Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos Barreiro | + 12840 word(s) | 12840 | 2021-11-19 03:00:05 | | | |
| 2 | Lindsay Dong | -4265 word(s) | 8575 | 2021-11-22 06:46:30 | | | | |
| 3 | Lindsay Dong | -4265 word(s) | 8575 | 2021-11-22 06:58:33 | | | | |
| 4 | Lindsay Dong | -6388 word(s) | 2187 | 2022-04-13 12:47:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Barreiro, C.; Martinez, S.; Barredo, J.L. Main Carotenoids Produced by Microorganisms. Encyclopedia. Available online: https://encyclopedia.pub/entry/16176 (accessed on 07 February 2026).
Barreiro C, Martinez S, Barredo JL. Main Carotenoids Produced by Microorganisms. Encyclopedia. Available at: https://encyclopedia.pub/entry/16176. Accessed February 07, 2026.
Barreiro, Carlos, Sonia Martinez, Jose Luis Barredo. "Main Carotenoids Produced by Microorganisms" Encyclopedia, https://encyclopedia.pub/entry/16176 (accessed February 07, 2026).
Barreiro, C., Martinez, S., & Barredo, J.L. (2021, November 19). Main Carotenoids Produced by Microorganisms. In Encyclopedia. https://encyclopedia.pub/entry/16176
Barreiro, Carlos, et al. "Main Carotenoids Produced by Microorganisms." Encyclopedia. Web. 19 November, 2021.
Copy Citation
Carotenoids are the pigments present in plants, animals, and microorganisms which are responsible for a broad variety of colors found in nature. Their capacity as antioxidants mainly established their marketable success as health, food, and feed supplements, and cosmetics components. Currently, chemical synthesis dominates the worldwide market; however, due to the high biological value of natural carotenoids, the production scheme is moving towards microbial production as a profitable alternative.
fungi
bacteria
algae
carotenoids
carotene
xanthophyll
astaxanthin
beta-carotene
lutein
lycopene
zeaxanthin
canthaxanthin
The simplest way to understand what carotenoids are is to look for colors in living organisms in a natural environment. Those reddish, orange, or yellowish pigments observed in living (micro)organisms are mainly carotenoids. They are one of the most widespread and ubiquitous lipid-soluble and non-nitrogenous pigments. Carotenoids form a subfamily of isoprenoids (also named terpenoids), which result in a diverse group of secondary metabolites. A prevalent example of this is the autumn colors and hues of trees and bushes. Other common examples of carotenoids include the orange-red condiment and food coloring annatto (derived from seeds of the achiote tree (Bixa Orellana)), petals, pollen (e.g., saffron), fruits (e.g., papaya, mandarins, oranges), vegetables (e.g., paprika, tomato), roots (e.g., carrots), animal tissues (e.g., salmonid and goldfish skin, flamingo and canary plumage, invertebrate exoskeletons), and animal products (e.g., egg yolks) (Figure 1) [1][2][3][4].
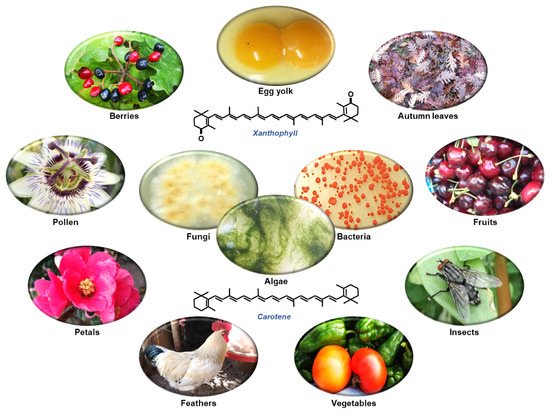
Figure 1. Examples of different colored structures from natural environments where diverse carotenoids can be found. The center panel presents some of the most common carotenoid producers (fungi, algae, and bacteria) and the structure of a xanthophyll (upper: canthaxanthin) and a carotene (lower: β-carotene).
Based on their oxygenation status, carotenoids can be divided in two main groups: (i) oxygenated molecules (oxycarotenoids) and (ii) non-oxygenated molecules. On the one hand, oxycarotenoids including carbonyl, carboxylic acid, ester, epoxy, hydroxy and methoxy groups are the xanthophylls (e.g., astaxanthin (C40H52O4), canthaxanthin (C40H52O2), lutein (C40H56O2) or zeaxanthin (C40H56O2)). On the other hand, those strictly non-oxygenated molecules (hydrocarbons) are the carotenes (C40H56) (e.g., lycopene, α-carotene, β-carotene) (Figure 2) [2][3][5].
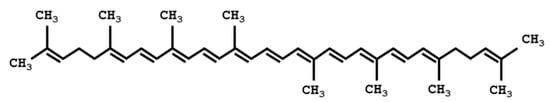
Figure 2. Acyclic C40H56 model structure of a carotenoid molecule.
Nowadays, 722 organisms have been described as the source of the 1204 natural carotenoids currently defined in the Carotenoid DataBase (http://carotenoiddb.jp (accessed on updated: September 2020)) [6][7]. They are naturally produced by photosynthetic species (plants, algae, and cyanobacteria), some groups of fungi and some non-photosynthetic bacteria [4][8][9]. Although it is generally recognized that humans and animals are not able to produce their own carotenoids, the genome sequencing of the pea aphid (Acyrthosiphon pisum) detected carotenoid biosynthetic genes due to horizontal gene transfer from fungi [10][11].
Brief History of Carotenoids Discovery and Production
Carotenoids are taxonomically widespread and serve as pigments in plants and animals, and are the reason for the diverse and intense colors present in nature (Figure 1) [12]. Because of this colorful characteristic, these compounds were one of the earliest studied phytochemicals [13], leading to the study of carotenoid pigments that began in the nineteenth century and spanning 200 years. This history can be divided into four periods, as Otto Isler indicated in his book entitled Carotenoids, in 1971 [14].
Beginning in the 19th century (first period), the core of carotenoid analysis was the isolation of the pigments and their characterization through their light absorption measurements, although their structures were still widely unknown [15]. Henri Braconnot (1780–1855) was likely in charge of the first research on carotenoids in 1817, which was carried out in paprika (Capsicum annuum). More than a hundred years later (1927), a pigment from paprika was purified in its crystalline form under the name of capsanthin [9]. In 1818, Aschoff isolated an apocarotenoid named crocin (from the word “crocos”, which means saffron in German [16]). The apocarotenoids are carotenoid cleavage products as a result of the activity of specific carotenoid cleavage oxygenases [17][18]. Crocin is the chemical ingredient primarily responsible for the saffron color (Crocus sativus) [16][19]. A few years later, in 1823, the research on crab (Brachyura) developed by Goebel suggested for the first time the presence of carotenoids in animals. However, Heinrich Wackenroder (1798–1854) reached the best-recognized milestone in 1831 because of research on carrots (Daucus carota L.). He isolated and described for the first time the β-carotene as part of his analysis of carrot juice when he searched for an effective anthelminthic drug [20]. Subsequently, in 1837, the Swedish chemist Jöns J. Berzelius (1779–1848) defined the xanthophyll present in autumn leaves. Later, lycopene was isolated from berries of Tamus communis by Hartsen in 1873. A few years later, in 1903 Schunk gave its name (lycopene) when noticed that this tomato pigment had a different absorption spectrum than carrot carotenes [7][20][21][22][23].
Following this first period (19th century) of carotenoid research, which focused on their isolation, the second period (1900–1927) was based on the determination of the empirical formula of carotenoids. These tentative efforts to discover carotenoid’s role in photosynthesis were also capital [14]. Because of the different carotenoid characterizations, Richard Willstätter (1872–1942) and Walter Mieg established the empirical formula of β-carotene (C40H56) in 1907 [24]. In 1919, Harry Steenbock (1886–1967) observed growth promoting activity due to the carotenoids found in colored vegetables. He noticed how the yellow corn and yellow vegetables (e.g., sweet potato, carrots), in contrast to white vegetables (e.g., potato, parsnip), decreased the effect of lacking vitamin A [25]. Ten years later, Hans von Euler (1873–1964) and Paul Karrer (1889–1971) defined β-carotene as a good growth factor closely related to vitamin A (retinol). Later, the β-carotene and vitamin A structures were elucidated in 1930–1931 by Karrer, who received the Nobel Prize in Chemistry in 1937 “for his investigations on carotenoids, flavins and vitamins A and B2” (the Nobel Prize: www.nobelprize.org, accessed on August 2021) [7][14].
The findings on vitamin A opened up the third period of carotenoid analysis. The provitamin A (β-carotene) concept was the core of this third carotenoid period (1928–1949). The idea of provitamins (molecules able to be transformed into vitamins by the organism) was a completely new concept, which resulted in great scientific and commercial significance. The elucidation of structural formula and the development of synthesis methods also fueled this third period [14]. This resulted in the number of known naturally occurring carotenoids growing from 15 to about 80 over 15 years (1933–1948). The structures of around 35 of these pigments were defined too. In 1947, the first chemical synthesis of crystalline vitamin A was announced by Isler and co-workers [26], which initiated the development of different industrial manufacturing procedures. In 1950, several groups allowed the total synthesis of β-carotene, which opened next period of carotenoid research.
The fourth period (1950–1990) increased the number of new carotenoids exponentially and led to extraordinary advances in carotenoid chemical synthesis, and their absolute configuration determination were reached. Thus, in 1960, Rhodia Inc. begun the production of methylheptenone from isoprene; BASF obtained iso-methylheptenone from isobutylene; Glidden Co. was able to synthesize citral from turpentine oil via myrcene [7]. Nowadays, the C40-carotenoids produced by industrial synthesis are reached by double Wittig condensation of symmetrical structures (e.g., identical end groups). However, just five out of the several hundred naturally occurring carotenoids (lycopene, canthaxanthin, astaxanthin, β,β–carotene, and zeaxanthin) and three apocarotenoids (β-apo-8′-carotenal, β-apo-8′-carotenoate, and citranaxanthin) are synthetically produced at industrial scales [27][28]. Only 50 carotenoids are valuable in human nutrition and up to 20 of them are typically detected in human blood and tissues, which come from the diet [3][29]. BASF SE, Kemin Industries, DSM Animal Nutrition, Novus International Inc, Biochem Products B.V., and Allied Biotech Corporation are the main worldwide players of the feed carotenoids market, which accounted for 81% of the overall revenue in 2020 (www.mordorintelligence.com, accessed on August 2021).
The need for commercial production of natural pigments boosted intensive research on the microbial biosynthesis of carotenoids, which can be considered the fifth period of carotenoids research (1991 to present). β-carotene and astaxanthin, for example, are extensively studied and industrially produced by microbial fermentation [3][28][30]. However, the screening of new producers of carotenoids (algae, bacteria, yeast and fungi), the development of fermentation processes or the use of genetic engineering or synthetic biology methodologies (e.g., search and use of genes involved in carotenoid biosynthesis, storage and catabolism) mark this fifth period [3][31][32][33].
What Are Carotenoids?
Based on their chemical characteristics, carotenoids are lipophilic compounds insoluble in water, which contain a chromophore. They comprise a long polyene central chain of conjugated double bonds (with a maximum absorbance of 400–500 nm), which is the reason for their characteristic yellow to reddish colors [3]. Following the IUPAC’s rule 1 about the definition of classes of compounds: “carotenoids are a class of hydrocarbons (carotenes) and their oxygenated derivatives (xanthophylls) consisting of eight isoprenoid units joined in such a manner that the arrangement of isoprenoid units is reversed at the centre of the molecule so that the two central methyl groups are in a 1,6-positional relationship and the remaining nonterminal methyl groups are in a 1,5-positional relationship. All carotenoids may be formally derived from the acyclic C40H56 structure, having a long central chain of conjugated double bonds, by (i) hydrogenation, (ii) dehydrogenation, (iii) cyclization, or (iv) oxidation. or any combination of these processes” (https://www.qmul.ac.uk/sbcs/iupac/carot/car1t7.html#p2, accessed on August 2021) (Figure 2) [2].
On the other hand, if the number of carbons are to be taken into account, the carotenoids, following the Carotenoid DataBase (http://carotenoiddb.jp, accessed on August 2021; https://www.qmul.ac.uk/sbcs/iupac/carot/car1t7.html#p2, accessed on August 2021) can be divided into: (i) C30 (37 carotenoids; e.g., 4,4′-diapolycopene), (ii) C40 (1121 carotenoids; e.g., lycopene), (iii) C45 (13 carotenoids; e.g., nonaflavuxanthin: intermediate in the biosynthesis of decaprenoxanthin (C50) by Corynebacterium glutamicum) and (iv) C50 (33 carotenoids; e.g., haloxanthin).
Biosynthetic Pathways of Carotenoids
Carotenoid biosynthetic pathways have been extensively studied over the past years. The starting molecule from which all carotenoids are synthesized is phytoene, a C40 colorless carotenoid. First, two molecules of geranylgeranyl diphosphate (GGPP) are condensed by the enzyme phytoene synthase (CrtYB) to produce phytoene. GGPP molecules are in turn synthesized by the condensation of dimethylallyl pyrophosphate (DMAPP) and three molecules of isopentenyl pyrophosphate (IPP), by means of the enzyme GGPP synthase (CrtE). Mainly in eukaryotes, these precursor molecules derive from the mevalonate pathway (MEV), whilst in prokaryotes and plants they derive from the methyl-erythritol phosphate pathway (MEP), also known as the non-mevalonate pathway [34].
Consequently, the enzyme phytoene desaturase (CrtI) catalyzes the transformation of phytoene into lycopene by introducing four double bonds. This enzyme can conduct further desaturation, synthesizing linear carotenoids, such as neurosporene or 3,4-didehydrolycopene.
In fungi (e.g., Xanthophyllomyces dendrorhous, Puccinia graminis [35][36]), the gene crtYB encodes a bifunctional enzyme that can perform as a phytoene synthase, as previously mentioned, and as a lycopene cyclase. The latter enzymatic activity is able to convert the acyclic ends of lycopene into β-rings, and generates carotenoid diversity, by producing β-carotene and γ-carotene. Subsequently, ketolation and hydroxylation reactions occur in order to synthesize other carotenoids (Figure 3). Depending on the microorganism, some carotenoids can be synthesized by enzymes encoded by different genes [37].
β-carotene hydroxylase is an enzyme encoded by the crtZ gene, which is able to hydroxylate β-carotene to form β-cryptoxanthin, and further add another hydroxy group to finally synthesize zeaxanthin.
Canthaxanthin is produced by the transformation of two methylene groups located in the β-ionone rings of β-carotene into keto groups. This reaction is carried out by the enzyme β-carotene ketolase (CrtW), which initially adds a keto group to β-carotene, forming echinenone, and afterwards synthesizes canthaxanthin by adding an extra keto group [38].
Furthermore, astaxanthin is formed by the addition of 4-keto groups and 3-hydroxy groups to the molecule of β-carotene, by means of the enzyme astaxanthin synthetase (CrtS), helped by a cytochrome P450 reductase (CrtR). These enzymes are naturally present in some microorganisms, such as X. dendrorhous [39]. Moreover, the biosynthesis of astaxanthin in other microorganisms has been described by means of the enzymes β-carotene hydroxylase and β-carotene ketolase, encoded by the genes crtZ and crtW, respectively [40].
The lutein biosynthetic pathway is mainly present in plants. Lutein can be synthesized from lycopene through the action of two lycopene cyclases (AtLCYe and AtLCYb) and two hydroxylases (AtCYP97A and AtCYP97C). The production of lutein has been observed in a strain of Synechocystis sp. transformed with the previously mentioned genes [34].
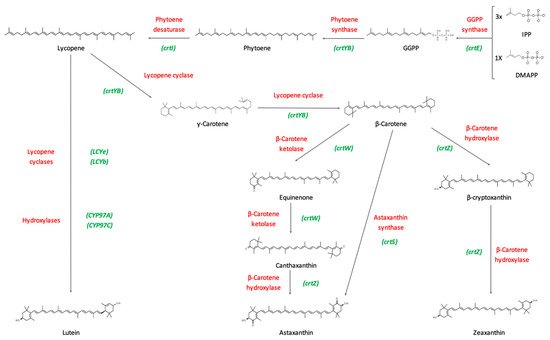
Figure 3. Biosynthetic pathway of main commercial carotenoids. The pathway begins with the condensation of three molecules of isopentenyl pyrophosphate (IPP) and one molecule of dimethylallyl pyrophosphate (DMAPP) by means of the enzyme GGPP synthase (crtE), forming geranylgeranyl diphosphate (GGPP). In some fungi, such as X. dendrorhous, the gene crtYB encodes for a bifunctional enzyme that can perform as a phytoene synthase and as a lycopene cyclase, it combines two molecules of GGPP forming phytoene. Afterwards, phytoene is transformed into lycopene by means of the enzyme phytoene desaturase (crtI). The enzyme encoded by the gene crtYB, and with its lycopene cyclase activity, is able to convert the acyclic ends of lycopene to β-ring, and to generate carotenoid diversity, by producing β-carotene and γ-carotene. Further ketolation and hydroxylation reactions synthesize the rest of carotenoids. Lutein can be synthesized from lycopene through the action of two lycopene cyclases (LCYe and LCYb) and two hydroxylases (CYP97A and CYP97C). Red color indicates the enzyme name, whereas green color shows the gene names. This figure is based on Rodríguez-Sáiz and co-workers and Barreiro and co-workers [8][39].
References
- Saini, R.K.; Keum, Y.S. Progress in Microbial Carotenoids Production. Indian J. Microbiol. 2017, 57, 129–130.
- Pfander, H.; Lanz, M.; Traber, B. Synthesis of carotenoids. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1997; Volume 20, pp. 561–612.
- Barreiro, C.; Barredo, J. (Eds.) Carotenoids production: A healthy and profitable industry. In Microbial Carotenoids: Methods and Protocols; Springer Science+Business Media: New York, NY, USA, 2018; pp. 45–55. ISBN 9781493987429.
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From plants to food and feed industries. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J., Eds.; Springer Science: Amsterdam, The Netherlands, 2018; pp. 57–71.
- Bhosale, P.; Bernstein, P.S. Microbial xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455.
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004.
- Fernandes, A.S.; Do Nascimento, T.C.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Carotenoids—A brief overview on its structure, biosynthesis, synthesis, and applications. Prog. Carotenoid Res. 2018, 1–16.
- Barredo, J.; García-Estrada, C.; Kosalkova, K.; Barreiro, C. Biosynthesis of astaxanthin as a main carotenoid in the eterobasidiomycetous yeast Xanthophyllomyces dendrorhous. J. Fungi 2017, 3, 44.
- Gómez-García, M.R.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053.
- Moran, N.A.; Jarvik, T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 2010, 328, 624–627.
- Barreiro, C.; Gutiérrez, S.; Olivera, E.R. Fungal Horizontal Gene Transfer: A History Beyond the Phylogenetic Kingdoms. In Horizontal Gene Transfer; Villa, T., Viñas, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 315–336. ISBN 9783030218621.
- Klassen, J.L. Phylogenetic and evolutionary patterns in microbial carotenoid biosynthesis are revealed by comparative genomics. PLoS ONE 2010, 5, e11257.
- Hammond, B.R.; Renzi, L.M. Carotenoids. Adv. Nutr. 2013, 4, 474–476.
- Isler, O. Introduction. In Carotenoids; Isler, O., Gutmann, H., Ulrich, S., Eds.; Springer Basel AG: Basel, Switzerland, 1971; pp. 12–25. ISBN 978-3-0348-5832-8.
- Isler, O. History and Industrial Application of Carotenoids and Vitamin A (1). Pure Appl. Chem. 1979, 51, 447–462.
- José Bagur, M.; Alonso Salinas, G.; Jiménez-Monreal, A.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2017, 23, 30.
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692.
- Pandita, D. Saffron (Crocus sativus L.): Phytochemistry, therapeutic significance and omics-based biology. In Medicinal and Aromatic Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 325–396. ISBN 978-0-12-819590-1.
- Singla, R.K.; Bhat, V.G. Crocin: An overview. Indo Glob. J. Pharm. Sci. 2014, 1, 281–286.
- Sourkes, T.L. The discovery and early history of carotene. Bull. Hist. Chem. 2009, 34, 33.
- Vogele, A.C. Effect of environmental factors upon the color of the tomato and the watermelon. Plant Physiol. 1937, 12, 929–955.
- Meroni, E.; Raikos, V. Lycopene in beverage emulsions: Optimizing formulation design and processing effects for enhanced delivery. Beverages 2018, 4, 14.
- The xanthophyll group of yellow colouring matters. Proc. R. Soc. Lond. 1904, 72, 165–176.
- Willstätter, R.; Mieg, W. Untersuchungen über Chlorophyll; IV. Ueber die gelben Begleiter des Chlorophylls. Justus Liebig’s Ann. Chem. 1907, 355, 1–28.
- Buttriss, J.L.; Welch, A.A.; Kearney, J.M.; Lanham-New, S.A. (Eds.) Public Health Nutrition, 2nd ed.; Wiley-Blackwell: Hoboken, FJ, USA, 2017; ISBN 978-1-118-66097-3.
- Isler, O.; Huber, W.; Ronco, A.; Kofler, M. Synthese des Vitamin A. Helv. Chim. Acta 1947, 30, 1911–1927.
- Ernst, H. Recent advances in industrial carotenoid synthesis. Pure Appl. Chem. 2002, 74, 2213–2226.
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and production methods. Food Qual. Saf. 2018, 2, 69–74.
- Khachik, F. Distribution and metabolism of dietary carotenoids in humans as a criterion for development of nutritional supplements. Pure Appl. Chem. 2006, 78, 1551–1557.
- Demain, A.L.; Sánchez, S. Advancement of biotechnology by genetic modifications. In Microbial Carotenoids: Methods and Protocols; Barreiro, C., Barredo, J.L., Eds.; Springer Nature: New York, NY, USA, 2018; Volume 1852, pp. 1–43. ISBN 9781493987429.
- Xue, D.; Abdallah, I.I.; de Haan, I.E.M.; Sibbald, M.J.J.B.; Quax, W.J. Enhanced C30 carotenoid production in Bacillus subtilis by systematic overexpression of MEP pathway genes. Appl. Microbiol. Biotechnol. 2015, 99, 5907–5915.
- Furubayashi, M.; Ikezumi, M.; Takaichi, S.; Maoka, T.; Hemmi, H.; Ogawa, T.; Saito, K.; Tobias, A.V.; Umeno, D. A highly selective biosynthetic pathway to non-natural C50 carotenoids assembled from moderately selective enzymes. Nat. Commun. 2015, 6, 7534.
- Gong, G.; Liu, L.; Zhang, X.; Tan, T. Multi-omics metabolism analysis on irradiation-induced oxidative stress to Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2019, 103, 361–374.
- Lehmann, M.; Vamvaka, E.; Torrado, A.; Jahns, P.; Dann, M.; Rosenhammer, L.; Aziba, A.; Leister, D.; Rühle, T. Introduction of the carotenoid biosynthesis α-branch into Synechocystis sp. PCC 6803 for lutein production. Front. Plant Sci. 2021, 12.
- Visser, H.; van Ooyen, A.J.J.; Verdoes, J.C. Metabolic engineering of the astaxanthin-biosynthetic pathway of Xanthophyllomyces dendrorhous. FEMS Yeast Res. 2003, 4, 221–231.
- Wang, E.; Dong, C.; Zhang, P.; Roberts, T.H.; Park, R.F. Carotenoid biosynthesis and the evolution of carotenogenesis genes in rust fungi. Fungal Biol. 2021, 125, 400–411.
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a red-hot carotenoid: Applications, synthesis, and biosynthetic evolution. Plants 2020, 9, 1039.
- Misawa, N.; Kajiwara, S.; Kondo, K.; Yokoyama, A.; Satomi, Y.; Saito, T.; Miki, W.; Ohtani, T. Canthaxanthin biosynthesis by the conversion of methylene to keto groups in a hydrocarbon β-carotene by a single gene. Biochem. Biophys. Res. Commun. 1995, 209, 867–876.
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658.
- Misawa, N.; Satomi, Y.; Kondo, K.; Yokoyama, A.; Kajiwara, S.; Saito, T.; Ohtani, T.; Miki, W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 1995, 177, 6575–6584.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.3K
Book:
Encyclopedia of Fungi
Online Date:
19 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No

