freedman, A.S.; Jacobson, C.A.; Armand, P.A.; Joyce, R.M.; Arnason, J.E.; Rassenti, L.Z.; et al. Efficacy results of a phase 2 trial of first-line idelalisib plus ofatumumab in chronic lymphocytic leukemia. Blood Adv. 2019, 3, 1167–1174.
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.-M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236.
- Timár, B.; Fülöp, Z.; Csernus, B.; Angster, C.; Bognár, Á.; Szepesi, Á.; Kopper, L.; Matolcsy, A. Relationship between the mutational status of VH genes and pathogenesis of diffuse large B-cell lymphoma in Richter’s syndrome. Leukemia 2004, 18, 326–330.
- Parikh, S.A.; Rabe, K.G.; Call, T.G.; Zent, C.S.; Habermann, T.M.; Ding, W.; Leis, J.F.; Schwager, S.M.; Hanson, C.A.; Macon, W.R.; et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): A cohort study of newly diagnosed patients. Br. J. Haematol. 2013, 162, 774–782.
- Rossi, D.; Cerri, M.; Capello, D.; Deambrogi, C.; Rossi, F.M.; Zucchetto, A.; De Paoli, L.; Cresta, S.; Rasi, S.; Spina, V.; et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br. J. Haematol. 2008, 142, 202–215.
- Rezvani, S.; Tominna, M.; Al-Katib, S.; Smith, M.D.; Cousineau, C.; Al-Katib, A. Lymphomatoid Granulomatosis in a Patient with Chronic Lymphocytic Leukemia and Rapidly Progressing Peribronchovascular Pulmonary Infiltrates. Case Rep. Pulmonol. 2019, 2019, 1–5.
- Rossi, D.; Spina, V.; Deambrogi, C.; Rasi, S.; Laurenti, L.; Stamatopoulos, K.; Arcaini, L.; Lucioni, M.; Rocque, G.B.; Xu-Monette, Z.Y.; et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood 2011, 117, 3391–3401.
- Rossi, D.; Bodoni, C.L.; Genuardi, E.; Monitillo, L.; Drandi, D.; Cerri, M.; Deambrogi, C.; Ricca, I.; Rocci, A.; Ferrero, S.; et al. Telomere length is an independent predictor of survival, treatment requirement and Richter’s syndrome transformation in chronic lymphocytic leukemia. Leukemia 2009, 23, 1062–1072.
- Rossi, D.; Spina, V.; Cerri, M.; Rasi, S.; Deambrogi, C.; De Paoli, L.; Laurenti, L.; Maffei, R.; Forconi, F.; Bertoni, F.; et al. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to richter syndrome. Clin. Cancer Res. 2009, 15, 4415–4422.
- Visentin, A.; Rigolin, G.M.; Mauro, F.R.; Martines, A.; Frezzato, F.; Imbergamo, S.; Pravato, S.; Gargarella, L.R.; Bardi, M.A.; Nanni, M.; et al. Complex Karyotype Subtypes at Chronic Lymphocytic Leukemia Diagnosis Refine the Risk of Developing a Richter Syndrome. The Richter Syndrome Scoring System. Blood 2020, 136 (Suppl. S1), 33–34.
- Visentin, A.; Bonaldi, L.; Rigolin, G.M.; Mauro, F.R.; Martines, A.; Frezzato, F.; Pravato, S.; Gargarella, L.R.; Bardi, M.A.; Cavallari, M.; et al. The complex karyotype landscape in chronic lymphocytic leukemia allows to refine the risk of Richter syndrome transformation. Haematologica 2021.
- Kohlhaas, V.; Blakemore, S.J.; Al-Maarri, M.; Nickel, N.; Pal, M.; Roth, A.; Hövelmeyer, N.; Schäfer, S.C.; Knittel, G.; Lohneis, P.; et al. Active Akt signaling triggers CLL toward Richter transformation via overactivation of Notch1. Blood 2021, 137, 646–660.
- Augé, H.; Notarantonio, A.B.; Morizot, R.; Quinquenel, A.; Fornecker, L.M.; Hergalant, S.; Feugier, P.; Broséus, J. Microenvironment Remodeling and Subsequent Clinical Implications in Diffuse Large B-Cell Histologic Variant of Richter Syndrome. Front. Immunol. 2020, 11, 594841.
- Wang, Y.; Sinha, S.; Wellik, L.E.; Secreto, C.R.; Rech, K.L.; Call, T.G.; Parikh, S.A.; Kenderian, S.S.; Muchtar, E.; Hayman, S.R.; et al. Distinct immune signatures in chronic lymphocytic leukemia and Richter syndrome. Blood Cancer J. 2021, 11, 86.
- Khan, N.; Chitalia, A.; Ozdemirli, M.; Ray, G.; Gehan, E.; Cheson, B.D. Cell of Origin in Richter’s Transformation of CLL. Blood 2012, 120, 4574.
- Jain, P.; O’Brien, S. Richter’s transformation in chronic lymphocytic leukemia. Oncology 2012, 26, 1146–1152.
- Soilleux, E.J.; Wotherspoon, A.; Eyre, T.A.; Clifford, R.; Cabes, M.; Schuh, A.H. Diagnostic dilemmas of high-grade transformation (Richter’s syndrome) of chronic lymphocytic leukaemia: Results of the phase II National Cancer Research Institute CHOP-OR clinical trial specialist haemato-pathology central review. Histopathology 2016, 69, 1066–1076.
- Federmann, B.; Mueller, M.R.; Steinhilber, J.; Horger, M.S.; Fend, F. Diagnosis of Richter transformation in chronic lymphocytic leukemia: Histology tips the scales. Ann. Hematol. 2018, 97, 1859–1868.
- Falchi, L.; Keating, M.J.; Marom, E.M.; Truong, M.T.; Schlette, E.J.; Sargent, R.L.; Trinh, L.; Wang, X.; Smith, S.C.; Jain, N.; et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood 2014, 123, 2783–2790.
- Khan, M.; Siddiqi, R.; Thompson, P.A. Approach to Richter transformation of chronic lymphocytic leukemia in the era of novel therapies. Ann. Hematol. 2018, 97, 1–15.
- Condoluci, A.; Rossi, D. Treatment of Richter’s Syndrome. Curr. Treat. Options Oncol. 2017, 18, 75.
- Rossi, D.; Spina, V.; Gaidano, G. Biology and treatment of Richter syndrome. Blood 2018, 131, 2761–2772.
- Pontoizeau, C.; Girard, A.; Mesbah, H.; Haumont, L.A.; Devillers, A.; Tempescul, A.; Salaün, P.Y.; Lamy, T.; Le Jeune, F.; Palard-Novello, X. Prognostic Value of Baseline Total Metabolic Tumor Volume Measured on FDG PET in Patients with Richter Syndrome. Clin. Nucl. Med. 2020, 45, 118–122.
- Albano, D.; Camoni, L.; Rodella, C.; Giubbini, R.; Bertagna, F. 2--FDG PET/CT Role in Detecting Richter Transformation of Chronic Lymphocytic Leukemia and Predicting Overall Survival. Clin. Lymphoma, Myeloma Leuk. 2021, 21, e277–e283.
- Musanhu, E.; Sharma, R.K.; Attygalle, A.; Wotherspoon, A.; Chau, I.; Cunningham, D.; Dearden, C.; El-Sharkawi, D.; Iyengar, S.; Sharma, B. Chronic lymphocytic leukaemia and Richter’s transformation: Multimodal review and new imaging paradigms. Clin. Radiol. 2021, 76, 789–800.
- Cwynarski, K.; Van Biezen, A.; De Wreede, L.; Stilgenbauer, S.; Bunjes, D.; Metzner, B.; Koza, V.; Mohty, M.; Remes, K.; Russell, N.; et al. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter’s syndrome): A retrospective analysis from the Chronic Lymphocytic Leukemia Subcommittee of the Chronic Leukemia Working Party and Lymphoma Working P. J. Clin. Oncol. 2012, 30, 2211–2217.
- Lahoud, O.B.; Devlin, S.M.; Maloy, M.A.; Roeker, L.E.; Dahi, P.B.; Ponce, D.M.; Gyurkocza, B.; Koehne, G.; Young, J.W.; Castro-Malaspina, H.R.; et al. Reduced-intensity conditioning hematopoietic stem cell transplantation for chronic lymphocytic leukemia and Richter’s transformation. Blood Adv. 2021, 5, 2879–2889.
- Kharfan-Dabaja, M.A.; Kumar, A.; Stingo, F.E.; Khimani, F.; Hussaini, M.; Ayala, E.; Nishihori, T.; Shah, B.; Locke, F.L.; Pinilla-Ibarz, J.; et al. Allogeneic Hematopoietic Cell Transplantation for Richter Syndrome: A Single-Center Experience. Clin. Lymphoma Myeloma Leuk. 2018, 18, e35–e39.
- Kim, H.T.; Baker, P.O.; Parry, E.; Davids, M.; Alyea, E.P.; Ho, V.T.; Cutler, C.; Koreth, J.; Gooptu, M.; Romee, R.; et al. Allogeneic hematopoietic cell transplantation outcomes in patients with Richter’s transformation. Haematologica 2021.
- Aulakh, S.; Reljic, T.; Yassine, F.; Ayala, E.; Chavez, J.C.; Chanan-Khan, A.; Pinilla-Ibarz, J.; Kumar, A.; Kharfan-Dabaja, M.A. Allogeneic hematopoietic cell transplantation is an effective treatment for patients with Richter syndrome: A systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther. 2021, 14, 33–40.
- Behdad, A.; Griffin, B.; Chen, Y.H.; Ma, S.; Kelemen, K.; Lu, X.; Chen, Q.C. PD-1 is highly expressed by neoplastic B-cells in Richter transformation. Br. J. Haematol. 2019, 185, 370–373.
- Rogers, K.A.; Huang, Y.; Dotson, E.; Lundberg, J.; Andritsos, L.A.; Awan, F.T.; Woyach, J.A.; Byrd, J.C. Use of PD-1 (PDCD1) inhibitors for the treatment of Richter syndrome: Experience at a single academic centre. Br. J. Haematol. 2019, 185, 363–366.
- Ding, W.; LaPlant, B.R.; Call, T.G.; Parikh, S.A.; Leis, J.F.; He, R.; Shanafelt, T.D.; Sinha, S.; Le-Rademacher, J.; Feldman, A.L.; et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017, 129, 3419–3427.
- Jain, N.; Ferrajoli, A.; Basu, S.; Thompson, P.A.; Burger, J.A.; Kadia, T.M.; Estrov, Z.E.; Pemmaraju, N.; Lopez, W.; Thakral, B.; et al. A Phase II Trial of Nivolumab Combined with Ibrutinib for Patients with Richter Transformation. Blood 2018, 132 (Suppl. S1), 296.
- Armand, P.; Murawski, N.; Molin, D.; Zain, J.; Eichhorst, B.; Gulbas, Z.; Hawkes, E.A.; Pagel, J.M.; Phillips, T.; Ribrag, V.; et al. Pembrolizumab in relapsed or refractory Richter syndrome. Br. J. Haematol. 2020, 190, e117–e120.
- Tsang, M.; Shanafelt, T.D.; Call, T.G.; Ding, W.; Chanan-Khan, A.; Leis, J.F.; Nowakowski, G.S.; Bowen, D.; Conte, M.; Schwager, S.M.; et al. The efficacy of ibrutinib in the treatment of Richter syndrome. Blood 2015, 125, 1676–1678.
- Visentin, A.; Imbergamo, S.; Scomazzon, E.; Pravato, S.; Frezzato, F.; Bonaldi, L.; Pizzi, M.; Vio, S.; Gregianin, M.; Burei, M.; et al. BCR kinase inhibitors, idelalisib and ibrutinib, are active and effective in Richter syndrome. Br. J. Haematol. 2019, 185, 193–197.
- Jaglowski, S.M.; Jones, J.A.; Nagar, V.; Flynn, J.M.; Andritsos, L.A.; Maddocks, K.J.; Woyach, J.A.; Blum, K.A.; Grever, M.R.; Smucker, K.; et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: A phase 1b/2 study. Blood 2015, 126, 842–850.
- Hillmen, P. Acalabrutinib Monotherapy Effective for Richter Transformation. ASH Annu. Meet. 2016, 19, 471.
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 826–833.
- Bouclet, F.; Calleja, A.; Dilhuydy, M.S.; Véronèse, L.; Pereira, B.; Amorim, S.; Cymbalista, F.; Herbaux, C.; de Guibert, S.; Roos-Weil, D.; et al. Real-world outcomes following venetoclax therapy in patients with chronic lymphocytic leukemia or Richter syndrome: A FILO study of the French compassionate use cohort. Ann. Hematol. 2021, 100, 987–993.
- Davids, M.S.; Rogers, K.A.; Tyekucheva, S.; Pazienza, S.; Renner, S.K.; Montegaard, J.; Rocchio, M.; Ihuoma, U.; Jacobson, C.A.; Fisher, D.C.; et al. A multicenter phase II study of venetoclax plus dose- adjusted R-EPOCH (VR- EPOCH) for Richter ’ s POPULAR. J. Clin. Oncol. 2020, 38, 8004.
- Alderuccio, J.P.; Mackrides, N.; Chapman, J.R.; Vega, F.; Lossos, I.S. Rapid complete response to blinatumomab as a successful bridge to allogeneic stem cell transplantation in a case of refractory Richter syndrome. Leuk. Lymphoma 2019, 60, 230–233.
- Turtle, C.J.; Hay, K.A.; Hanafi, L.A.; Li, D.; Cherian, S.; Chen, X.; Wood, B.; Lozanski, A.; Byrd, J.C.; Heimfeld, S.; et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-Specific chimeric antigen Receptor-modified T cells after failure of ibrutinib. J. Clin. Oncol. 2017, 35, 3010–3020.
- Gauthier, J.; Hirayama, A.V.; Purushe, J.; Hay, K.A.; Lymp, J.; Li, D.H.; Yeung, C.C.S.; Sheih, A.; Pender, B.S.; Hawkins, R.M.; et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 2020, 135, 1650–1660.
- Benjamini, O.; Shimoni, A.; Besser, M.; Shem-tov, N.; Danylesko, I.; Yerushalmi, R.; Merkel, D.G.; Tadmor, T.; Lavie, D.; Fineman, R.; et al. Safety and Efficacy of CD19-CAR T Cells in Richter’s Transformation after Targeted Therapy for Chronic Lymphocytic Leukemia. Blood 2020, 136 (Suppl. S1), 40.
- Kittai, A.S.; Bond, D.A.; William, B.; Saad, A.; Penza, S.; Efebera, Y.; Larkin, K.; Wall, S.A.; Choe, H.K.; Bhatnagar, B.; et al. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Adv. 2020, 4, 4648–4652.
- Mato, A.R.; Schuster, S.J.; Foss, F.M.; Isufi, I.; Kothari, S.K.; Ding, W.; Brander, D.M.; Sitlinger, A.; Rosenthal, A.C.; Leis, J.F.; et al. A Once Daily, Oral, Triple Combination of BTK Inhibitor, mTOR Inhibitor and IMiD for Treatment of Relapsed/Refractory Richter’s Transformation and De Novo Diffuse Large B-Cell Lymphoma. Blood 2020, 136, 21–22.
- Holstein, S.A.; Lunning, M.A. CAR T-Cell Therapy in Hematologic Malignancies: A Voyage in Progress. Clin. Pharmacol. Ther. 2020, 107, 112–122.
