
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abdul Khalil H. P. S. | + 4038 word(s) | 4038 | 2020-08-10 07:43:10 | | | |
| 2 | Catherine Yang | + 7 word(s) | 4045 | 2020-08-19 11:26:17 | | |
Video Upload Options
Cellulose nanomaterials from plant fibre provide potential applications in biomedical. The biomedical application of nanocellulose isolated from plant fibre, which is a carbohydrate-based source, is very viable in the 21st century. The essential characteristics of plant fibre-based nanocellulose, which include its molecular, tensile and mechanical
properties, as well as its biodegradability potential, have been widely explored for functional materials in the preparation of aerogel. Plant cellulose nano fibre (CNF)-based aerogels are novel functional materials that have attracted remarkable interest. In recent years, CNF aerogel has been extensively used in the biomedical field due to its biocompatibility, renewability and biodegradability. The effective surface area of CNFs influences broad applications in biological and medical studies such as sustainable antibiotic delivery for wound healing, the preparation of scaffolds for tissue cultures, the development of drug delivery systems, biosensing and an antimicrobial film for wound healing. Many researchers have a growing interest in using CNF-based aerogels in the mentioned applications. The application of cellulose-based materials is widely reported in the literature. However, only a few studies discuss the potential of cellulose nanofibre aerogel in detail. The potential applications of CNF aerogel include composites, organic–inorganic hybrids, gels, foams, aerogels/xerogels, coatings and nano-paper, bioactive and wound dressing materials and bioconversion.
1. Introduction
Cellulose fibres derived from plant fibre have been widely used as reinforcements in polymers for packaging and biomedical applications. The use of aerogel in the biomedical application is on the increase, especially in tissue repair. The reparation of aerogel is difficult, time consuming and a major challenge in this research phase. However, extensive studies to develop materials of cheaper and better properties with high prospects and effectiveness for many applications are the focus of the present work. Aerogel contains more than 99% air and is a lightweight material, often prepared and manufactured in multi-shape structures depending on the required application. It has been manufactured from many inorganic and organic sources [1][2].
More recently, the development of nanobiotechnology aerogels has been possible from a variety of nanomaterials such as chitosan nanoparticles, cellulose nano fibres (CNFs), metals like silver and alginate, etc. Many scientists have worked on studies to develop aerogel with cheaper and better properties for several applications [3]. The classification of aerogels is based on the nature of the materials used for the preparation. Some examples are mesoporous silica aerogels, graphene-based aerogels, composite aerogels, and aerogel catalysts. Previous studies reported that hydroxyapatite silica aerogel from amorphous silica rice husk served as a biomaterial for in vitro biocompatibility as an alternative biomaterial for biomedical applications. The sol–gel ambient pressure drying method was used for the fabrication of silica aerogel; the results obtained from the characterisation studies revealed that the synthesised aerogel was effective for the nucleation of apatite (Sani et al., 2017). Due to its high electrical conductivity, ultra-lightweight, large specific surface area graphene aerogel has been applied for temperature and pressure sensing, as well for material elasticity (Mao et al., 2020). Silica aerogel–polyvinyl alcohol composite aerogel was synthesised at ambient pressure for drug delivery. Bioaerogels made from polysaccharides have received remarkable attention (Soorbaghi et al., 2019). They have been used widely in medical applications such as in tissue engineering and drug delivery.
The majority of nanomaterials used in the manufacturing of aerogels are biopolymers. Biopolymers have been preferably used in biomedical and environmental applications due to their abundance, renewable source, low toxicity, biocompatibility and biodegradability. Various plant and animal biopolymers have been used in the preparation of aerogels. This includes cellulose, collagen, chitosan, alginate, etc. However, plant biopolymers are preferred due to their availability, biocompatibility, biodegradability and renewability potential. Cellulose has been reported as the most abundant natural polymer, and is mostly used in the preparation of aerogels more than other biopolymers. Cellulose is mainly derived from biomass, and it is the main composite of the plant cell walls [4]. It has also been widely isolated from other living organisms such as bacteria [4]. Bacterial cellulose does not contain impurities such as wax, lignin, pectin, and hemicelluloses, which were commonly present in cellulose derived from plants [4]. Plant cellulose can be isolated from different plants, parts of a plant or plant waste. According to the literature, the most used plants are soybean hulls, wheat straw, sugarcane, rice straw and husks, palm oil residue, pineapple, banana leaf fibre, bagasse, hemp and flax straws [5][6][7].
Thin cellulose fibres, often called cellulose nanofibres (CNFs), have a high crystallinity, great mechanical stiffness and strength [8]. Their diameter is approximately 3 nm, and their micron-scale lengths have both crystalline and amorphous sections. CNF can be isolated from the microfibres of plant cellulose [9]. It is often prepared by the mechanical fibrillation of cellulose and its yield depends on the source of the cellulose and the preparation method. The obtained CNF can include the ultrafine grinding of cellulose biomass or homogenisation and microfluidisation [10]. In some cases, chemical, enzymatic or even mechanical pre-treatment of CNF is done to enhance the quality of materials, reduce the energy input or achieve other purposes [11]. Owing to the unique properties of CNF and its ability to be incorporated with different materials, various potential applications have been suggested, tested and achieved.
The applications of CNF-based materials include composites, organic–inorganic hybrids, the formation of gels, foams, aerogels/xerogels, coatings and nano-paper. Those materials have been widely studied and experimented in many medical and biomedical applications. Biomedical applications of CNF include drug delivery, scaffold fabrication, biosensing and diagnostic purposes, antimicrobial wound dressing, medical implants and vascular grafts, as shown in Figure 1.
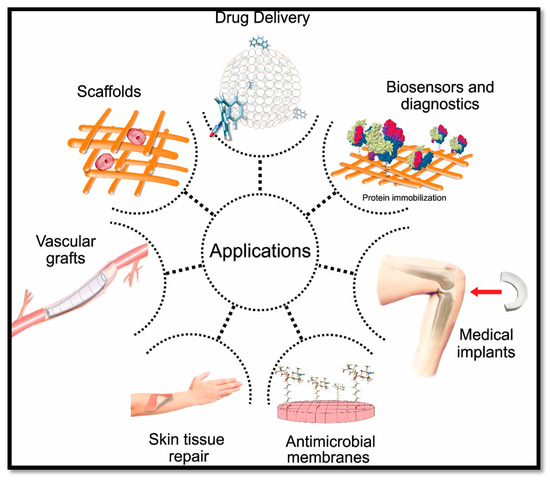
Figure 1. Cellulose nanofibre (CNF)-based aerogel in biomedical applications.
The application of cellulose-based materials is widely reported in the literature. However, only a few studies discuss the potential of cellulose nanofibre in detail, owing to the fact that these properties, which include CNF applications in composites, organic–inorganic hybrids, gels, foams, aerogels/xerogels, coatings, nano-paper production, bioactive and wound dressing materials, bioconversion and other future potential applications, have not been extensively reviewed. Moreover, CNF use in the production of aerogels has a wide application in many areas. However, the use of sustainable aerogel in biomedical applications has not been discussed in detail. Publications on cellulose nanofibre aerogel used in biomedical applications continue to attract the interest of readers; however, there has not been an updated review on this subject. Figure 2 summarises the number of publications in the past 10 years in the ScienceDirect database using cellulose nanofibre. The focus of the present review is on the recent advancements in the preparations, properties, chronological studies and stages of development of aerogels for biomedical applications. This review includes recent trends regarding the application of cellulose nanofibre aerogels, as well as the prospects and challenges of the potential applications, especially in sustainable biomedical applications.
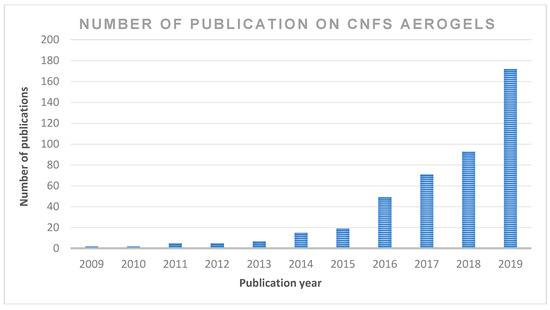
Figure 2. The number of scientific publications contributing to the subject “cellulose nanofibre; CNF and aerogel” by year (search done through ScienceDirect on 6 June 2020 (from 2009 to 2019).
2. Cellulose Nanofibre (CNF) Aerogels in Medical Application
Cellulose nanofibres (CNFs) are usually converted to aerogel during drug absorption. The properties of CNF aerogels such as their biodegradability, biocompatibility, low toxicity and renewability have had an impact on the prospects of aerogels in a wide range of biological and medical applications under investigation (Table 1). CNF aerogel has been tested in diverse medical applications such as biosensing, tissue engineering [12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][94] and drug delivery [13]].
Table 1. Chronological examples of some studies with cellulose and CNF-based materials in biomedical applications.
|
Material |
Advantage |
Method |
Application |
Reference |
|
Super critically dried silica sol-gel discs |
Facilitate the detection of chemicals and organisms |
Use of viruses to trigger a response in immobilised bacteria and chemicals |
Biosensors and diagnostics |
[14]. |
|
Cellulose-based hydrogel |
Superabsorbent capacity and satisfying biodegradability |
Tested for biodegradability and antibacterial activity against E.coli |
Antibacterial activity |
[15] |
|
Ultrafine cellulose acetate fibres with silver nanoparticles |
Very strong antimicrobial activity |
Direct electrospinning of a CA solution with small amounts of AgNO3 and then photoreduction |
Antimicrobial film |
[16] |
|
Cellulose acetate nanofibre |
Inhibit the growth of G+ and G- bacteria |
cellulose acetate nanofibre membrane using supercritical carbon dioxide |
Strong antibacterial film |
[17] |
|
Hydroxyapatite/bacterial cellulose (HAp/BC) nanocomposite |
Better adhesion and activity and faster proliferated |
HAp/BC nanocomposite scaffolds were prepared to utilise the biomimetic technique |
Bone tissue engineering. |
[18] |
|
Bacterial cellulose (BC) aerogel |
Easily equipped No aide interactions |
BC aerogel matrix loaded with drug and the release behaviour from the matrix were studied |
Drug delivery |
[19] |
|
Bacterial CNF incorporated with gold nanoparticles |
Biocatalytic activity and fast response in low conc. of H2O2 |
Immobilisation of heme proteins and enzymes |
Fabrication of H2O2 biosensors. |
[20] |
|
Hydrophobic nanocellulose aerogels |
Increase oral availability of drugs |
Physical adsorption of a drug to aerogel for oral administration |
Drug delivery system |
[21] |
|
Nanofibrillated cellulose (NFC) aerogels |
Controlled drug delivery |
NFC hydrogels are incorporated with the drug then convert it to aerogel |
Drug delivery system |
[22] |
|
NCF/collagen composite aerogels |
Strong absorption Biocompatible High proliferation. |
Di-aldehyde NCFs and collagen were cross-linked together and formed the composite aerogels. |
Tissue engineering and wound dressing |
[23] |
|
Nanocellulose aerogel (NCA) |
Significant increase in cell count. |
Cultured NIH 3T3 cells for two weeks on NCA. |
Scaffolds for 3D cell culture |
[24] |
|
Nanocellulose aerogel (NA) |
Monitor the level of protease in chronic wounds |
The complex of polypeptide-NA (PepNA) to detect the sensitivity of PepNA for human neutrophil. |
Biosensors |
[25] |
|
Antibacterial cellulose-based aerogel |
Bacterial inhibition rate of >99.99%. |
Fixing antibacterial substances on the surface of cellulose aerogels. |
Bacterial growth inhibition |
[26] |
|
CNF composite aerogel |
Significant increase in cell count. |
Cultured 3T3 NIH cells on poly (vinyl alcohol). |
Scaffolds for 3D cell culture |
[27] |
|
NFC aerogel |
Noticeable increase in drug release |
Loaded of NFC aerogel with alkylating antineoplastic agent. |
Cancer treatments |
[28] |
|
Nanocellulose derivate aerogel |
Complete inhibition of tested bacteria. |
Loading lysozymes and silver nanoparticles on CNF aerogel. |
Bacterial growth inhibition |
[29] |
|
Strain-sensing protonated CNF aerogel |
Stretchable and sensitive |
Cross-linking CNF surface with PSS in PEDOT/PSS generated PEDOT/PSS/CNF aerogels |
Biosensors |
[30] |
|
Nanocellulose/gelatine composite cryogels |
Controllable porosity, and good biocompatibility |
Used cross-linked di-aldehyde starch as carriers for controlled 5-fluorouracil (5-FU) release. |
Controlled drug release |
[31] |
2.1. Tissue Engineering
In tissue engineering technologies, to maintain regular growth of tissue, the cells must have a 3D scaffold to permit a proper exchange of waste or nutrients for the growing cells [24]. CNF aerogels have been used as a scaffold for tissue engineering and are proven to enhance the growth and the proliferation of cells [23][32][33]. The high porosity (more than 99%) of CNF-based aerogels allows high oxygen permeability and accelerates the exchange of metabolic requirements in growing cells, leading to enhanced cell activities, better adhesion, and increased proliferation. Unlike the other scaffolds, CNF and bio-based scaffolds have less cytotoxicity to growing cells, and their biocompatibility has proved to be higher. Liu et al. [32] reported that CNF and bio-based scaffolds had less than 5% cell death after 72 h of cell growth.
Similarly, Cai et al. concluded that CNF aerogel microspheres significantly facilitated the growth and proliferation of fibroblasts [24]. Scaffolding is an essential part of tissue engineering, besides giving the oxygen, adhesion and metabolic requirements to cells, it also directs the shape and structure of the tissue. Figure 3 presents a schematic diagram of a CNF-based scaffold and its usage in tissue engineering. Scaffolds can be designed based on the needed tissue, thus allowing the target cells to proliferate accordingly. Numerous composite aerogels have been prepared recently, containing different materials; Lu et al. [23] prepared composite aerogels using cellulose and collagen as precursors to reduces the influence of protease, thereby curing chronic wounds. This is because collagen is biocompatible, biodegradable and has strong adhesive characteristics on the skin. Hence, it is applicable for wound dressing.
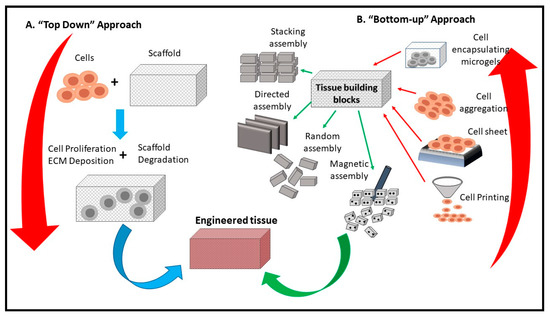
Figure 3. Schematic diagram of the use of CNF in tissue engineering (adapted from Tingli Lu et al. [34]. Copyright 2013. Reproduced with permission from the Dove Medical Press publisher.
2.1.1. Wound Healing
The process of wound healing requires normal cellular function, moisture control, and optimal oxygen permeability [35][36][37]. The effective surface area of filamentous biomaterials has been explored to develop nanoparticle-based cellulose. For example, a nanocellulose polymer was developed for cellular uptake. Similarly, a synthesised polymer was used as a folate receptor for the testing of cancer [38]. However, CNF aerogel can be incorporated with an antimicrobial material during the preparation process and can be used as a wound dressing material (Figure 4). Many plant extracts have been investigated for their antimicrobial activity. An evaluation of the effect of aqueous and alcoholic extracts of the peels of Punica granatum showed better activity than some of the common antibiotics. Similar materials can be incorporated in the aerogel to generate an antimicrobial aerogel for wound dressing [39].
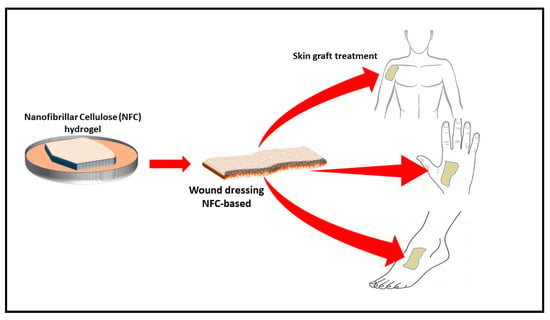
Figure 4. Schematic drawing of production of biodegradable antimicrobial wound dressing material (adapted from T. Hakkarainen [40] et al., 2016). Copyright 2016. Reproduced with permission from Elsevier Ltd.
Antimicrobial-incorporated CNF aerogel prevents infection and provides moisture and optimal oxygen permeability to the cells for proliferation. CNF aerogel has an advantage in that regular wound dressing sheets may not provide oxygen permeability or microbial control. The use of cellulose nanofibre aerogels with their high porosity and surface area provide oxygen permeability, thereby preventing the growth of anaerobic bacteria. Moreover, they can be incorporated with an antibacterial ingredient to inhibit aerobic bacterial as well. Wang et al. [36] investigated CNF and copper-containing mesoporous bioactive glass as antibacterial ingredients in aerogel composites for the potential treatment of chronic wounds. The obtained aerogel completely inhibited the growth of E. coli and other inflammatory bacteria. However, using metal particles like copper and sulphur is not desirable for many researchers, due to the toxicity potential. CNF incorporated with natural antimicrobial material aerogel (CNF/AM aerogel) could serve as a potential treatment for chronic diabetic ulcers. CNF/AM aerogel provides the sustainable inhibition of the microbial growth on wounds. The high porosity of aerogel provides aerobic conditions, preventing the growth of anaerobic bacteria such as Clostridium perfringens and gangrene development. Moreover, antimicrobials incorporated with the aerogel could prevent the growth of infectious aerobic bacteria and accelerate wound healing.
2.1.2. Biosensing and Diagnostics
A biosensor is known as an analytical device that detects low concentrations of a desirable parameter, and it combines a biological component with a physicochemical detector. Nowadays, with the development of nanotechnology, the use of 3D frame materials like bioaerogels is a crucial strategy to overcome the challenges of the low instability and sensitivity associated with 1D and 2D materials. Low-density nanocellulose aerogel from cotton has been applied as a transducer biosensor surface for protease wound dressing (Edward et al., 2016). Bacterial CNFs incorporated with gold nanoparticles have been used to immobilise heme proteins and enzymes due to the extensive surface area and biocompatibility of nanofibres. This matrix of bacterial CNF incorporated with gold nanoparticles was used to fabricate hydrogen peroxide biosensors. The findings revealed that they have the capability of detecting low concentrations of hydrogen peroxide [41].
Similarly, Weishaupt et al. [42] developed a biosensor for heavy metals. The sensing of cyanobacterial biomolecule C-phycocyanin (CPC) was enhanced with genetic modified bacteria and integrated into CNF films as a carrier material, after which CPC–CNF films were able to detect free copper ions in human blood serum, and heavy metal sensitive fluorescent emission was observed. CNFs in aerogels can also be linked to either immobilised antigen or antibodies that conjugate to an enzyme or fluorophore label used to detect the specific antibody or antigen, respectively, as presented in Figure 5.
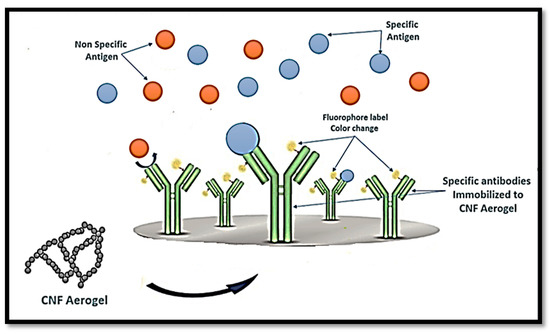
Figure 5. Schematic diagram of the potential use of CNF aerogel in the diagnosis of specific antigens.
2.1.3. Drug Delivery
In recent decades, wide varieties of novel delivery systems were developed and reported on in relation to the prolonged and controlled release of pharmaceuticals and other bioactive compounds through different routes of administration. Hydrophobic nanocellulose aerogels are one of these controlled systems, and can increase drug bioavailability compared to the intravenous and oral bioavailability of the pure drug solution. Moreover, the other advantages include the mucoadhesive properties and the floating tendency of cellulose bioaerogel [21]. The use of CNFs has been explored in drug formulations due to their unique physicochemical properties. These unique properties include their rheological and barrier properties. These properties are responsible for the stability of CNFs in oil–water and air–water interfaces. This unique characteristic can also be attributed to their large surface area to volume ratio, which means that molecular interactions will require less soluble drugs. However, by varying the porous structure of CNF aerogels, and controlling the interactions between them and the desired drug to be delivered, the drug release profile can be tuned [22][43].
Paclitaxel is an anticancer drug that has been effectively delivered to human-derived tumours in a mouse model with aerogels. A wide variety of other drugs were recently delivered in animal models following the same approaches, as illustrated in Figure 6. The drug delivery system can be controlled by controlling the size of the pores, surface area and drug and aerogel interactions. This approach could be a novel treatment for type one diabetes. Beta cell (β cell) pancreatic islets found in the pancreas that synthesise and secrete insulin could be immobilised in CNF aerogel and masked from our bodies’ immune systems. These aerogel-immobilised β cells could be introduced to the human body as a potential biosynthetic pancreas.
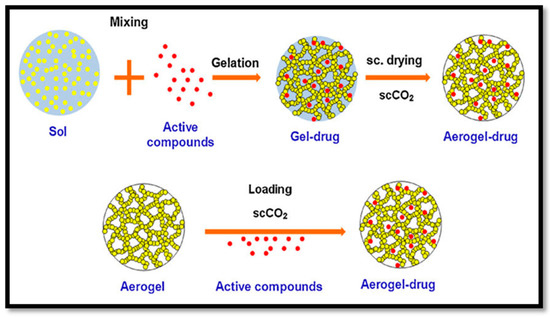
Figure 6. Fabrication process of drug delivery aerogel (cited from García-González et al. [44]. Copyright 2011. Reproduced with permission from Elsevier Ltd.
2.1.4. Antimicrobial Immobilisation
Cellulose nanofibre incorporated with natural or synthetic antimicrobial material can be immobilised inside the nanocellulose fibres (NCFs) based aerogels. This complex can be fabricated and then applied as an antimicrobial film for wound dressing. Many studies have been carried out regarding the incorporation of CNFs with silver and zinc as antibacterial inhibitors acting on different targets inside the microbial cell. Uymin et al. [29] have incorporated lysozymes and silver nanoparticles on the surface of CNF aerogels, resulting in the inhibition of the growth of 99.9% of all tested microbes. Table 2 summarises some examples of the immobilisation of antimicrobial materials inside the NCFs. In the study conducted by Xiao et al. [45]], the authors investigated the efficacy of antibacterial nanocellulose-based sponges through the covalent immobilisation of gentamicin. The result obtained revealed that the incorporation of 0.33 wt% gentamicin in the CNF sponge has a non-leaching effect and a durable contact-active antibacterial effect. Although there was no significant effect on the surface morphology, the findings revealed that cross-linkages slightly influenced the mechanical properties.
Table 5. Immobilisation of antimicrobial ingredients into CNFs.
|
Antimicrobial Agent |
Function |
Reference |
|
Silver nanoparticles (average size of 21 nm) incorporated into the cellulose acetate nanofibre |
Excellent antibacterial action against Gram-positive S. aureus and Gram-negative E. coli, K. pneumonia, and P. aeruginosa |
[46] |
|
Silver nitrate (size ranging from 10 to 20 nm) incorporated into the cellulose acetate nanofibre |
Very strong antimicrobial activity against S. aureus, K. pneumonia, E. coli, and P. aeruginosa |
[47] |
|
Composition of nanofibrillated Cellulose with silver nanoclusters (NFC/AgNC) |
Antibacterial activity against E. coli |
[48] |
|
ZnO incorporated into the cellulose acetate nanofibre |
Exhibited strong antibacterial activity against S. aureus, E. coli, and Citrobacter |
[49] |
|
Silver nanoparticles incorporated into bacterial cellulose nanofibres |
Strong antimicrobial potential against E. coli and S. aureus bacteria |
[50] |
|
T4 bacteriophage incorporated into core/shell electrospun fibres of polyethene oxide, cellulose diacetate (CDA) and their blends |
Prevent bacterial growth on contaminated food surfaces |
[51] |
|
Porous CNFs with biomass tar, polyacrylonitrile (PAN), and silver nanoparticles |
Excellent antimicrobial performance against Gram-positive S. aureus and Gram-negative E. coli, |
[52] |
|
Chitosan adsorbed cellulose nanofibre (CNF) films |
Prepared CNF film even with low Mw of chitosan exhibited antibacterial activity against L. innocua and E. coli. |
[53] |
|
Covalent grafting of gentamicin to nanocellulose-based sponge |
Excellent antibacterial performance against E. coli and S. aureus, with bactericidal rates of over 99.9% |
[45] |
|
Ag nanoparticle/cellulose nanofibre (Ag NP/CNF) composite aerogels |
The aerogel exhibited good antibacterial (for E. coli) and antifungal (for A. niger) activity. |
[54] |
|
Cellulose nanofibres (CNFs) and thyme essential oil (EO) |
Sustained antibacterial release for fresh food preservation. |
[55] |
2.2. Potential and Challenges of with CNF Aerogel in Biomedical Application
The potential use of CNF-based aerogels as sustainable materials for biomedical applications is cost effective. However, thorough toxicity testing of all nano-biomaterials remains essential, yet few studies have been reported in the literature. Specifically, detailed in vivo and biocompatibility studies on the interaction between CNFs and biological tissue have not been unexplored. The available literature failed to compare the CNFs’ sources with the types of animal models used. Many studies have been reported regarding the use of CNF-based materials for different applications. Nevertheless, CNFs still have some drawbacks and challenges that may restrict their usage in biomedical applications due to their high production costs. This is because most of the production routes use supercritical drying, which is expensive and risky. This shows that investigations into CNF production on the laboratory scale are yet to be considered for wide-scale commercialisation purposes. Microstructural images of some of the previously produced CNF-based aerogels are presented in Table 3. Their morphological characteristics, as illustrated by the SEM images, show a good porous nested network, which promotes the growth of damaged tissue when they are used as implants.
Table 3. SEM images of cellulose nanofibrillate aerogel. Reproduced with permission from Springer Nature © 2018, Scrivener © 2017, and the Royal Society of Chemistry © 2013.
However, at present, the main challenge of CNFs is related to the development of a green isolation process of nanocellulose from the natural cellulosic biomass. The current utilisation of harsh chemicals increases the toxicity of the isolated CNF. The preparation of bacterial celluloses (BCs) via the use of enzymes make the process greener and mostly acceptable in the laboratory as well as on an industrial scale. On the contrary, they have a high yield of production and less energy consumption. They are also less costly compared to chemical processes, and they also avoid pollution. Therefore, the utilisation of the enzymatic approach for the production and further modification of nanocellulose has lots of advantages compared to the adoption of the chemical approach.
Many studies have been conducted on the creation of a mass synthesis process for bacterial cellulose nanofibre, which significantly reduces the production cost. However, large-scale industrial production of bacterial cellulose nanofibre has not been achieved. Furthermore, more biochemical and genetic studies are needed to fully comprehend and enhance the cellulose production process. Another challenge is the poor processing potential of cellulose nanofibres due to their insolubility in most solvents. The poor solubility behaviour of cellulose nanofibres is attributed to their high crystallinity. Studies have been conducted to enhance the solubility of the cellulose nanofibres with the use of ionic liquids. The crystal structure of the cellulose is often destroyed with the solubility enhancement procedures and this results in poor mechanical properties in the cellulose. Despite extensive advancements in tissue engineering, no materials have been found to capture the intricacies of the native tissue nor its functionality to an ideal level. The challenge of innovating new composite materials with nanoscale engineering methods to produce fully biomimetic tissues has not yet been achieved.
According to the Cryogel Safety Data Sheet (2007), from a health perspective, aerogel may irritate the eyes and skin of some people. Furthermore, the nanosized particles of aerogels can cause silicosis in the respiratory tract if inhaled. The particles may also cause dryness in the eyes, mucous membranes and even skin. Other challenges such as CNFs’ toxicity, their environmental impact and the high-energy consumption of their production route need to be researched in detail. Furthermore, the biological and environmental toxicity of CNF-based materials’ remediation may be the greatest obstacle for their application and marketability, as the eco-toxicology studies on CNF-based materials are still limited and are at the primary stage [59]. However, more research needs to be performed to confirm whether CNF-based materials have any toxicity to humans, animals, or even microorganisms.
3. Conclusions
Plant cellulosic nanofibres (CNFs) have attracted the interest of scientists for use in the production of sustainable biomedical materials. The effective design of plant CNF-incorporated aerogel materials can be a useful tool for the development of sustainable anti-infection materials for wound dressing, scaffolds for tissue culture and drug delivery systems. The primary role of their design is defined based on the possibilities, limitations, and suitability of CNF aerogels for the development of sustainable material. Previous researchers have achieved significant progress; however, in the literature, there have been reports of challenges in the commercialisation of sustainable biomedical materials based on CNF aerogels. It has been reported that further lab-scale toxicity experiments and animal model studies on the use of CNF aerogels as sustainable materials in biomedical applications are necessary.
References
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27.
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Biomedical applications of polysaccharide and protein based aerogels. Biobased Aerogels 2018, 295–323.
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741.
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412.
- Rehman, N.; Alam, S.; Amin, N.U.; Mian, I.; Ullah, H. Ecofriendly isolation of cellulose from eucalyptus lenceolata: A novel approach. Int. J. Polym. Sci. 2018.
- Draman, S.F.S.; Daik, R.; Mohd, N. Eco-friendly extraction and characterization of cellulose from lignocellulosoic fiber. ARPN J. Eng. Appl. Sci. 2016, 11, 9591–9595.
- Chávez-Guerrero, L.; Sepúlveda-Guzmán, S.; Silva-Mendoza, J.; Aguilar-Flores, C.; Pérez-Camacho, O. Eco-friendly isolation of cellulose nanoplatelets through oxidation under mild conditions. Carbohydr. Polym. 2018, 181, 642–649.
- Rizal, S.; Gopakumar, D.A.; Huzni, S.; Thalib, S.; Syakir, M.; Owolabi, F.T.; Aprilla, N.S.; Paridah, M.; Abdul Khalil, H.P.S. Tailoring the effective properties of typha fiber reinforced polymer composite via alkali treatment. BioResources 2019, 14, 5630–5645.
- Jawaid, M.; Khan, M.M. Polymer-Based Nanocomposites for Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2018.
- Wang, Q.; Wei, W.; Chang, F.; Sun, J.; Xie, S.; Zhu, Q. Controlling the size and film strength of individualized cellulose nanofibrils prepared by combined enzymatic pretreatment and high pressure microfluidization. BioResources 2016, 11, 2536–2547.
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329.
- Weng, L.; Boda, S.K.; Wang, H.; Teusink, M.J.; Shuler, F.D.; Xie, J. Novel 3D hybrid nanofiber aerogels coupled with BMP-2 peptides for cranial bone regeneration. Adv. Healthc. Mater. 2018, 7, 1701415.
- Salgado, M.; Santos, F.; Rodríguez-Rojo, S.; Reis, R.L.; Duarte, A.R.C.; Cocero, M.J. Development of barley and yeast β-glucan aerogels for drug delivery by supercritical fluids. J. CO2 Util. 2017, 22, 262–269.
- Power, M.; Hosticka, B.; Black, E.; Daitch, C.; Norris, P. Aerogels as biosensors: Viral particle detection by bacteria immobilized on large pore aerogel. J. Non-Cryst. Solids 2001, 285, 303–308.
- Yoshii, F.; Zhao, L.; Wach, R.A.; Nagasawa, N.; Mitomo, H.; Kume, T. Hydrogels of polysaccharide derivatives crosslinked with irradiation at paste-like condition. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 320–324.
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637.
- Cardea, S.; De Marco, I. Cellulose acetate and supercritical carbon dioxide: Membranes, nanoparticles, microparticles and nanostructured filaments. Polymers 2020, 12, 162.
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098.
- Liebner, F.; Haimer, E.; Wendland, M.; Neouze, M.A.; Schlufter, K.; Miethe, P.; Heinze, T.; Potthast, A.; Rosenau, T. Aerogels from unaltered bacterial cellulose: Application of scCO2 drying for the preparation of shaped, ultra-lightweight cellulosic aerogels. Macromol. Biosci. 2010, 10, 349–352.
- Wang, W.; Zhang, T.-J.; Zhang, D.-W.; Li, H.-Y.; Ma, Y.-R.; Qi, L.-M.; Zhou, Y.-L.; Zhang, X.-X. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 2011, 84, 71–77.
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.; Ikkala, O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816.
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77.
- Lu, T.; Li, Q.; Chen, W.; Yu, H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138.
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547.
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Pircher, N.; Liebner, F.; Condon, B.D. Preparation, characterization and activity of a peptide-cellulosic aerogel protease sensor from cotton. Sensors 2016, 16, 1789.
- Henschen, J.; Illergård, J.; Larsson, P.A.; Ek, M.; Wågberg, L. Contact-active antibacterial aerogels from cellulose nanofibrils. Colloids Surf. B Biointerfaces 2016, 146, 415–422.
- Zhang, C.; Zhai, T.; Turng, L.-S. Aerogel microspheres based on cellulose nanofibrils as potential cell culture scaffolds. Cellulose 2017, 24, 2791–2799.
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021.
- Eluszkiewicz, J.; Uymin, G.; Flittner, D.; Cady-Pereira, K.; Mlawer, E.; Henderson, J.; Moncet, J.-L.; Nehrkorn, T.; Wolff, M. A fast code for channel limb radiances with gas absorption and scattering in a spherical atmosphere. J. Quant. Spectrosc. Radiat. Transf. 2017, 193, 31–39.
- Zhou, J.; Hsieh, Y.-L. Conductive polymer protonated nanocellulose aerogels for tunable and linearly responsive strain sensors. ACS Appl. Mater. Interfaces 2018, 10, 27902–27910.
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/gelatin composite cryogels for controlled drug release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389.
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271.
- Bukhari, N.; Joseph, J.P.; Hussain, J.; Adeeb, M.A.M.; Wakim, M.J.Y.; Yahya, E.B.; Arif, A.; Saleem, A.; Sharif, N. Prevalence of human papilloma virus sub genotypes following head and neck squamous cell carcinomas in asian continent, a systematic review Article. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3269.
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337.
- Wang, Q.; Yao, Q.; Liu, J.; Sun, J.; Zhu, Q.; Chen, H. Processing nanocellulose to bulk materials: A review. Cellulose 2019, 26, 1–33.
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298.
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231.
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and their composites for drug delivery: A brief review. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2018.
- Yahya, E.B.; Alhawari, S.M.; Amhimmid, K.; AbuAeshah, R.H.A.; Saada, A.O. Evaluation of in-vitroantibacterial activity of aqueous and alcoholic extracts of the peels punica granatum and olea europaea leaves. J. Sci. Technol. (Med. Sci.) 2018, 2, 36–44.
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301.
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative study of aerogels obtained from differently prepared nanocellulose fibers. ChemSusChem 2014, 7, 154–161.
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Kämpf, M.M.; Zimmermann, T.; Maniura-Weber, K.; Faccio, G. A protein-nanocellulose paper for sensing copper ions at the nano-to micromolar level. Adv. Funct. Mater. 2017, 27, 1604291.
- Svagan, A.J.; Benjamins, J.-W.; Al-Ansari, Z.; Shalom, D.B.; Müllertz, A.; Wågberg, L.; Löbmann, K. Solid cellulose nanofiber based foams–towards facile design of sustained drug delivery systems. J. Control. Release 2016, 244, 74–82.
- García-González, C.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438.
- Xiao, Y.; Rong, L.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Sui, X. A light-weight and high-efficacy antibacterial nanocellulose-based sponge via covalent immobilization of gentamicin. Carbohydr. Polym. 2018, 200, 595–601.
- Son, W.K.; Youk, J.H.; Park, W.H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434.
- Chen, L.; Bromberg, L.; Hatton, T.A.; Rutledge, G.C. Electrospun cellulose acetate fibers containing chlorhexidine as a bactericide. Polymer 2008, 49, 1266–1275.
- Díez, I.; Eronen, P.; Österberg, M.; Linder, M.B.; Ikkala, O.; Ras, R.H. Functionalization of nanofibrillated cellulose with silver nanoclusters: Fluorescence and antibacterial activity. Macromol. Biosci. 2011, 11, 1185–1191.
- Anitha, S.; Brabu, B.; Thiruvadigal, D.J.; Gopalakrishnan, C.; Natarajan, T. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2012, 87, 1065–1072.
- Yang, X.; Cranston, E.D. Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater. 2014, 26, 6016–6025.
- Korehei, R.; Kadla, J.F. Encapsulation of T4 bacteriophage in electrospun poly (ethylene oxide)/cellulose diacetate fibers. Carbohydr. Polym. 2014, 100, 150–157.
- Song, K.; Wu, Q.; Zhang, Z.; Ren, S.; Lei, T.; Negulescu, I.I.; Zhang, Q. Porous carbon nanofibers from electrospun biomass tar/polyacrylonitrile/silver hybrids as antimicrobial materials. ACS Appl. Mater. Interfaces 2015, 7, 15108–15116.
- Deng, Z.; Jung, J.; Zhao, Y. Development, characterization, and validation of chitosan adsorbed cellulose nanofiber (CNF) films as water resistant and antibacterial food contact packaging. LWT-Food Sci. Technol. 2017, 83, 132–140.
- Matsuyama, K.; Morotomi, K.; Inoue, S.; Nakashima, M.; Nakashima, H.; Okuyama, T.; Kato, T.; Muto, H.; Sugiyama, H. Antibacterial and antifungal properties of Ag nanoparticle-loaded cellulose nanofiber aerogels prepared by supercritical CO2 drying. J. Supercrit. Fluids 2019, 143, 1–7.
- Zhang, Z.; Wang, X.; Gao, M.; Zhao, Y.; Chen, Y. Sustained release of an essential oil by a hybrid cellulose nanofiber foam system. Cellulose 2020, 27, 2709–2721.
- Tan, S.; Li, J.; Zhou, L.; Chen, P.; Xu, D.; Xu, Z. Fabrication of a flexible film electrode based on cellulose nanofibers aerogel dispersed with functionalized graphene decorated with SnO2 for supercapacitors. J. Mater. Sci. 2018, 53, 11648–11658.
- Muthuraj, R.; Sachan, A.; Castro, M.; Feller, J.-F.; Seantier, B.; Grohens, Y. Vapor and pressure sensors based on cellulose nanofibers and carbon nanotubes aerogel with thermoelectric properties. J. Renew. Mater. 2018, 6, 277–287.
- Zheng, Q.; Cai, Z.; Gong, S. Green synthesis of polyvinyl alcohol (PVA)–cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118.
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325.




