
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kevin Zhai | + 943 word(s) | 943 | 2020-06-16 08:59:53 | | | |
| 2 | Rita Xu | -90 word(s) | 853 | 2020-06-28 11:48:19 | | | | |
| 3 | Kevin Zhai | + 224 word(s) | 1077 | 2020-07-14 02:13:28 | | | | |
| 4 | Rita Xu | Meta information modification | 1077 | 2020-10-27 04:11:37 | | | | |
| 5 | Rita Xu | + 692 word(s) | 1769 | 2020-11-09 09:59:02 | | |
Video Upload Options
Intracellular calcium (Ca2+) concentration ([Ca2+]i) is a key determinant of cell fate and is implicated in carcinogenesis. Membrane ion channels are structures through which ions enter or exit the cell, depending on the driving forces. The opening of transient receptor potential vanilloid 1 (TRPV1) ligand-gated ion channels facilitates transmembrane Ca2+ and Na+ entry, which modifies the delicate balance between apoptotic and proliferative signaling pathways. Proliferation is upregulated through two mechanisms: (1) ATP binding to the G-protein-coupled receptor P2Y2, commencing a kinase signaling cascade that activates the serine-threonine kinase Akt, and (2) the transactivation of the epidermal growth factor receptor (EGFR), leading to a series of protein signals that activate the extracellular signal-regulated kinases (ERK) 1/2. The TRPV1-apoptosis pathway involves Ca2+ influx and efflux between the cytosol, mitochondria, and endoplasmic reticulum (ER), the release of apoptosis-inducing factor (AIF) and cytochrome c from the mitochondria, caspase activation, and DNA fragmentation and condensation. While proliferative mechanisms are typically upregulated in cancerous tissues, shifting the balance to favor apoptosis could support anti-cancer therapies. TRPV1, through [Ca2+]i signaling, influences cancer cell fate; therefore, the modulation of the TRPV1-enforced proliferation–apoptosis balance is a promising avenue in developing anti-cancer therapies and overcoming cancer drug resistance.
1. [Ca2+]i and the Critical Balance between Apoptosis and Proliferation
Molecular mechanisms that mediate cell death and proliferation exist in balance in functional physiological systems. Proliferation is involved in structural development and renewal, while programmed cell death is necessary to eliminate defective cells and prevent uncontrolled growth. Carcinogenesis results from imbalances in the described pathways, which favor proliferation and reduce apoptosis [1][2]. Therefore, anti-cancer therapies shift the balance in the opposite direction by reducing proliferation and upregulating apoptosis.
Apoptosis is defined as programmed cell death, characterized by fragmentation of inter-nucleosomal DNA [3]. Two major mechanisms of apoptosis are an extrinsic, death-receptor mediated mechanism, and an intrinsic, mitochondria-mediated mechanism [4]. The extrinsic mechanism involves the linking of membrane death receptors to adapter proteins, which bind and position pro-caspase 8 for conversion into caspase 8; the intrinsic mechanism is triggered by the release of cytochrome c from mitochondria, which promotes caspase 9 activation [4][5]. The Bcl-2 family of proteins, which includes the proapoptotic proteins Bax and Bak and the antiapoptotic protein Bcl-2, is implicated in the intrinsic mechanism of apoptosis [6]. Both the intrinsic and extrinsic apoptotic mechanisms lead to the activation of caspase 3, which mediates apoptosis through nuclear activity.
Calcium (Ca2+) is a second messenger that influences the proliferation–apoptosis balance. Intracellular Ca2+ ([Ca2+]i) is modulated by receptor-operated, store-operated (SOC), and voltage-sensitive ion channels, ion exchangers, pumps, Ca2+ binding proteins, mitochondrial Ca2+ ([Ca2+]m), and endoplasmic reticulum (ER) and sarcoplasmic reticulum (SR) Ca2+ ([Ca2+]ER and [Ca2+]SR) [7][8]. Intracellular Ca2+ release channels comprise one subset of ion channels; these include the ryanodine receptor (RyR) and inositol 1,4,5-triphosphosphate (IP3) receptor (IP3R) channels, both of which are localized to the ER and SR. RyR channels, which are activated by elevated [Ca2+]i or protein signaling, and IP3R channels, which are activated by IP3 binding, release Ca2+ from the ER and SR. Through [Ca2+]i signaling, these two channel types modulate muscle contraction and nerve impulse transmission [9][10]. Abberant Ca2+ transport from the ER or SR to the cytosol may elevate [Ca2+]m and consequently induce mitochondrial dysfunction [11][12].
Beyond locomotion and neurotransmission, shifts in [Ca2+]i homeostasis may also mediate cell death or proliferation. For instance, while [Ca2+]i signaling via IP3R contributes to proliferation and oncogenesis, RyR [Ca2+]i signaling supports apoptosis in lung cancer cells [10][13]. Furthermore, Ca2+ influx through T-type voltage-gated Ca2+ channels (VGCC) is implicated in the proliferation of cancerous and noncancerous cells, while the blockage of such channels promotes apoptosis in glioblastoma cells [14][15]. In contrast, Ca2+ influx through L-type VGCC causes death in bovine chromaffin cells [16]. Notably, [Ca2+]i-mediated cell death may be apoptotic or necrotic in nature, depending on the time of exposure and [Ca2+] i involved [17].
Significantly, [Ca2+]I signaling regulates proliferation, invasion, and metastasis in cancerous tissues [18]. A variety of oncologic therapies, including cisplatin, arsenic trioxide, trimethyltin chloride, and some candidate epigenetic drugs, induce their proapoptotic and anti-proliferative effects (in part or in whole) through the modulation of [Ca2+]i [19][20]. Therefore, specific [Ca2+]i-affecting proteins, including transmembrane ion channels, which mediate Ca2+ flow between the extracellular space and the cytosol, are potential targets for chemotherapeutic agents.
Transient receptor potential (TRP) channels comprise a large family of membrane Ca2+ channels, which respond to a wide variety of environmental stimuli [21][22][23]. Transient receptor potential vanilloid 1, or vanilloid receptor 1 (TRPV1/VR1), known as the capsaicin receptor, is a member of the TRPV subfamily of TRP channels. TRPV1 is a ligand-gated ion channel which is activated by capsaicin and capsaicin analogues (e.g., resiniferatoxin, RTX), heat, and endogenous cannabinoids such as anandamide (AEA); its antagonists include capsazepine and ruthenium red [24][25]. The stimulation of TRPV1 causes Ca2+ and Na+ influx through transmembrane ion channels. While these channels generally exhibit selectivity for Ca2+ over Na+, the precise nature of this selectivity depends on a variety of factors, including the nature and concentration of the agonist [26]. TRPV1 is involved in thermoregulation, circadian rhythms, energy intake and metabolism, and acute, chronic, and inflammatory nociception; as such, the ion channel receptor is a target in the development of analgesic therapies [27][28][29][30][31][32]. Furthermore, given its role in modulating [Ca2+]i, TRPV1 influences the balance between proliferation and apoptosis [33].
2. Balance Between Apoptosis and Proliferation Mediated by TRPV1
The activation of the TRPV1 ion channel is a critical signal involved in numerous intracellular processes, some of which trigger either apoptosis or proliferation (Figure 1). While the apoptotic effects of TRPV1 are well characterized, literature on TRPV1-related proliferation remains sparse. The binding of exogenous agonists to the TRPV1 receptor and subsequent Ca2+ influx from the cytosol into the cell are characteristics shared between the apoptotic and proliferative pathways. However, both the positive allosteric modulation of cell membrane TRPV1 receptors and the activation of endoplasmic reticulum (ER)-localized TRPV1 channels are associated exclusively with the pro-apoptotic pathway [34][35].
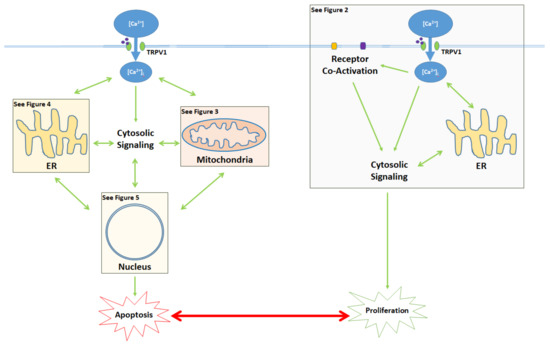
Figure 1. Activation of the TRPV1 ligand-gated ion channel causes Ca2+ influx into the cytosol and influences the balance between proliferation and apoptosis. Apoptotic signaling occurs through the cytosol, mitochondria, ER, and the nucleus. In contrast, the proliferative effects of TRPV1 are mediated by the activation of other cell membrane receptors, ER signaling, and cytosolic protein signaling cascades. The proliferative, proapoptotic mitochondrial, proapoptotic ER, and proapoptotic nuclear signaling mechanisms are highlighted in the colored boxes, and specified in Figure 2 Figure 3 Figure 4 Figure 5, respectively.
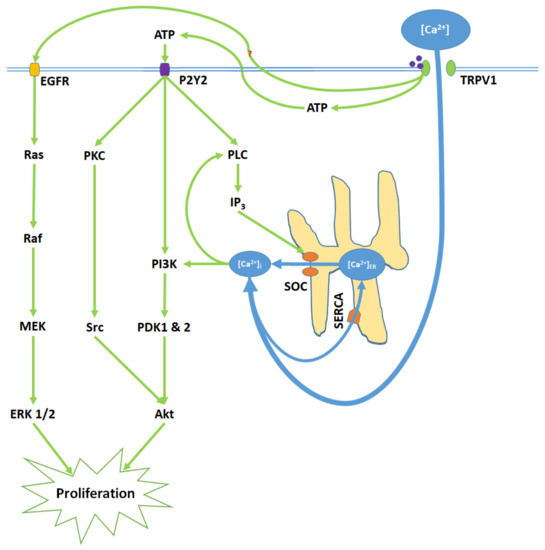
Figure 2. TRPV1 induces proliferation through Ca2+ entry, ATP release and membrane P2Y purinoceptor 2 (P2Y2) activation, and the transactivation of epidermal growth factor receptor (EGFR). Elevated [Ca2+]i and ATP-P2Y2 binding upregulate intracellular inositol triphosphate (IP3) via phospholipase C (PLC); IP3 opens store-operated channels (SOC) and thereby causes Ca2+ release from the ER. Activated P2Y2 receptors also begin the phosphoinositide-3-kinase (PI3K)/Akt pathway, a kinase signaling cascade that ultimately activates Akt. TRPV1 additionally transactivates EGFR; this prompts Ras/Raf/MAPK-ERK kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling, which upregulates ERK 1/2 mitogen-activated protein kinases (MAPK). Akt and ERK 1/2 MAPK promote proliferation through nuclear activity.
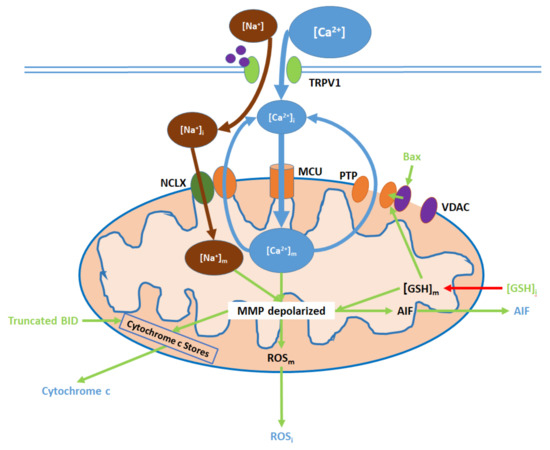
Figure 3. TRPV1 induces mitochondrial dysfunction through Ca2+ and Na+ entry, membrane depolarization, reactive oxygen species (ROS) production, and the release of cytochrome c and apoptosis-inducing factor (AIF). Initial Ca2+ and Na+ influx causes the hyperpolarization of the mitochondrial membrane, while consequent Ca2+ export through the permeability transition pore (PTP) and active Ca2+ removal via the Na+/Ca2+ exchanger (NCLX) depolarize the membrane. As inputs, downregulated intracellular glutathione ([GSH]i) and upregulated Bax arise from nuclear activity. Upon their release (driven by membrane depolarization), AIF translocates directly to the nucleus, cytochrome c participates in intracellular caspase 9 activation, and intracellular ROS (ROSi) supports the activation of p38 MAPKs.
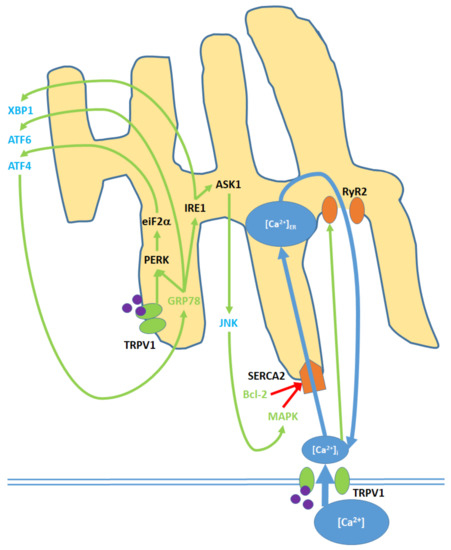
Figure 4. Cell membrane and ER TRPV1 activation promote ER stress through the modulation of [Ca2+]ER, activation of various kinases, the upregulation of nuclear transcription factors, and the release of c-Jun N-terminal kinase (JNK) into the cytosol. TRPV1 proteins localized to the ER membrane contribute only to protein signaling within the ER, while TRPV1 channels in the cell membrane promote both [Ca2+]i and protein signaling. Initial Ca2+ entry into the ER occurs through the sarco/endoplasmic reticulum Ca2+ ATPase 2 (SERCA2) pump, which is eventually blocked, causing net Ca2+ export via the ryanodine receptor 2 (RyR2) channels. Glucose regulated protein 78 (GRP78) upregulation and Bcl-2 downregulation, as inputs, arise from nuclear activity. MAPK is both an input and output of ER stress, as it is upregulated via both mitochondrial activity and JNK. Activating transcription factors 4 (ATF4) and 6 (ATF6) and X-box binding protein 1 (XBP1) are transcription factors that constitute the downstream nuclear targets of ER stress; ATF4, in particular, feeds back to the ER by upregulating GRP78.
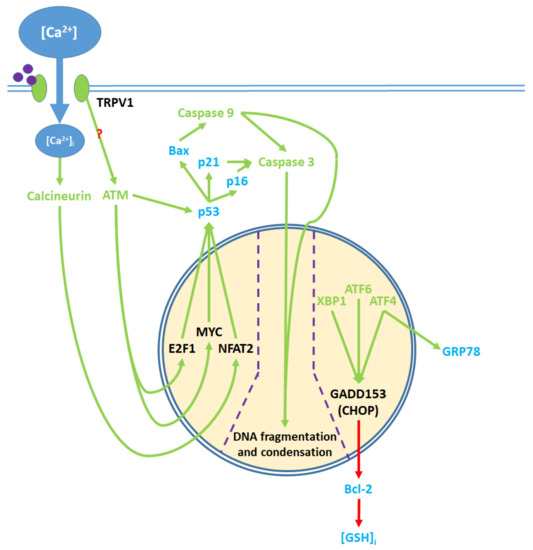
Figure 5. Pro-apoptotic processes induced by TRPV1 localized in the nucleus. The nuclear component of ER stress occurs as ATF4, ATF6, and XBP1 are activated by ER stress and upregulate GADD153, which in turn downregulates Bcl-2 protein production. The upregulation of GRP78 by ATF4 feeds back to and enhances ER stress. The cytosolic activation of the ATM serine-threonine kinase by TRPV1 protein signaling and calcineurin by elevated [Ca2+]i promote the nuclear transcription factors E2F1, MYC, and NFAT2, which upregulate p53. The precise mechanism through which TRPV1 activates ATM remains unclear. p53 upregulates the apoptotic mediators Bax, p16, and p21, which activate caspase 9 and 3. Activated caspases translocate from the cytosol to the nucleus, where they mediate DNA fragmentation and condensation.
Abbreviations: ATP, Adenosine TriPhosphate; AIF, Apoptosis Inducing Factor; Akt, Akt serine-threonine protein kinase; ASK1, Apoptosis Signal-regulating Kinase 1; ATF4, Activating Transcription Factor 4; ATF6, Activating Transcription Factor 6; ATM, ATM serine-threonine kinase; Bax, Bcl-2 associated x protein; Bcl-2, B-cell lymphoma 2; BID, BH3 Interacting-domain Death agonist; E2F1, E2F transcription factor 1; EGFR, Epidermal Growth Factor Receptor; eIF2, eukaryotic Initiation Factor 2; ERK, Extracellular signal-Regulated Kinase; GADD153, DNA Damage-Inducible Transcript 3; GRP78, Glucose Regulated Protein 78; [GSH]i, intracellular glutathione; [GSH]m, mitochondrial glutathione; IP3, Inositol triPhosphate; IRE1, Inositol-Requiring Enzyme 1; JNK, c-Jun N-terminal Kinase; MAPK, Mitogen Activated Protein Kinase; MCU, Mitochondrial Ca2+ Uniporter; MEK, MAPK-ERK Kinase; MMP, Mitochondrial Membrane Potential; MYC, Myc proto-oncogene; NCLX, Na+/Ca2+ exchanger; NFAT2, Nuclear Factor of Activated T-cells 2; P2Y2, P2Y purinoceptor 2; PDK, Phosphoinositide-Dependent Kinase; PERK, PKR-like Endoplasmic Reticulum Kinase; PI3K, PhosphoInositide-3-Kinase; PKC, Protein Kinase C; PLC, PhosphoLipase C; PTP, Permeability Transition Pore; Raf, Rapidly accelerated fibrosarcoma; Ras, Rat sarcoma; ROS, Reactive Oxygen Species; ROSi, intracellular Reactive Oxygen Species; ROSm, mitochondrial Reactive Oxygen Species; RyR2, Ryanodine Receptor 2; SERCA, Sarco/Endoplasmic Reticulum Ca2+ ATPase; SOC, Store-Operated Channel; Src, proto-oncogene tyrosine-protein kinase Src; TRPV1, Transient Receptor Potential Vanilloid 1; VDAC, Voltage Dependent Anion Channel; XBP1, X-box Binding Protein 1.
References
- Cvejic, D.; Selemetjev, S.; Savin, S.; Paunovic, I.; Tatic, S. Changes in the balance between proliferation and apoptosis during the progression of malignancy in thyroid tumours. Eur. J. Histochem. 2009, 53, e8.
- Hao, X.; Du, M.; Bishop, A.E.; Talbot, I.C. Imbalance between proliferation and apoptosis in the development of colorectal carcinoma. Virchows Arch. 1998, 433, 523–527.
- Afanas’ev, V.N.; Korol, B.A.; Mantsygin Yu, A.; Nelipovich, P.A.; Pechatnikov, V.A.; Umansky, S.R. Flow cytometry and biochemical analysis of DNA degradation characteristic of two types of cell death. FEBS Lett. 1986, 194, 347–350, doi:10.1016/0014-5793(86)80115-6.
- Reed, J.C. Mechanisms of apoptosis. Am. J. Pathol. 2000, 157, 1415–1430, doi:10.1016/S0002-9440(10)64779-7.
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312.
- Kuwana, T.; Newmeyer, D.D. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 2003, 15, 691–699, doi:10.1016/j.ceb.2003.10.004.
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565, doi:10.1038/nrm1150.
- Varghese, E.; Samuel, S.M.; Sadiq, Z.; Kubatka, P.; Liskova, A.; Benacka, J.; Pazinka, P.; Kruzliak, P.; Busselberg, D. Anti-Cancer Agents in Proliferation and Cell Death: The Calcium Connection. Int. J. Mol. Sci. 2019, 20, doi:10.3390/ijms20123017.
- Santulli, G.; Lewis, D.; des Georges, A.; Marks, A.R.; Frank, J. Ryanodine Receptor Structure and Function in Health and Disease. Subcell. Biochem. 2018, 87, 329–352, doi:10.1007/978-981-10-7757-9_11.
- Gambardella, J.; Lombardi, A.; Morelli, M.B.; Ferrara, J.; Santulli, G. Inositol 1,4,5-Trisphosphate Receptors in Human Disease: A Comprehensive Update. J. Clin. Med. 2020, 9, doi:10.3390/jcm9041096.
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051, doi:10.1113/JP272781.
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Front. Oncol. 2017, 7, 140, doi:10.3389/fonc.2017.00140.
- Shin, D.-H.; Leem, D.-G.; Shin, J.-S.; Kim, J.-I.; Kim, K.-T.; Choi, S.Y.; Lee, M.-H.; Choi, J.-H.; Lee, K.-T. Compound K induced apoptosis via endoplasmic reticulum Ca2+ release through ryanodine receptor in human lung cancer cells. J. Ginseng Res. 2018, 42, 165–174.
- Panner, A.; Wurster, R.D. T-type calcium channels and tumor proliferation. Cell Calcium 2006, 40, 253–259, doi:10.1016/j.ceca.2006.04.029.
- Valerie, N.C.; Dziegielewska, B.; Hosing, A.S.; Augustin, E.; Gray, L.S.; Brautigan, D.L.; Larner, J.M.; Dziegielewski, J. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem. Pharm. 2013, 85, 888–897, doi:10.1016/j.bcp.2012.12.017.
- Cano-Abad, M.A.F.; Villarroya, M.; Garcı‘a, A.G.; Gabilan, N.H.; Lo´pez, M.G. Calcium entry through L-type calcium channels causes mitochondrial disruption and chromaffin cell death. J. Biol. Chem. 2001, 276, 39695–39704.
- Nicotera, P.; Orrenius, S. The role of calcium in apoptosis. Cell Calcium. 1998, 23, 173–180, doi:10.1016/s0143-4160(98)90116-6.
- Stewart, T.A.; Yapa, K.T.; Monteith, G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta 2015, 1848, 2502–2511, doi:10.1016/j.bbamem.2014.08.016.
- Florea, A.M.; Busselberg, D. Anti-cancer drugs interfere with intracellular calcium signaling. Neurotoxicology 2009, 30, 803–810, doi:10.1016/j.neuro.2009.04.014.
- Raynal, N.J.; Lee, J.T.; Wang, Y.; Beaudry, A.; Madireddi, P.; Garriga, J.; Malouf, G.G.; Dumont, S.; Dettman, E.J.; Gharibyan, V.; et al. Targeting Calcium Signaling Induces Epigenetic Reactivation of Tumor Suppressor Genes in Cancer. Cancer Res. 2016, 76, 1494–1505, doi:10.1158/0008-5472.CAN-14-2391.
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524.
- Huang, J.; Liu, J.; Qiu, L. Transient receptor potential vanilloid 1 promotes EGFR ubiquitination and modulates EGFR/MAPK signalling in pancreatic cancer cells. Cell Biochem. Funct. 2020, 10.1002/cbf.3483, doi:10.1002/cbf.3483.
- So, C.L.; Milevskiy, M.J.G.; Monteith, G.R. Transient receptor potential cation channel subfamily V and breast cancer. Lab. Invest. 2020, 100, 199–206, doi:10.1038/s41374-019-0348-0.
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; Di Marzo, V.; Julius, D.; Hogestatt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457, doi:10.1038/22761.
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824, doi:10.1038/39807.
- Bautista, D.; Julius, D. Fire in the hole: Pore dilation of the capsaicin receptor TRPV1. Nat. Neurosci. 2008, 11, 528-529, doi:10.1038/nn0508-528.
- Satheesh, N.J.; Uehara, Y.; Fedotova, J.; Pohanka, M.; Büsselberg, D.; Kruzliak, P. TRPV currents and their role in the nociception and neuroplasticity. Neuropeptides 2016, 57, 1–8.
- Szallasi, A.; Cruz, F.; Geppetti, P. TRPV1: A therapeutic target for novel analgesic drugs? Trends Mol. Med. 2006, 12, 545–554, doi:10.1016/j.molmed.2006.09.001.
- Christie, S.; Wittert, G.A.; Li, H.; Page, A.J. Involvement of TRPV1 Channels in Energy Homeostasis. Front. Endocrinol. (Lausanne) 2018, 9, 420, doi:10.3389/fendo.2018.00420.
- Jeong, K.Y. Changes in TRPV1-Mediated Physiological Function in Rats Systemically Treated With Capsaicin on the Neonate. Int. J. Mol. Sci. 2020, 21, doi:10.3390/ijms21093143.
- Inprasit, C.; Lin, Y.-W. TRPV1 Responses in the Cerebellum Lobules V, VIa and VII Using Electroacupuncture Treatment for Inflammatory Hyperalgesia in Murine Model. Int. J. Mol. Sci. 2020, 21, 3312.
- Shah, S.; Carver, C.M.; Mullen, P.; Milne, S.; Lukacs, V.; Shapiro, M.S.; Gamper, N. Local Ca(2+) signals couple activation of TRPV1 and ANO1 sensory ion channels. Sci. Signal. 2020, 13, doi:10.1126/scisignal.aaw7963.
- Buch, T.R.H.; Buch, E.A.M.; Boekhoff, I.; Steinritz, D.; Aigner, A. Role of Chemosensory TRP Channels in Lung Cancer. Pharmaceuticals 2018, 11, doi:10.3390/ph11040090.
- Kristin Stock; Jitender Kumar; Michael Synowitz; Stefania Petrosino; Roberta Imperatore; Ewan St. John Smith; Peter Wend; Bettina Purfürst; Ulrike A. Nuber; Ulf Gurok; et al.Vitali MatyashJoo-Hee WälzleinSridhar R. ChirasaniGunnar DittmarBenjamin F. CravattStefan MommaGary R. LewinAlessia LigrestiLuciano De PetrocellisLuigia CristinoVincenzo Di MarzoHelmut KettenmannRainer Glass Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nature Medicine 2012, 18, 1232, 10.1038/nm.2827.
- Mustafa Nazıroğlu; Bilal Çiğ; Walter Blum; Csaba Vizler; Andrea Buhala; Annamária Marton; Róbert Katona; Katalin Josvay; Beat Schwaller; Zoltan Olah; et al.Laszlo Pecze Targeting breast cancer cells by MRS1477, a positive allosteric modulator of TRPV1 channels. PLOS ONE 2017, 12, e0179950, 10.1371/journal.pone.0179950.




