Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Motoi Kanagawa | + 2215 word(s) | 2215 | 2021-12-13 11:14:07 | | | |
| 2 | Lindsay Dong | + 231 word(s) | 2446 | 2022-01-12 04:27:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kanagawa, M. Dystroglycanopathy. Encyclopedia. Available online: https://encyclopedia.pub/entry/18093 (accessed on 07 February 2026).
Kanagawa M. Dystroglycanopathy. Encyclopedia. Available at: https://encyclopedia.pub/entry/18093. Accessed February 07, 2026.
Kanagawa, Motoi. "Dystroglycanopathy" Encyclopedia, https://encyclopedia.pub/entry/18093 (accessed February 07, 2026).
Kanagawa, M. (2022, January 12). Dystroglycanopathy. In Encyclopedia. https://encyclopedia.pub/entry/18093
Kanagawa, Motoi. "Dystroglycanopathy." Encyclopedia. Web. 12 January, 2022.
Copy Citation
Dystroglycanopathy is a collective term referring to muscular dystrophies with abnormal glycosylation of dystroglycan. At least 18 causative genes of dystroglycanopathy have been identified, and its clinical symptoms are diverse, ranging from severe congenital to adult-onset limb-girdle types.
muscular dystrophy
glycosylation
dystroglycan
1. Introduction
Muscular dystrophy is a group of genetic disorders with progressive muscle weakness, and >50 causative genes have been identified (www.musclegenetable.fr, accessed on 1 November 2021). Dystroglycanopathy (DGpathy) is a collective term referring to muscular dystrophies with abnormal glycosylation of dystroglycan (DG). DG was first identified as a glycoprotein that interacts with dystrophin, the causative gene product of Duchenne muscular dystrophy, in skeletal muscle [1]. Once translated, DG is cleaved into α and β subunits, both of which are expressed in muscle cell membranes, interacting with each other in a non-covalent fashion. αDG is an extracellular subunit containing an exceedingly large number of sugar chains, and its binding to basement membrane and synaptic proteins is mediated by the sugar chains. Its known ligands include molecules containing laminin G-domain, such as laminin and agrin [2]. On the other hand, βDG is a transmembrane protein that binds to αDG on the cell surface and to dystrophin intracellularly. Since dystrophin binds to the actin cytoskeleton, DG plays a role in connecting the basement membrane and cytoskeleton across the cell membrane.
In the early 2000s, αDG sugar chain abnormalities and loss of laminin-binding activity were reported in specimens of patients with Walker–Warburg syndrome (WWS), muscle–eye–brain disease (MEB), Fukuyama congenital muscular dystrophy (FCMD), congenital muscular dystrophy 1C, and limb-girdle muscular dystrophy (LGMD) 2I (alternatively called LGMDR9) [3][4][5][6]. Because DG and dystrophin are normally expressed in cell membranes in the tissues of these patients, αDG sugar chain abnormalities are thought to disrupt the coordination between the basement membrane and the cytoskeleton [7]. Since then, cases showing similar sugar chain abnormalities have been reported, which led to the establishment of the disease concept of DGpathy. To date, at least 18 causative genes of DGpathy have been identified [8] (Table 1).
Table 1. DGpathy genes and gene product functions.
| DGpathy Genes | Gene Functions |
|---|---|
| POMT1 | Protein O-mannosyl-transferase as a part of the POMT1/2 complex |
| POMT2 | Protein O-mannosyl-transferase as a part of the POMT1/2 complex |
| POMGNT1 | Protein O-mannose β1,2-N-acetylglucosaminyltransferase; Core M1 synthesis |
| POMGNT2 | Protein O-mannose β1,4-N-acetylglucosaminyltransferase; Core M3 synthesis |
| B3GALNT2 | β1,3-N-acetylgalactosaminyltransferase; Core M3 synthesis |
| POMK | Protein O-mannose kinase; phosphorylation of Core M3 |
| FKTN | Ribitol phosphate transferase; tandem ribitol synthesis |
| FKRP | Ribitol phosphate transferase; tandem ribitol synthesis |
| ISPD/CRPPA | CDP-ribitol pyrophosphorylase; synthesis of CDP-ribitol (donor substrate of FKTN/FKRP) |
| TMEM5/ RXYLT1 |
Ribitol-5-phosphate xylosyltransferase; synthesis of linker structure between tandem ribitol and matriglycan |
| B4GAT1 | β1,4-Glucuronyltransferase; synthesis of linker structure between tandem ribitol and matriglycan |
| LARGE | Xylosyl- and glucuronyltransferase; matriglycan synthesis |
| DAG1 | Dystroglycan |
| GMPPB | GDP-mannose pyrophosphorylase required for the formation of GDP-Man; Dolichol-phosphate-mannose synthesis |
| DPM1 | Dolichol-phosphate-mannose synthase; Dolichol-phosphate-mannose synthesis |
| DPM2 | Dolichol-phosphate-mannose synthase; Dolichol-phosphate-mannose synthesis |
| DPM3 | Dolichol-phosphate-mannose synthase; Dolichol-phosphate-mannose synthesis |
| DOLK | Dolichol kinase required for the formation of dolichol-phosphate; Dolichol-phosphate-mannose synthesis |
DGpathy exhibit a broad clinical spectrum, ranging from severe congenital muscular dystrophies, such as WWS and FCMD, to mild, adult-onset LGMD [9]. This is likely due to the effects of mutations involving the functions of gene products (enzymatic activity), rather than to variation in causative genes. In addition, one of the characteristics of DGpathy is the involvement of central nervous system disorders, such as malformation of the brain (type II lissencephaly) and mental retardation. Brain lesions in DGpathy have also shown a wide range of clinical symptoms, ranging from severe malformation of the brain to lesions with only mental retardation without structural abnormalities [10][11].
2. Sugar Chain Structure of DG and Functions of Causative Genes of DGpathy
The sugar chain structure involved in the ligand binding activity of DG and the enzymes involved in its biosynthesis are shown in Figure 1. O-Mannosyl glycosylation is required for the binding of DG to its ligands, and some of the causative genes of DGpathy encode enzymes involved in the biosynthesis of sugar chains named Core M1 (galactose-β1,4-N-acetylglucosamine-β1,2-mannose-; Gal-β1,4-GlcNAc-β1,2-Man-) and Core M3 (N-acetylgalactosamine-β1,3-N-acetylglucosamine-β1,4-mannose-; GalNAc-β1,3-GlcNAc-β1,4-Man-). The protein-O-mannose transferase (POMT) 1-POMT2 complex functions to transfer a mannose moiety to serine/threonine residues of DG [12]. Because the POMT1-POMT2 complex uses dolichol phosphate mannose (Dol-P-Man) as a donor substrate, mutations in the genes (GMPPB, DPM1/2/3, and DOLK) that encode enzymes involved in its biosynthesis have been reported to cause DGpathy (Table 1) [8]. The sugar chains of Core M1 are extended by protein O-mannose N-acetylglucosaminyltransferase (POMGNT1) [13], while those of Core M3 are extended by POMGNT2 and β1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2) [14].
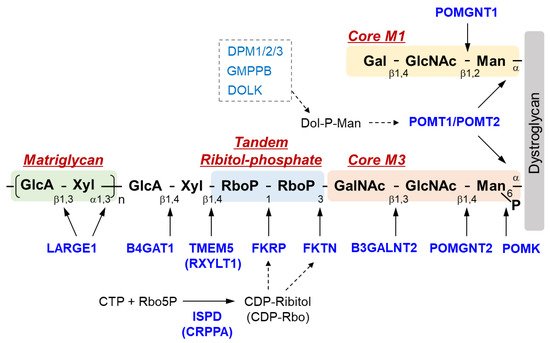
Figure 1. Overview of DG sugar chain structure and modifying enzymes. Man, mannose; GlcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine; RboP, ribitol phosphate; Xyl, xylose; GlcA, glucronic acid; Gal, galactose; Rbo5P, ribitol-5-phosphate; POMK, Protein O-mannose kinase; POMGNT, Protein O-mannose N-acetylglucosaminyltransferase; B3GALNT2, β1,3-N-acetylgalactosaminyltransferase 2; TMEM 5, Transmembrane protein 5; RXYLT1, ribitol-5-phosphate xylosyltransferase 1; B4GAT1, β-1,4-glucuronyltransferase 1; LARGE1, like-acetylglucosaminyltransferase 1/ LARGE xylosyl- and glucuronyltransferase 1; POMT, protein O-mannosyl-transferase; FKTN, fukutin; FKRP, Fukutin-related protein; ISPD, isoprenoid synthase domain-containing protein; CRPPA, CDP-ribitol pyrophosphorylase A.
After the Core M3 trisaccharides have been modified, mannose is phosphorylated by protein O-mannose kinase (POMK) [14]. On the non-reducing terminal side of Core M3, two tandemly-connected sugar alcohol phosphates, called ribitol-phosphates, are modified (tandem ribitol-phosphate) [15]. This modification is carried out by two ribitol-phosphate transferases, fukutin (FKTN) and fukutin-related protein (FKRP); the first ribitol-phosphate is modified by fukutin, while the second is modified by FKRP. The donor substrate for ribitol-phosphate is CDP-ribitol (CDP-Rbo), which is produced from ribitol-5-phosphate (Rbo5P) and CTP by the enzymatic action of isoprenoid synthase domain-containing protein (ISPD) [15][16][17]. Additionally, the names CDP-ribitol pyrophosphorylase A (CRPPA) and D-ribitol-5-phosphate cytidylyltransferase have also been proposed for ISPD based on its enzymatic activity. FCMD, which is caused by mutations in FKTN, is the predominant form of DGpathy in Japan, and LGMD2I, which is caused by mutations in FKRP, is the most frequent form of DGpathy in the United States and Europe. DGpathy patients with ISPD mutations have been often reported (see review [2]). Together, diseases caused by defects in ribitol-phosphate modification can account for the majority of DGpathy.
The end of tandem ribitol-phosphate is followed by xylose and glucuronic acid modified by ribitol-5-phosphate xylosyltransferase 1 (RXYLT1; previously transmembrane protein 5, TMEM5) and β1,4-glucuronyltransferase 1 (B4GAT1) [18][19]. This is then extended with repeating unit of disaccharides consisting of xylose and glucuronic acid, which is formed by LARGE1 (like-acetylglucosaminyltransferase 1/LARGE xylosyl- and glucuronyltransferase 1) [20]. As this repeat structure functions as a binding site for matrix ligands [21][22], it is also called matriglycan. The loss of function of any of the causative genes of DGpathy results in the termination of sugar chains synthesis, preventing the formation of matriglycan. Thus, abnormalities in any of the genes result in matriglycan deficiency. POMGNT1, which is a modifying enzyme of Core M1, is thought to be involved in matriglycan modification by binding to Core M3 and fukutin [23]. Mannosyl phosphorylation of Core M3 is required for ribitol-phosphate modification by FKRP and for matriglycan extension by LARGE1 [24][25]. Thus, many enzymes are required for DG glycosylation, and modification specificity may be created by a series of enzymatic reactions that proceed in an extremely orderly manner.
3. Pathological Mechanism of DGpathy
3.1. Muscular Dystrophy
In DGpathy, the loss of ligand binding activity of DG due to sugar chain abnormalities is thought to disrupt association with the basement membrane, which reduces the physical stability of the muscle cell membrane, making necrosis more likely to occur [7]. This pathological mechanism suggests that DGpathy patients exhibit muscle pathology similar to those of patients with dystrophin-deficient Duchenne-type muscular dystrophy (DMD). However, some DGpathy patients clearly have more severe disease manifestations than DMD patients, which cannot be explained by only the disruption of association between the basement membrane and cell membrane. Muscle fiber-selective MCK-fukutin-cKO mice present only extremely mild muscular dystrophy, suggesting that there are factors other than susceptibility of muscle fibers to necrosis [26][27]. Muscle progenitor cell-selective Myf5-fukutin-cKO mice exhibit a decreased number of muscle satellite cells and decreased proliferative activity and differentiation activity of muscle progenitor cells with progression of the pathological condition, which results in impaired muscle regeneration [27].
In addition, a recent study using induced pluripotent stem (iPS) cells derived from FKRP-deficient LGMD2I or WWS patients revealed decreased autophagy and increased apoptosis in myotubes, indicating that alterations in cell homeostasis may be involved in DGpathy pathogenesis [28].
3.2. Central Nervous System Abnormalities
Brain abnormalities in DGpathy patients are diverse, ranging from severe brain malformation and associated mental retardation/refractory epilepsy to average intelligence with little brain malformation [10][11]. Although an association between the type of gene mutation and the severity of the disease has been suggested, the underlying mechanism of the broad clinical spectrum of brain abnormalities in DGpathy patients remains unclear. DG is expressed in the termini of radial glia in the developing cerebral cortex, contributing to maintenance of the glia limitans-basement membrane complex. DG sugar chain abnormalities impair its binding to the basement membrane, disrupting the glia limitans-basement membrane complex [29][30][31]. The protrusion of neuronal cells from the site of basement membrane disruption into the subarachnoid space is thought to be the major pathological mechanism leading to type II lissencephaly [32].
3.3. Cardiomyopathy
Heart failure, along with respiratory disorders, is the leading cause of death in DGpathy patients. Many FCMD patients, for example, have left ventricular systolic impairment despite the absence of left ventricular hypertrophy and fibrosis is found at autopsy [33]. In addition, dilated cardiomyopathy in fukutin-mutated patients with very mild muscular lesions has been reported [34]. As with skeletal muscle, sugar chain abnormalities are thought to cause impaired association between the basement membrane and cell membrane [35], but cardiomyopathy associated with DGpathy is generally mild in many cases. With regards to model mice, no myocardial lesions or cardiac dysfunction are observed in young (up to 24 weeks old) striated muscle-selective MCK-fukutin-cKO mice. However, despite the absence of cardiac hypertrophy, mice older than 1 year of age exhibit fibrosis in addition to decreased cardiac function, such as chamber dilation during diastole and decreased fractioning shortening, reproducing the cardiac pathology seen in FCMD patients [36]. Moreover, the contractile property of cardiomyocytes isolated from MCK-fukutin-cKO mice is reduced, and intracellular Ca2+ handling during excitation-contraction coupling is disrupted. Similarly, mild pathological changes and decreased cardiac function have been reported in FKRP-mutant mice [37].
4. Treatment Methods
4.1. Gene Therapy
Because DGpathy is a single-gene disorder, gene therapy is considered a simple treatment strategy. As mentioned above, we showed that vulnerability of muscle fibers triggers the onset of DGpathy and that poor muscle regeneration due to decreased function of muscle progenitor cells is associated with pathological severity and progression, using skeletal muscle-selective fukutin cKO mice [27]. Since glycosylation status in muscle progenitor cells and myoblasts changes during differentiation [21], the expression of glycosyltransferases may also be strictly controlled. Therefore, these cells are not suitable targets for gene therapy that uses promoters constitutively inducing gene expression. On the other hand, selective gene rescue in muscle fibers is expected to suppress myonecrosis that triggers muscle regeneration and disease onset, although it does not improve poor muscle regeneration. In fact, the administration of an adeno-associated viral vector that allow a muscle fiber-selective expression of the fukutin gene dramatically ameliorates muscular dystrophy in Myf5-fukutin-cKO mice that exhibit muscle regeneration abnormalities [27]. The effectiveness of gene therapy for DGpathy has also been demonstrated in FKRP-mutant mice and Largemyd mice [38][39][40]. Dhoke et al. recently reported a gene correction approach with the CRISPR-Cas9 gene editing for FKRP-deficient DGpathy to enable cell therapy [41]. In this study, the authors corrected FKRP mutations in iPS cells derived from FKRP-deficient DGpathy patients using homology-directed repair to knock in the wild-type sequence, differentiated the gene-corrected cells into myogenic progenitors, and then transplanted them in the FKRP-mutant mice. Results showed restoration of glycosylation in engrafted muscles, indicating a potential of the combination of gene editing and iPS cell-derived myogenic cell transplantation as a therapeutic approach in the future.
4.2. Pharmacotherapy
Foltz et al. reported that the mTOR pathway is activated in the muscle of fukutin-deficient mice with advanced pathology and that inhibition of the mTOR pathway with rapamycin suppresses muscular lesions such as fibrosis [42]. Binding between DG and matrix proteins is known to be involved not only in the structural maintenance of myocytes, but also in intracellular signal transduction, but DG sugar chain abnormalities may not directly activate the mTOR pathway. Thus, rapamycin may act on fibroblasts to inhibit the synthesis of matrix proteins, resulting in the suppression of fibrosis. Selective estrogen receptor modulators (SERMs) are expected to be effective against muscular dystrophy because of their features such as anti-inflammation, anti-fibrosis, bone loss suppression, and muscle mass gain. Wu et al. conducted long-term administration of two SERMs, tamoxifen and raloxifene, in FKRP-mutant mice, showing improvements in muscle function and muscle pathology [43].
Screening for low molecular-weight compounds effective in the treatment of DGpathy has been performed using FKRP-mutant zebrafish [44], which identified hits including steroids, non-steroidal anti-inflammatory drugs (NSAIDs), antibacterial drugs, and calcium chelators. Steroids have been used clinically in muscular dystrophy patients, and the beneficial effects of glucocorticoid (predonisolone) administration on the pathological condition has been suggested in FKRP-mutant mice [45].
A unique treatment strategy for FCMD based on molecular pathology has been reported. Many FCMD patients have a transposon insertion in the fukutin gene [46]. A strong RNA splice-acceptor site exists in this insertion, which activates a cryptic splicing donor site in the protein-encoding final exon, resulting in abnormal splicing of fukutin [47]. Administration of antisense nucleotides capable of correcting this splicing abnormality restores the normal function of fukutin in both fukutin KI mice and human patient-derived cells. Because this antisense nucleotide therapy can be applied to almost all FCMD patients, it represents a promising fundamental molecular-targeted therapy, and rapid progress is expected in the future.
4.3. Ribitol Supplementation Therapy
A treatment strategy for DGpathy that has received the most attention recently is perhaps ribitol supplementation therapy (Figure 2). The donor substrate for fukutin and FKRP, which are ribitol phosphate transferases, is CDP-Rbo, which is produced from ribitol-5-phosphate and CTP by the enzymatic action of ISPD. Although the biosynthetic mechanism of ribitol-5-phosphate has not been understood, the pentose phosphate pathway is thought to be involved [17]. The addition of ribitol and ribose increases the level of intracellular CDP-Rbo in normal cells and wild type mice possibly because they are somehow converted to ribitol-5-phosphate. CDP-Rbo production was shown to increase even if ribose or ribitol was added to fibroblasts derived from patients with ISPD mutations [48]. An increase in the ISPD substrate level likely enhances the enzymatic activity of the mutant protein. However, this effect likely depends on the mutation type, and it is thereby dependent on residual ISPD activity. To overcome this issue, CDP-Rbo replacement therapy has been proposed [15]. This treatment may be applicable to all forms of ISPD mutations, although the efficiency of CDP delivery into cells and its in vivo stability need to be improved.
References
- Ibraghimov-Beskrovnaya, O.; Ervasti, J.M.; Leveille, C.J.; Slaughter, C.A.; Sernett, S.W.; Campbell, K.P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 1992, 355, 696–702.
- Kanagawa, M.; Toda, T. Ribitol-phosphate-a newly identified posttranslational glycosylation unit in mammals: Structure, modification enzymes and relationship to human diseases. J. Biochem. 2018, 163, 359–369.
- Hayashi, Y.K.; Ogawa, M.; Tagawa, K.; Noguchi, S.; Ishihara, T.; Nonaka, I.; Arahata, K. Selective deficiency of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 2001, 57, 115–121.
- Michele, D.E.; Barresi, R.; Kanagawa, M.; Saito, F.; Cohn, R.D.; Satz, J.S.; Dollar, J.; Nishino, I.; Kelley, R.I.; Somer, H.; et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 2002, 418, 417–422.
- Brockington, M.; Blake, D.J.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Ponting, C.P.; Estournet, B.; Romero, N.B.; Mercuri, E.; et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am. J. Hum. Genet. 2001, 69, 1198–1209.
- Brockington, M.; Yuva, Y.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Herrmann, R.; Anderson, L.V.; Bashir, R.; Burgunder, J.M.; et al. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum. Mol. Genet. 2001, 10, 2851–2859.
- Han, R.; Kanagawa, M.; Yoshida-Moriguchi, T.; Rader, E.P.; Ng, R.A.; Michele, D.E.; Muirhead, D.E.; Kunz, S.; Moore, S.A.; Iannaccone, S.T.; et al. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of alpha-dystroglycan. Proc. Natl. Acad. Sci. USA 2009, 106, 12573–12579.
- Kanagawa, M.; Toda, T. Muscular dystrophy with ribitol-phosphate deficiency: A novel post-translational mechanism in dystroglycanopathy. J. Neuromuscul. Dis. 2017, 4, 259–267.
- Godfrey, C.; Foley, A.R.; Clement, E.; Muntoni, F. Dystroglycanopathies: Coming into focus. Curr. Opin. Genet. Dev. 2011, 21, 278–285.
- Godfrey, C.; Clement, E.; Mein, R.; Brockington, M.; Smith, J.; Talim, B.; Straub, V.; Robb, S.; Quinlivan, R.; Feng, L.; et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain 2007, 130, 2725–2735.
- Clement, E.; Mercuri, E.; Godfrey, C.; Smith, J.; Robb, S.; Kinali, M.; Straub, V.; Bushby, K.; Manzur, A.; Talim, B.; et al. Brain involvement in muscular dystrophies with defective dystroglycan glycosylation. Ann. Neurol. 2008, 64, 573–582.
- Manya, H.; Chiba, A.; Yoshida, A.; Wang, X.; Chiba, Y.; Jigami, Y.; Margolis, R.U.; Endo, T. Demonstration of mammalian protein O-mannosyltransferase activity: Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. USA 2004, 101, 500–505.
- Yoshida, A.; Kobayashi, K.; Manya, H.; Taniguchi, K.; Kano, H.; Mizuno, M.; Inazu, T.; Mitsuhashi, H.; Takahashi, S.; Takeuchi, M.; et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell 2001, 1, 717–724.
- Yoshida-Moriguchi, T.; Willer, T.; Anderson, M.E.; Venzke, D.; Whyte, T.; Muntoni, F.; Lee, H.; Nelson, S.F.; Yu, L.; Campbell, K.P. SGK196 is a glycosylation-specific O-mannose kinase required for dystroglycan function. Science 2013, 341, 896–899.
- Kanagawa, M.; Kobayashi, K.; Tajiri, M.; Manya, H.; Kuga, A.; Yamaguchi, Y.; Akasaka-Manya, K.; Furukawa, J.I.; Mizuno, M.; Kawakami, H.; et al. Identification of a post-translational modification with ribitol-phosphate and its defect in muscular dystrophy. Cell Rep. 2016, 14, 2209–2223.
- Riemersma, M.; Froese, D.S.; van Tol, W.; Engelke, U.F.; Kopec, J.; van Scherpenzeel, M.; Ashikov, A.; Krojer, T.; von Delft, F.; Tessari, M.; et al. Human ISPD is a cytidyltransferase required for dystroglycan O-mannosylation. Chem. Biol. 2015, 22, 1643–1652.
- Gerin, I.; Ury, B.; Breloy, I.; Bouchet-Seraphin, C.; Bolsée, J.; Halbout, M.; Graff, J.; Vertommen, D.; Muccioli, G.G.; Seta, N.; et al. ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto alpha-dystroglycan. Nat. Commun. 2016, 7, 11534.
- Manya, H.; Yamaguchi, Y.; Kanagawa, M.; Kobayashi, K.; Tajiri, M.; Akasaka-Manya, K.; Kawakami, H.; Mizuno, M.; Wada, Y.; Toda, T.; et al. The muscular dystrophy gene TMEM5 encodes a ribitol beta1,4-xylosyltransferase required for the functional glycosylation of dystroglycan. J. Biol. Chem. 2016, 291, 24618–24627.
- Willer, T.; Inamori, K.; Venzke, D.; Harvey, C.; Morgensen, G.; Hara, Y.; Beltrán Valero de Bernabé, D.; Yu, L.; Wright, K.M.; Campbell, K.P. The glucuronyltransferase B4GAT1 is required for initiation of LARGE-mediated alpha-dystroglycan functional glycosylation. eLife 2014, 3, e03941.
- Inamori, K.; Yoshida-Moriguchi, T.; Hara, Y.; Anderson, M.E.; Yu, L.; Campbell, K.P. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science 2012, 335, 93–96.
- Goddeeris, M.M.; Wu, B.; Venzke, D.; Yoshida-Moriguchi, T.; Saito, F.; Matsumura, K.; Moore, S.A.; Campbell, K.P. LARGE glycans on dystroglycan function as a tunable matrix scaffold to prevent dystrophy. Nature 2013, 503, 136–140.
- Briggs, D.C.; Yoshida-Moriguchi, T.; Zheng, T.; Venzke, D.; Anderson, M.E.; Strazzulli, A.; Moracci, M.; Yu, L.; Hohenester, E.; Campbell, K.P. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat. Chem. Biol. 2016, 12, 810–814.
- Kuwabara, N.; Manya, H.; Yamada, T.; Tateno, H.; Kanagawa, M.; Kobayashi, K.; Akasaka-Manya, K.; Hirose, Y.; Mizuno, M.; Ikeguchi, M.; et al. Carbohydrate-binding domain of the POMGnT1 stem region modulates O-mannosylation sites of α-dystroglycan. Proc. Natl. Acad. Sci. USA 2016, 113, 9280–9285.
- Walimbe, A.S.; Okuma, H.; Joseph, S.; Yang, T.; Yonekawa, T.; Hord, J.M.; Venzke, D.; Anderson, M.E.; Torelli, S.; Manzur, A.; et al. POMK regulates dystroglycan function via LARGE1-mediated elongation of matriglycan. eLife 2020, 9, e61388.
- Kuwabara, N.; Imae, R.; Manya, H.; Tanaka, T.; Mizuno, M.; Tsumoto, H.; Kanagawa, M.; Kobayashi, K.; Toda, T.; Senda, T.; et al. Crystal structures of fukutin-related protein (FKRP), a ribitol-phosphate transferase related to muscular dystrophy. Nat. Commun. 2020, 11, 303.
- Beedle, A.M.; Turner, A.J.; Saito, Y.; Lueck, J.D.; Foltz, S.J.; Fortunato, M.J.; Nienaber, P.M.; Campbell, K.P. Mouse fukutin deletion impairs dystroglycan processing and recapitulates muscular dystrophy. J. Clin. Investig. 2012, 122, 3330–3342.
- Kanagawa, M.; Yu, C.C.; Ito, C.; Fukada, S.; Hozoji-Inada, M.; Chiyo, T.; Kuga, A.; Matsuo, M.; Sato, K.; Yamaguchi, M.; et al. Impaired viability of muscle precursor cells in muscular dystrophy with glycosylation defects and amelioration of its severe phenotype by limited gene expression. Hum. Mol. Genet. 2013, 22, 3003–3015.
- Ortiz-Cordero, C.; Bincoletto, C.; Dhoke, N.R.; Selvaraj, S.; Magli, A.; Zhou, H.; Kim, D.H.; Bang, A.G.; Perlingeiro, R.C.R. Defective autophagy and increased apoptosis contribute toward the pathogenesis of FKRP-associated muscular dystrophies. Stem Cell Rep. 2021, 16, 2752–2767.
- Moore, S.A.; Saito, F.; Chen, J.; Michele, D.E.; Henry, M.D.; Messing, A.; Cohn, R.D.; Ross-Barta, S.E.; Westra, S.; Williamson, R.A.; et al. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 2002, 418, 422–425.
- Satz, J.S.; Ostendorf, A.P.; Hou, S.; Turner, A.; Kusano, H.; Lee, J.C.; Turk, R.; Nguyen, H.; Ross-Barta, S.E.; Westra, S.; et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J. Neurosci. 2010, 30, 14560–14572.
- Myshrall, T.D.; Moore, S.A.; Ostendorf, A.P.; Satz, J.S.; Kowalczyk, T.; Nguyen, H.; Daza, R.A.; Lau, C.; Campbell, K.P.; Hevner, R.F. Dystroglycan on radial glia end feet is required for pial basement membrane integrity and columnar organization of the developing cerebral cortex. J. Neuropathol. Exp. Neurol. 2012, 71, 1047–1063.
- Nakano, I.; Funahashi, M.; Takada, K.; Toda, T. Are breaches in the glia limitans the primary cause of the micropolygyria in Fukuyama-type congenital muscular dystrophy (FCMD)? Pathological study of the cerebral cortex of an FCMD fetus. Acta Neuropathol. 1996, 91, 313–321.
- Nakanishi, T.; Sakauchi, M.; Kaneda, Y.; Tomimatsu, H.; Saito, K.; Nakazawa, M.; Osawa, M. Cardiac involvement in Fukuyama-type congenital muscular dystrophy. Pediatrics 2006, 117, e1187–e1192.
- Murakami, T.; Hayashi, Y.K.; Noguchi, S.; Ogawa, M.; Nonaka, I.; Tanabe, Y.; Ogino, M.; Takada, F.; Eriguchi, M.; Kotooka, N.; et al. Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann. Neurol. 2006, 60, 597–602.
- Kabaeva, Z.; Meekhof, K.E.; Michele, D.E. Sarcolemma instability during mechanical activity in Largemyd cardiac myocytes with loss of dystroglycan extracellular matrix receptor function. Hum. Mol. Genet. 2011, 20, 3346–3355.
- Ujihara, Y.; Kanagawa, M.; Mohri, S.; Takatsu, S.; Kobayashi, K.; Toda, T.; Naruse, K.; Katanosaka, Y. Elimination of fukutin reveals cellular and molecular pathomechanisms in muscular dystrophy-associated heart failure. Nat. Commun. 2019, 10, 5754.
- Blaeser, A.; Awano, H.; Wu, B.; Lu, Q.L. Progressive dystrophic pathology in diaphragm and impairment of cardiac function in FKRP P448L mutant mice. PLoS ONE 2016, 11, e0164187.
- Qiao, C.; Wang, C.H.; Zhao, C.; Lu, P.; Awano, H.; Xiao, B.; Li, J.; Yuan, Z.; Dai, Y.; Martin, C.B.; et al. Muscle and heart function restoration in a limb girdle muscular dystrophy 2I (LGMD2I) mouse model by systemic FKRP gene delivery. Mol. Ther. 2014, 22, 1890–1899.
- Gicquel, E.; Maizonnier, N.; Foltz, S.J.; Martin, W.J.; Bourg, N.; Svinartchouk, F.; Charton, K.; Beedle, A.M.; Richard, I. AAV-mediated transfer of FKRP shows therapeutic efficacy in a murine model but requires control of gene expression. Hum. Mol. Genet. 2017, 26, 1952–1965.
- Barresi, R.; Michele, D.E.; Kanagawa, M.; Harper, H.A.; Dovico, S.A.; Satz, J.S.; Moore, S.A.; Zhang, W.; Schachter, H.; Dumanski, J.P.; et al. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat. Med. 2004, 10, 696–703.
- Dhoke, N.R.; Kim, H.; Selvaraj, S.; Azzag, K.; Zhou, H.; Oliveira, N.A.J.; Tungtur, S.; Ortiz-Cordero, C.; Kiley, J.; Lu, Q.L.; et al. A universal gene correction approach for FKRP-associated dystroglycanopathies to enable autologous cell therapy. Cell Rep. 2021, 36, 109360.
- Foltz, S.J.; Luan, J.; Call, J.A.; Patel, A.; Peissig, K.B.; Fortunato, M.J.; Beedle, A.M. Four-week rapamycin treatment improves muscular dystrophy in a fukutin-deficient mouse model of dystroglycanopathy. Skelet. Muscle 2016, 6, 20.
- Wu, B.; Shah, S.N.; Lu, P.; Bollinger, L.E.; Blaeser, A.; Sparks, S.; Harper, A.D.; Lu, Q.L. Long-term treatment of tamoxifen and raloxifene alleviates dystrophic phenotype and enhances muscle functions of FKRP dystroglycanopathy. Am. J. Pathol. 2018, 188, 1069–1080.
- Serafini, P.R.; Feyder, M.J.; Hightower, R.M.; Garcia-Perez, D.; Vieira, N.M.; Lek, A.; Gibbs, D.E.; Moukha-Chafiq, O.; Augelli-Szafran, C.E.; Kawahara, G.; et al. A limb-girdle muscular dystrophy 2I model of muscular dystrophy identifies corrective drug compounds for dystroglycanopathies. JCI Insight 2018, 3, e120493.
- Wu, B.; Shah, S.N.; Lu, P.; Richardson, S.M.; Bollinger, L.E.; Blaeser, A.; Madden, K.L.; Sun, Y.; Luckie, T.M.; Cox, M.D.; et al. Glucocorticoid steroid and alendronate treatment alleviates dystrophic phenotype with enhanced functional glycosylation of alpha-dystroglycan in mouse model of limb-girdle muscular dystrophy with FKRPP448L mutation. Am. J. Pathol. 2016, 186, 1635–1648.
- Kobayashi, K.; Nakahori, Y.; Miyake, M.; Matsumura, K.; Kondo-Iida, E.; Nomura, Y.; Segawa, M.; Yoshioka, M.; Saito, K.; Osawa, M.; et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature 1998, 394, 388–392.
- Taniguchi-Ikeda, M.; Kobayashi, K.; Kanagawa, M.; Yu, C.C.; Mori, K.; Oda, T.; Kuga, A.; Kurahashi, H.; Akman, H.O.; DiMauro, S.; et al. Pathogenic exon-trapping by SVA retrotransposon and rescue in Fukuyama muscular dystrophy. Nature 2011, 478, 127–131.
- Van Tol, W.; van Scherpenzeel, M.; Alsady, M.; Riemersma, M.; Hermans, E.; Kragt, E.; Tasca, G.; Kamsteeg, E.J.; Pennings, M.; van Beusekom, E.; et al. Cytidine diphosphate-ribitol analysis for diagnostics and treatment monitoring of cytidine diphosphate-l-ribitol pyrophosphorylase A muscular dystrophy. Clin. Chem. 2019, 65, 1295–1306.
More
Information
Subjects:
Biochemistry & Molecular Biology; Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
12 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




