Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Faiza Amin | + 1784 word(s) | 1784 | 2021-10-25 11:14:07 | | | |
| 2 | Rita Xu | Meta information modification | 1784 | 2021-11-30 05:27:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Amin, F. Glass Ionomer Cement. Encyclopedia. Available online: https://encyclopedia.pub/entry/16537 (accessed on 07 February 2026).
Amin F. Glass Ionomer Cement. Encyclopedia. Available at: https://encyclopedia.pub/entry/16537. Accessed February 07, 2026.
Amin, Faiza. "Glass Ionomer Cement" Encyclopedia, https://encyclopedia.pub/entry/16537 (accessed February 07, 2026).
Amin, F. (2021, November 30). Glass Ionomer Cement. In Encyclopedia. https://encyclopedia.pub/entry/16537
Amin, Faiza. "Glass Ionomer Cement." Encyclopedia. Web. 30 November, 2021.
Copy Citation
The glass ionomer cement (GIC) is a translucent, water-based cement invented in 1972 by Wilson and Kent.
nanostructures
nanotechnology
glass ionomer cement
1. Introduction
The glass ionomer cement (GIC) is a translucent, water-based cement invented in 1972 by Wilson and Kent. The terminology is based on fluorosilicate glass and polyacrylic acid (PAA) as its original components [1]. The reaction occurs by acid-base interaction between ion leachable fluorosilicate glass powder and aqueous solution of PAA [2]. The bioactivity of GIC is possible due to their capability to supply the therapeutic ions when PAA reacts with ion leachable glasses [3][4][5]. Hydrophilic characteristics of PAA in these cements increase the bioactivity compared to resin-based materials [6]. After mixing the base (powder) and acid (liquid), the cement hardens in 2–10 min [7]. GlCs bond chemically via a carboxyl group from cement itself to the dentin or enamel of the tooth structure through calcium. In addition, these cements release fluoride ions that induce anticariogenic property for a fairly long period [8][9]. Many mechanisms are involved in the anticariogenic effects of fluoride, including the inhibition of bacterial growth and metabolism in the oral cavity, decreased demineralization, increased remineralization of the dental hard tissues and inhibition of pellicle and plaque formation on the tooth surface. It is assumed that caries formation is restricted through all these mechanisms after the release of fluoride from GICs [10]. Moreover, their stability in an aqueous environment, similar coefficient of thermal expansion to the tooth structure, biocompatibility, good marginal seal properties and high retention have led to their extensive use as cavity bases and liners, direct filling materials and luting materials [11][12].
Despite many advantages, the GICs are not widely used as permanent restorative materials for stress bearing areas because of their poor mechanical properties. During high masticatory stresses, the material is likely to fail due to its poor fracture toughness, tensile strength, wear resistance and hardness [9]. To enhance the physical and mechanical properties of GICs without compromising the biological or handling properties, many modifications have endeavored to the inorganic component of GICs. In this regard, fibers, metals, and other nonreactive fillers have been assessed. The most challenging task in this context is to achieve adhesion between the cement matrix and reinforcing agents. Moreover, to strengthen the GICs, various chemical modifications have been explored [13]. One method is that high powder to liquid ratio was achieved by incorporating glass particles with controlled particle sizes. This approach resulted in high-viscosity GICs. The properties of these high viscosity materials were superior compared to the conventional GICs [14]. Other approaches have involved the addition of aluminosilicate fibres, amalgam alloys, carbon, hydroxyapatite (HA) and stainless-steel particles [15][16][17]. GICs modified by incorporating nanostructures exhibited fewer air voids and internal microcracks. In addition, apparently modified materials are easier to handle than unmodified cements, which resulted in greater strengths in compression [18]. To modify the chemical, biological, and physical properties of dental restorative materials, manufacturers incorporated a myriad of nanoparticles (NPs), making them innovative [19]. Over the past decade, the inclination of researchers towards the field of nanotechnology has been reflected by increased number of patent applications. Nanostructures incorporated in dental biomaterials enhanced their properties [20]. A brief review of nanostructures added in various dental materials and their characteristics and applications is presented in Table 1.
Table 1. Brief Overview of Nanostructures and their Dental Applications.
| Nanostructures | Characteristics and Applications | References |
|---|---|---|
| Nanorods |
|
[21][22] |
| Nanospheres |
|
[21][22][23][24] |
| Nanotubes |
|
[25][26][27] |
| Nanofibers |
|
[28][29][30][31] |
| Dendrimers or dendritic copolymers |
|
[32][33][34] |
| Nanopores |
|
[35][36] |
| Quantum dots (QD) |
|
[37] |
| Nanoshells |
|
[38] |
| Liposomes |
|
[39][40] |
| Fullerenes |
|
[41][42] |
| Nanowires |
|
[23][43] |
| Nanobelts |
|
[44][45] |
| Nanocapsules |
|
[46][47][48] |
Due to the distinctive properties, GICs have been widely used in dentistry for more than four decades. Common dental applications of GICs include permanent restorative materials in pediatric dentistry, liners, bases and fissure sealants. One of the most exclusive applications of GICs in orthodontics includes bonding of orthodontic brackets and bands with the tooth structure [1]. In addition, GICs exhibited good clinical outcomes and high durability when used for Atraumatic Restorative Treatment (ART) [49][50]. However, their mechanical characteristics are not adequate to sustain the masticatory forces as described above; hence, to improve their strength, several modifications have been investigated. For example, amalgam alloys, aluminosilicate fibres, carbon, fillers, HA powder and stainless steel have been added to strengthen the GICs [51]. Moreover, in dentistry, the addition of nanostructures has become an important field of research. Various types of nanomaterials including ceramic, metal or polymers, are added in GICs to improve the mechanical properties [17][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74]. The properties of GICs have been successfully enhanced by incorporating metal alloys at the nanoscale level (i.e., silver–tin or silver–palladium/titanium) into glass powder [75][76]. Similarly, nanoionomers have been amalgamated with GICs to improve the surface properties. Oxman and colleagues [77] compared two fluoroalumiosilicate resin modified glass ionomer cements (RMGIs) and a nanohybrid composite with nanoionomeric hybrid resin-modified glass ionomer (NHRMGI) and reported a significantly higher gloss with the material reinforced with the nanostructures. Wear rates for nanohybrid were significantly higher than other RMGIs [77]. Inorganic silica nanofillers (~40 nm size) into the liquid of GIC is another recent development that increases the strength of the polymer matrix. This reinforcement decreased the initial setting time, improved the wear resistance, better resistance to dissolution and disintegration. The reinforced ionomers retained a polished surface for a longer period of time compared to the conventional GICs. In addition, these newer cements were also far better in terms of optical properties and translucency [78]. Friedl et al. [79] incorporated nanofillers into the GICs and concluded that GICs with nanofillers can be used for posterior fillings due to improved mechanical properties [79]. Nano-hydroxyapatite (Nano-HA) and nanoflouroapatite (Nano-FA) were added to conventional GICs [80] that improved mechanical properties such as biaxial flexural strength, compressive strength, and diametral tensile strength compared to the conventional GICs [81].
2. Structure and Composition of Conventional GICs
The powder component of GICs (fluoroaluminosilicate) has the capability to leach ions upon reaction with PAA. Alumina (Al2O3), aluminium phosphate (AlPO4), calcium fluoride (CaF2), cryolite (Na3AlF6), sodium fluoride (NaF) and silica (SiO2) are the basic constituents of the powder [82]. Currently, the liquids are homo and copolymers of itaconic, maleic, or tricarboxylic acids. Formerly, PAA (~40% to 50%) were used but had a short shelf life because of high viscosity and gelation tendency. The gelation occurs due to excessive intermolecular hydrogen bonding. The liquid also contains tartaric acid that controls the setting characteristics [83]. GICs comprise an aluminosilicate network which is the three-dimensional structure. In the glass matrix, aluminum ions (Al) play a dual role of network forming and network dwelling ions, whereas silicone (Si) ions exist in interstices formed by four oxygen anions. Reaction initiates when loosely bound negative charges from the glass are attacked by carboxylic acid. Carboxylic acid attack at the Al ions network sites results in the disturbance in the three-dimensional matrix. After the attack, ionic bonds with polymers are formed when Al ions and other ions are released. The rate of setting reaction in GICs is controlled by Al/Si ratio [84][85] (Figure 1). This process involves formation of ionic bonds between calcium ions on the tooth surface and carboxylate groups on the polyacid molecules [1]. The ratio of counter ions should be close to the Al/Si ratio so that proper glass network is formed prior to mixing of acidic polymer into the glass. Reaction is initiated when loosely bound negative charges from the glass attack by carboxylic acid. Carboxylic acid attack at the Al ions network sites resulting in the disturbance in the three-dimensional matrix. After the attack, ionic bonds with polymers are formed when Al ions and other ions are released. Al/Si ratio controlled the rate of setting reaction in GICs. The ratio of Al2O3 to SiO2 (1:2 or more by mass) is critical for the cement’s accurate reactivity and hydrolytic stability. Over time, an ion-exchange layer is created through a diffusion process in which ions from the tooth and GIC move into the interfacial zone, establishing a strong chemical bond between tooth and the cement. In Figure 2, scanning electron microscopy exhibited the interfacial layer formed between glass-ionomer cement, Fuji IX (GC, Tokyo, Japan), and the tooth [1]. Regarding the setting characteristics and properties of GICs, the cements set within 2–3 min by an acid base reaction. Ion concentrations other than Al and Si also play a major role in the setting of GICs. Numerous spectroscopic techniques, such as 13C NMR, infrared and Fourier transform infrared (FTIR), are used to investigate the setting characteristics. These techniques concluded that the setting of GICs take place through the diffusion-controlled process in two steps. The first step is the formation of ionic cross links, which is responsible for the initial hardening of the cement. The second step is the maturation step, which is a continuous and ongoing process for a day [1]. To enhance the aesthetics property and the transparency, the Al2O3/SiO2 ratio has been changed along with reducing the amount of CaF2. To decrease the melting point, CaF2 was added as a flux. The radiopacity is achieved by the incorporation of barium (Ba), strontium (Sr), lanthanum (La), or zinc (Zn) [1][24].
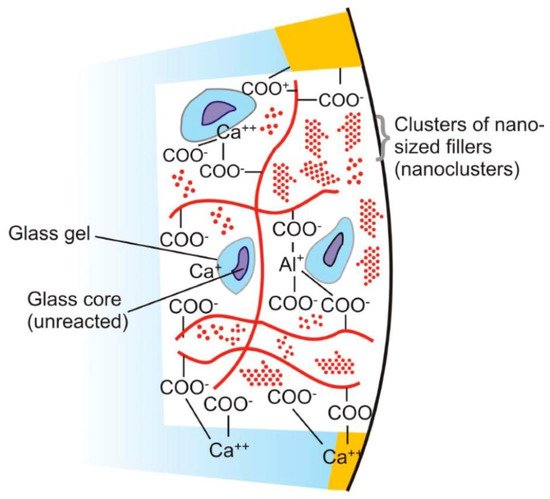
Figure 1. Diagram represents the structure of glass ionomer cement and its chemical bonding with tooth structure. Adopted from reference [24].

Figure 2. SEM Analysis representing Interfacial ion-exchange layer formed between tooth surface (above) and glass-ionomer cement (below). The circle indicates part of the ion-exchange layer. Reprinted from ref. [1].
References
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16.
- Wilson, A.D.; Kent, B. The glass-ionomer cement, a new translucent dental filling material. J. Chem. Technol. Biotechnol. 1971, 21, 313.
- Frencken, J.E.; Peters, M.C.; Manton, D.J.; Leal, S.C.; Gordan, V.V.; Eden, E. Minimal intervention dentistry for managing dental caries—A review: Report of a FDI task group. Int. Dent. J. 2012, 62, 223–243.
- Dos Santos, V.E.; Filho, A.V.; Targino, A.G.; Flores, M.A.; Galembeck, A.; Caldas, A.F.; Rosenblatt, A. A new “Silver-Bullet” to treat caries in children–Nano Silver Fluoride: A randomised clinical trial. J. Dent. 2014, 42, 945–951.
- Berg, M.C.J.J.; Momsen, N.C.R.; Benetti, A.R.; Telling, M.T.F.; Seydel, T.; Bordallo, H.N. Water dynamics in glass ionomer cements. Eur. Phys. J. Spec. Top. 2016, 225, 773–777.
- Zohuriaan, M.J.; Mehr, K.K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 447–451.
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972, 132, 133–135.
- Nicholson, J.W. Adhesion of glass-ionomer cements to teeth: A review. Int. J. Adhes. Adhes. 2016, 69, 33–38.
- Chau, N.P.T.; Pandit, S.; Cai, J.-N.; Lee, M.-H.; Jeon, J.-G. Relationship between fluoride release rate and anti-cariogenic biofilm activity of glass ionomer cements. Dent. Mater. 2015, 31, e100–e108.
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials—Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362.
- Alobiedy, A.N.; Alhille, A.H.; Al-Hamaoy, A.R. Mechanical Properties Enhancement of Conventional Glass Ionomer Cement by Adding Zirconium Oxide Micro and Nanoparticles. J. Eng. 2019, 25, 72–81.
- Frencken, J.E. Atraumatic restorative treatment and minimal intervention dentistry. Br. Dent. J. 2017, 223, 183–189.
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.; Schricker, S.R. A review of polyelectrolyte modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2012, 22, 2824–2833.
- Guggenberger, R.; May, R.; Stefan, K. New trends in glass-ionomer chemistry. Biomaterials 1998, 19, 479–483.
- Xu, H.; Eichmiller, F.; Antonucci, J.; Schumacher, G.; Ives, L. Dental resin composites containing ceramic whiskers and precured glass ionomer particles. Dent. Mater. 2000, 16, 356–363.
- Kawano, F.; Kon, M.; Kobayashi, M.; Miyai, K. Reinforcement effect of short glass fibers with CaO–P2O5–SiO2–Al2O3 glass on strength of glass-ionomer cement. J. Dent. 2001, 29, 377–380.
- Kerby, R.E.; Bleiholder, R.F. Physical properties of stainless-steel and silver-reinforced glass-ionomer cements. J. Dent. Res. 1991, 70, 1358–1361.
- Gjorgievska, E.; Tendeloo, V.; Nicholson, G.; Coleman, J.W.; Slipper, N.J.; Booth, I.J.S. The Incorporation of Nanoparticles into Conventional Glass-Ionomer Dental Restorative Cements. Microsc. Microanal. 2015, 21, 392–406.
- Soares, L.E.S.; Nahórny, S.; Braga, V.D.F.; Marciano, F.R.; Bhattacharjee, T.T.; Lobo, A.O. Raman spectroscopy-multivariate analysis related to morphological surface features on nanomaterials applied for dentin coverage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 228, 117818.
- Foong, L.K.; Foroughi, M.M.; Mirhosseini, A.F.; Safaei, M.; Jahani, S.; Mostafavi, M.; Ebrahimpoor, N.; Sharifi, M.; Varma, R.S.; Khatami, M. Applications of nano-materials in diverse dentistry regimes. RSC Adv. 2020, 10, 15430–15460.
- Chen, H.; Clarkson, B.H.; Sun, K.; Mansfield, J.F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid Interface Sci. 2005, 288, 97–103.
- Sadat-Shojai, M.; Atai, M.; Nodehi, A.; Khanlar, L.N. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010, 26, 471–482.
- Fan, Y.; Sun, Z.; Wang, R.; Abbott, C.; Moradian-Oldak, J. Enamel inspired nanocomposite fabrication through amelogenin supramolecular assembly. Biomaterials 2007, 28, 3034–3042.
- Kim, T.-H.; Eltohamy, M.; Kim, M.; Perez, R.; Kim, J.-H.; Yun, Y.-R.; Jang, J.-H.; Lee, E.-J.; Knowles, J.C.; Kim, H.-W. Therapeutic foam scaffolds incorporating biopolymer-shelled mesoporous nanospheres with growth factors. Acta Biomater. 2014, 10, 2612–2621.
- Oh, S.-H.; Finõnes, R.R.; Daraio, C.; Chen, L.-H.; Jin, S. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938–4943.
- Suo, L.; Li, Z.; Luo, F.; Chen, J.; Jia, L.; Wang, T.; Pei, X.; Wan, Q. Effect of dentin surface modification using carbon nanotubes on dental bonding and antibacterial ability. Dent. Mater. J. 2018, 37, 229–236.
- Hahn, B.-D.; Lee, J.-M.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Lee, B.-K.; Shin, D.-S.; Kim, H.-E. Mechanical and in vitro biological performances of hydroxyapatite–carbon nanotube composite coatings deposited on Ti by aerosol deposition. Acta Biomater. 2009, 5, 3205–3214.
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946.
- Kim, H.W.; Kim, H.E. Nanofiber generation of hydroxyapatite and fluor-hydroxyapatite bioceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 323–328.
- Tian, M.; Gao, Y.; Liu, Y.; Liao, Y.; Hedin, N.E.; Fong, H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing nano fibrillar silicate. Dent. Mater. 2008, 24, 235–243.
- Tian, M.; Gao, Y.; Liu, Y.; Liao, Y.; Xu, R.; Hedin, N.E.; Fong, H. Bis-GMA/TEGDMA dental composites reinforced with electrospun nylon 6 nanocomposite nanofibers containing highly aligned fibrillar silicate single crystals. Polymers 2007, 48, 2720–2728.
- Viljanen, E.; Langer, S.; Skrifvars, M.O.V.; Vallittu, P. Analysis of residual monomers in dendritic methacrylate copolymers and composites by HPLC and headspace-GC/MS. Dent. Mater. 2006, 22, 845–851.
- Viljanen, E.K.; Skrifvars, M.; Vallittu, P.K. Dendritic copolymers and particulate filler composites for dental applications: Degree of conversion and thermal properties. Dent. Mater. 2007, 23, 1420–1427.
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959.
- Luo, J.; Seghi, R.; Lannutti, J. Effect of silane coupling agents on the wear resistance of polymer-nanoporous silica gel dental composites. Mater. Sci. Eng. C 1997, 5, 15–22.
- Thorat, S.B.; Diaspro, A.; Salerno, M. In vitro investigation of coupling-agent-free dental restorative composite based on nano-porous alumina fillers. J. Dent. 2014, 42, 279–286.
- Alves, L.P.; Pilla, V.; Murgo, D.O.; Munin, E. Core–shell quantum dots tailor the fluorescence of dental resin composites. J. Dent. 2010, 38, 149–152.
- Wong, D.T. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J. Am. Dent. Assoc. 2006, 137, 313–321.
- Yamakami, K.; Tsumori, H.; Sakurai, Y.; Shimizu, Y.; Nagatoshi, K.; Sonomoto, K. Sustainable inhibition efficacy of liposome-encapsulated nisin on insoluble glucan-biofilm synthesis by Streptococcus mutans. Pharm. Biol. 2012, 51, 267–270.
- Yamakami, K.; Tsumori, H.; Shimizu, Y.; Sakurai, Y.; Nagatoshi, K.; Sonomoto, K. Effect of liposomal phosphatidylcholine acyl chain length on the bactericidal activity of liposome-encapsulated nisin on cariogenic Streptococcus mutans. J. Dent. Health Oral Disord. Ther. 2014, 1, 72–75.
- Mizuno, K.; Zhiyentayev, T.; Huangv, L.; Khalil, S.; Nasim, F. Antimicrobial Photodynamic Therapy with Functionalized Fullerenes: Quantitative Structure-activity Relationships. J. Nanomed. Nanotechnol. 2011, 2, 1–9.
- Pacor, S.; Grillo, A.; Đorđević, L.; Zorzet, S.; Lucafò, M.; Da Ros, T.; Prato, M.; Sava, G. Effects of Two Fullerene Derivatives on Monocytes and Macrophages. BioMed Res. Int. 2015, 2015, 1–13.
- Chen, H.; Sun, K.; Tang, Z.; Law, R.; Mansfield, J.F.; Czajka-Jakubowska, A.; Clarkson, B.H. Synthesis of Fluorapatite Nanorods and Nanowires by Direct Precipitation from Solution. Cryst. Growth Des. 2006, 6, 1504–1508.
- Bhuvaneswarri, J.; Alam, M.; Chandrasekaran, S.; Sathya, M. Future impact of nanotechnology in dentistry—A review. Int. J. Nanotechnol. App. 2013, 3, 15–20.
- Wang, X.; Sun, Y.; Lin, K. Facile synthesis of dental enamel-like hydroxyapatite nanorod arrays via hydrothermal transformation of hillebrandite nanobelts. J. Mater. Chem. B 2015, 3, 7334–7339.
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410.
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647.
- Ouyang, X.; Huang, X.; Pan, Q.; Zuo, C.; Huang, C.; Yang, X.; Zhao, Y. Synthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resin. J. Dent. 2011, 39, 825–833.
- Frencken, J.E. The ART approach using glass-ionomers in relation to global oral health care. Dent. Mater. 2010, 26, 1–6.
- Frencken, J.E.; Leal, S.; Navarro, M.F.L. Twenty-five-year atraumatic restorative treatment (ART) approach: A comprehensive overview. Clin. Oral Investig. 2012, 16, 1337–1346.
- Ching, H.S.; Luddin, N.; Kannan, T.P.; Ab Rahman, I.; Abdul Ghani, N.R. Modification of glass ionomer cements on their physical-mechanical and antimicrobial properties. J. Esthet. Dent. 2018, 30, 557–571.
- AB Rahman, I.; Masudi, S.M.; Luddin, N.; Shiekh, R.A. One-pot synthesis of hydroxyapatite–silica nanopowder composite for hardness enhancement of glass ionomer cement (GIC). Bull. Mater. Sci. 2014, 37, 213–219.
- Nuri Sari, M.; Seyed Tabaii, E.; Salehi Vaziri, A.; Ghaffari, H.; Araghbidi Kashani, M.; Eslami Amirabadi, G. Effect of nano-hydroxyapatite incorporation into resin modified glass ionomer cement on ceramic bracket debonding. J. Islam. Dent. Assoc. 2014, 26, 208–213.
- Pagano, S.; Chieruzzi, M.; Balloni, S.; Lombardo, G.; Torre, L.; Bodo, M.; Cianetti, S.; Marinucci, L. Biological, thermal and mechanical characterization of modified glass ionomer cements: The role of nanohydroxyapatite, ciprofloxacin and zinc l-carnosine. Mater. Sci. Eng. C 2019, 94, 76–85.
- Moheet, I.; Luddin, N.; Ab Rahman, I.; Masudi, S.M.; Kannan, T.P.; Ghani, N.R.N.A. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram. Int. 2018, 44, 9899–9906.
- Lee, J.J.; Lee, Y.K.; Choi, B.J.; Lee, J.H.; Choi, H.J.; Son, H.K.; Hwang, J.W.; Kim, S.O. Physical properties of resin-reinforced glass ionomer cement modified with micro and nano-hydroxyapatite. J. Nanosci. Nanotechnol. 2010, 10, 5270–5276.
- Ibrahim, M.A.; Priyadarshini, B.M.; Neo, J.; Fawzy, A.S. Characterization of chitosan/TiO2 nano-powder modified glass-ionomer cement for restorative dental applications. JERD 2017, 2, 146–156.
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R. A review of powder modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2011, 21, 1319–1328.
- Abbas, H.M.A.A. Effect of adding aluminium microparticles to conventional glass ionomer cement. Int. J. Mech Eng. Technol. 2019, 10, 1437–1451.
- De Caluwé, T.; Vercruysse, C.W.; Fraeyman, S.; Verbeeck, R.M. The influence of particle size and fluorine content of aluminosilicate glass on the glass ionomer cement properties. Dent. Mater. J. 2014, 30, 1029–1038.
- Souza, J.C.M.; Silva, J.B.; Aladim, A.; Carvalho, O.; Nascimento, R.M.D.; Silva, F.; Martinelli, A.E.; Henriques, B. Effect of Zirconia and Alumina Fillers on the Microstructure and Mechanical Strength of Dental Glass Ionomer Cements. Open Dent. J. 2016, 10, 58–68.
- Gu, Y.; Yap, A.; Cheang, P.; Khor, K. Zirconia–glass ionomer cement—A potential substitute for Miracle Mix. Scr. Mater. 2005, 52, 113–116.
- Arita, K.; Lucas, M.E.; Nishino, M. The effect of adding hydroxyapatite on the flexural strength of glass ionomer cement. J. Dent. Mater. 2003, 22, 126–136.
- Shiekh, R.; Ab Rahman, I.; Masudi, S.M.; Luddin, N. Modification of glass ionomer cement by incorporating hydroxyapatite-silica nano-powder composite: Sol–gel synthesis and characterization. Ceram. Int. 2014, 40, 3165–3170.
- Arita, K.; Yamamoto, A.; Shinonaga, Y.; Harada, K.; Abe, Y.; Nakagawa, K.; Sugiyama, S. Hydroxyapatite particle characteristics influence the enhancement of the mechanical and chemical properties of conventional restorative glassionomer cement. Dent. Mater. J. 2011, 30, 672–683.
- Rajabzadeh, G.; Salehi, S.; Nemati, A.; Tavakoli, R.; Solati-Hashjin, M. Enhancing glass ionomer cement features by using the HA/YSZ nanocomposite: A feed forward neural network modelling. J. Mech. Behav. Biomed. Mater. 2014, 29, 317–327.
- Moshaverinia, A.; Roohpour, N.; Ansari, S.; Moshaverinia, M.; Schricker, S.; Darr, J.A.; Rehman, I.U. Effects of N-vinylpyrrolidone (NVP) containing polyelectrolytes on surface properties of conventional glass-ionomer cements (GIC). Dent. Mater. 2009, 25, 1240–1247.
- Deb, S.; Nicholson, J.W. The effect of strontium oxide in glass–ionomer cements. J. Mater. Sci. Mater. Electron. 1999, 10, 471–474.
- Choudhary, K.; Nandlal, B. Comparative evaluation of shear bond strength of nano-hydroxyapatite incorporated glass ionomer cement and conventional glass ionomer cement on dense synthetic hydroxyapatite disk: An in vitro study. Indian J. Dent. Res. 2015, 26, 170–175.
- Moshaverinia, M.; Borzabadi-Farahani, A.; Sameni, A.; Moshaverinia, A.; Ansari, S. Effects of incorporation of nano-fluorapatite particles on microhardness, fluoride releasing properties, and biocompatibility of a conventional glass ionomer cement (GIC). Dent. Mater. J. 2016, 35, 817–821.
- Zoergiebel, J.; Ilie, N. Evaluation of a conventional glass ionomer cement with new zinc formulation: Effect of coating, aging and storage agents. Clin. Oral Investig. 2012, 17, 619–626.
- Cibim, D.D.; Saito, M.; Giovani, P.A.; Borges, A.F.S.; Pecorari, V.; Gomes, O.; Lisboa-Filho, P.N.; Nociti, F.; Puppin-Rontani, R.M.; Kantovitz, K.R. Novel Nanotechnology of TiO2 Improves Physical-Chemical and Biological Properties of Glass Ionomer Cement. Int. J. Biomater. 2017, 2017, 1–11.
- Felemban, N.H.; Ebrahim, M.I. Effects of adding silica particles on certain properties of resin-modified glass-ionomer cement. Eur. J. Dent. 2016, 10, 225–229.
- Lohbauer, U.; Walker, J.; Nikolaenko, S.; Werner, J.; Clare, A.; Petschelt, A.; Greil, P. Reactive fibre reinforced glass ionomer cements. Biomaterials 2003, 24, 2901–2907.
- Shahid, S.; Hassan, U.; Billington, R.; Hill, R.; Anderson, P. Glass ionomer cements: Effect of strontium substitution on esthetics, radiopacity and fluoride release. Dent. Mater. 2014, 30, 308–313.
- Baig, M.S.; Fleming, G.J. Conventional glass-ionomer materials: A review of the developments in glass powder, polyacid liquid and the strategies of reinforcement. J. Dent. 2015, 43, 897–912.
- Oxman, J.; Falsafi, A.; Mitra, S.; Ton, T.; Madsen, V.; Bui, H. Improved Polish, Wear-Resistance and Esthetics of a Nanoiomer Restorative Material; Pan European Federation of the IADR: London, UK, 2008.
- Basso, M. Teeth restoration using a high-viscosity glass ionomer cement: The Equia® system. J. Minim. Interv. Dent. 2011, 4, 74–76.
- Friedl, K.; Hiller, K.-A.; Friedl, K.-H. Clinical performance of a new glass ionomer based restoration system: A retrospective cohort study. Dent. Mater. 2011, 27, 1031–1037.
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440.
- McCabe, J.F.; Yan, Z.; Al Naimi, O.T.; Mahmoud, G.; Rolland, S.L. Smart materials in dentistry—Future prospects. Dent. Mater. J. 2009, 28, 37–43.
- Meclean, J.W.; Nicholson, J.W.; Wilson, A.D. Proposed nomenclature for glass-ionomer dental cements and reiated materiais. Quintessence Int. 1994, 25, 587–589.
- Permana, A.J.; Setyawati, H.; Hamami; Murwani, I.K. The influence of dicarboxylic acids: Oxalic acid and tartaric acid on the compressive strength of glass ionomer cements. In Proceedings of the 5th International Conference and Workshop on Basic and Applied Sciences (ICOWOBAS 2015), Surabaya, Indonesia, 16–17 October 2015; Volume 1718, p. 50003.
- McLean, J.W. The clinical use of glass-ionomer cements—Future and current developments. Clin. Mater. 1991, 7, 283–288.
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134.
More
Information
Subjects:
Agriculture, Dairy & Animal Science; Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
2 times
(View History)
Update Date:
30 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




