Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
|
Allergy
The glass ionomer cement (GIC) is a translucent, water-based cement invented in 1972 by Wilson and Kent.
- nanostructures
- nanotechnology
- glass ionomer cement
1. Introduction
The glass ionomer cement (GIC) is a translucent, water-based cement invented in 1972 by Wilson and Kent. The terminology is based on fluorosilicate glass and polyacrylic acid (PAA) as its original components [1]. The reaction occurs by acid-base interaction between ion leachable fluorosilicate glass powder and aqueous solution of PAA [2]. The bioactivity of GIC is possible due to their capability to supply the therapeutic ions when PAA reacts with ion leachable glasses [3,4,5]. Hydrophilic characteristics of PAA in these cements increase the bioactivity compared to resin-based materials [6]. After mixing the base (powder) and acid (liquid), the cement hardens in 2–10 min [7]. GlCs bond chemically via a carboxyl group from cement itself to the dentin or enamel of the tooth structure through calcium. In addition, these cements release fluoride ions that induce anticariogenic property for a fairly long period [8,9]. Many mechanisms are involved in the anticariogenic effects of fluoride, including the inhibition of bacterial growth and metabolism in the oral cavity, decreased demineralization, increased remineralization of the dental hard tissues and inhibition of pellicle and plaque formation on the tooth surface. It is assumed that caries formation is restricted through all these mechanisms after the release of fluoride from GICs [10]. Moreover, their stability in an aqueous environment, similar coefficient of thermal expansion to the tooth structure, biocompatibility, good marginal seal properties and high retention have led to their extensive use as cavity bases and liners, direct filling materials and luting materials [11,12].
Despite many advantages, the GICs are not widely used as permanent restorative materials for stress bearing areas because of their poor mechanical properties. During high masticatory stresses, the material is likely to fail due to its poor fracture toughness, tensile strength, wear resistance and hardness [9]. To enhance the physical and mechanical properties of GICs without compromising the biological or handling properties, many modifications have endeavored to the inorganic component of GICs. In this regard, fibers, metals, and other nonreactive fillers have been assessed. The most challenging task in this context is to achieve adhesion between the cement matrix and reinforcing agents. Moreover, to strengthen the GICs, various chemical modifications have been explored [13]. One method is that high powder to liquid ratio was achieved by incorporating glass particles with controlled particle sizes. This approach resulted in high-viscosity GICs. The properties of these high viscosity materials were superior compared to the conventional GICs [14]. Other approaches have involved the addition of aluminosilicate fibres, amalgam alloys, carbon, hydroxyapatite (HA) and stainless-steel particles [15,16,17]. GICs modified by incorporating nanostructures exhibited fewer air voids and internal microcracks. In addition, apparently modified materials are easier to handle than unmodified cements, which resulted in greater strengths in compression [18]. To modify the chemical, biological, and physical properties of dental restorative materials, manufacturers incorporated a myriad of nanoparticles (NPs), making them innovative [19]. Over the past decade, the inclination of researchers towards the field of nanotechnology has been reflected by increased number of patent applications. Nanostructures incorporated in dental biomaterials enhanced their properties [20]. A brief review of nanostructures added in various dental materials and their characteristics and applications is presented in Table 1.
Table 1. Brief Overview of Nanostructures and their Dental Applications.
| Nanostructures | Characteristics and Applications | References |
|---|---|---|
| Nanorods |
|
[21,22] |
| Nanospheres |
|
[21,22,23,24] |
| Nanotubes |
|
[25,26,27] |
| Nanofibers |
|
[28,29,30,31] |
| Dendrimers or dendritic copolymers |
|
[32,33,34] |
| Nanopores |
|
[35,36] |
| Quantum dots (QD) |
|
[37] |
| Nanoshells |
|
[38] |
| Liposomes |
|
[39,40] |
| Fullerenes |
|
[41,42] |
| Nanowires |
|
[23,43] |
| Nanobelts |
|
[44,45] |
| Nanocapsules |
|
[46,47,48] |
Due to the distinctive properties, GICs have been widely used in dentistry for more than four decades. Common dental applications of GICs include permanent restorative materials in pediatric dentistry, liners, bases and fissure sealants. One of the most exclusive applications of GICs in orthodontics includes bonding of orthodontic brackets and bands with the tooth structure [1]. In addition, GICs exhibited good clinical outcomes and high durability when used for Atraumatic Restorative Treatment (ART) [49,50]. However, their mechanical characteristics are not adequate to sustain the masticatory forces as described above; hence, to improve their strength, several modifications have been investigated. For example, amalgam alloys, aluminosilicate fibres, carbon, fillers, HA powder and stainless steel have been added to strengthen the GICs [51]. Moreover, in dentistry, the addition of nanostructures has become an important field of research. Various types of nanomaterials including ceramic, metal or polymers, are added in GICs to improve the mechanical properties [17,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74]. The properties of GICs have been successfully enhanced by incorporating metal alloys at the nanoscale level (i.e., silver–tin or silver–palladium/titanium) into glass powder [75,76]. Similarly, nanoionomers have been amalgamated with GICs to improve the surface properties. Oxman and colleagues [77] compared two fluoroalumiosilicate resin modified glass ionomer cements (RMGIs) and a nanohybrid composite with nanoionomeric hybrid resin-modified glass ionomer (NHRMGI) and reported a significantly higher gloss with the material reinforced with the nanostructures. Wear rates for nanohybrid were significantly higher than other RMGIs [77]. Inorganic silica nanofillers (~40 nm size) into the liquid of GIC is another recent development that increases the strength of the polymer matrix. This reinforcement decreased the initial setting time, improved the wear resistance, better resistance to dissolution and disintegration. The reinforced ionomers retained a polished surface for a longer period of time compared to the conventional GICs. In addition, these newer cements were also far better in terms of optical properties and translucency [78]. Friedl et al. [79] incorporated nanofillers into the GICs and concluded that GICs with nanofillers can be used for posterior fillings due to improved mechanical properties [79]. Nano-hydroxyapatite (Nano-HA) and nanoflouroapatite (Nano-FA) were added to conventional GICs [80] that improved mechanical properties such as biaxial flexural strength, compressive strength, and diametral tensile strength compared to the conventional GICs [81].
2. Structure and Composition of Conventional GICs
The powder component of GICs (fluoroaluminosilicate) has the capability to leach ions upon reaction with PAA. Alumina (Al2O3), aluminium phosphate (AlPO4), calcium fluoride (CaF2), cryolite (Na3AlF6), sodium fluoride (NaF) and silica (SiO2) are the basic constituents of the powder [82]. Currently, the liquids are homo and copolymers of itaconic, maleic, or tricarboxylic acids. Formerly, PAA (~40% to 50%) were used but had a short shelf life because of high viscosity and gelation tendency. The gelation occurs due to excessive intermolecular hydrogen bonding. The liquid also contains tartaric acid that controls the setting characteristics [83]. GICs comprise an aluminosilicate network which is the three-dimensional structure. In the glass matrix, aluminum ions (Al) play a dual role of network forming and network dwelling ions, whereas silicone (Si) ions exist in interstices formed by four oxygen anions. Reaction initiates when loosely bound negative charges from the glass are attacked by carboxylic acid. Carboxylic acid attack at the Al ions network sites results in the disturbance in the three-dimensional matrix. After the attack, ionic bonds with polymers are formed when Al ions and other ions are released. The rate of setting reaction in GICs is controlled by Al/Si ratio [84,85] (Figure 1). This process involves formation of ionic bonds between calcium ions on the tooth surface and carboxylate groups on the polyacid molecules [1]. The ratio of counter ions should be close to the Al/Si ratio so that proper glass network is formed prior to mixing of acidic polymer into the glass. Reaction is initiated when loosely bound negative charges from the glass attack by carboxylic acid. Carboxylic acid attack at the Al ions network sites resulting in the disturbance in the three-dimensional matrix. After the attack, ionic bonds with polymers are formed when Al ions and other ions are released. Al/Si ratio controlled the rate of setting reaction in GICs. The ratio of Al2O3 to SiO2 (1:2 or more by mass) is critical for the cement’s accurate reactivity and hydrolytic stability. Over time, an ion-exchange layer is created through a diffusion process in which ions from the tooth and GIC move into the interfacial zone, establishing a strong chemical bond between tooth and the cement. In Figure 2, scanning electron microscopy exhibited the interfacial layer formed between glass-ionomer cement, Fuji IX (GC, Tokyo, Japan), and the tooth [1]. Regarding the setting characteristics and properties of GICs, the cements set within 2–3 min by an acid base reaction. Ion concentrations other than Al and Si also play a major role in the setting of GICs. Numerous spectroscopic techniques, such as 13C NMR, infrared and Fourier transform infrared (FTIR), are used to investigate the setting characteristics. These techniques concluded that the setting of GICs take place through the diffusion-controlled process in two steps. The first step is the formation of ionic cross links, which is responsible for the initial hardening of the cement. The second step is the maturation step, which is a continuous and ongoing process for a day [1]. To enhance the aesthetics property and the transparency, the Al2O3/SiO2 ratio has been changed along with reducing the amount of CaF2. To decrease the melting point, CaF2 was added as a flux. The radiopacity is achieved by the incorporation of barium (Ba), strontium (Sr), lanthanum (La), or zinc (Zn) [1,24].
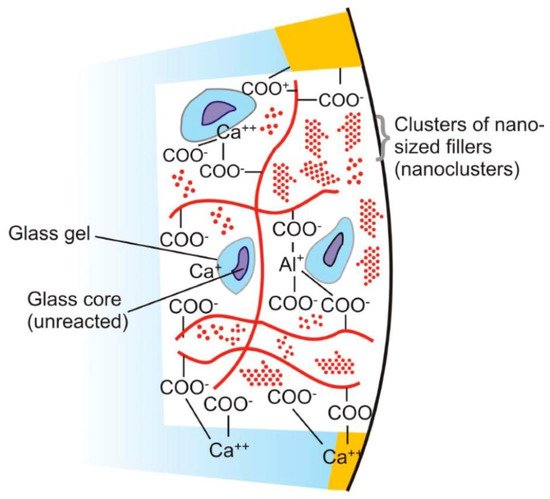
Figure 1. Diagram represents the structure of glass ionomer cement and its chemical bonding with tooth structure. Adopted from reference [24].

Figure 2. SEM Analysis representing Interfacial ion-exchange layer formed between tooth surface (above) and glass-ionomer cement (below). The circle indicates part of the ion-exchange layer. Reprinted from ref. [1].
This entry is adapted from the peer-reviewed paper 10.3390/ma14216260
This entry is offline, you can click here to edit this entry!
