
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Morgan Hamon | + 3548 word(s) | 3548 | 2021-11-08 04:46:50 | | | |
| 2 | Peter Viktor Hauser | + 2 word(s) | 3550 | 2021-11-09 20:52:53 | | | | |
| 3 | Conner Chen | Meta information modification | 3550 | 2021-11-10 04:42:09 | | |
Video Upload Options
Human organ function and physiology depend on a functional vascular system to facilitate oxygen and nutrient supply, as well as the removal of metabolic products. Ischemia is temporary reduction of blood supply that can cause physiological imbalance due to a lack of oxygen (hypoxia), nutrients, and a failure to eliminate metabolic waste products. Prolonged ischemia is associate with tissue damage and potentially necrosis. In this context, avoiding ischemia time remains critical to preventing hypoxic injury and potential damages to transplant tissues and organs. Despite substantial progress in creating three-dimensional (3D) blood vessels, fabricating a functional vascular multiscale system has remained a challenge . Many techniques have been developed to fabricate vascular networks that can mimic the complexity, the unique structures, and the functionality of human blood vessels. Among these advancements, 3D bioprinting has become an essential tool for the fabrication of vascularized bioconstructs due to improved control over vascular growth, reproducibility, and scalability of the fabrication process.
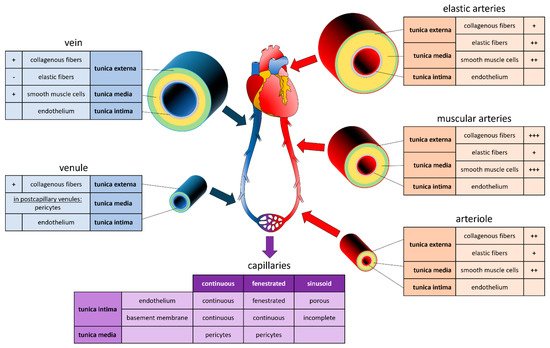
1. Vascular System
2. Printing Methods
| Materials Application | Printer Style | Advantages | Disadvantages |
|---|---|---|---|
| Extrusion-based | Bioplotting | wide range of bioinks | slow printing process |
| Fusion deposition modelling | wide range of bioinks | limited resolution only hydrogels slow |
|
| Laser-assisted | Stereolithography | high resolution possible | UV damage to cells small range of bioinks |
| Droplet-based | Inkjet | gentle to printed cells fast printing affordable |
limitations of cell density low resolution |
2.1. Sacrificial
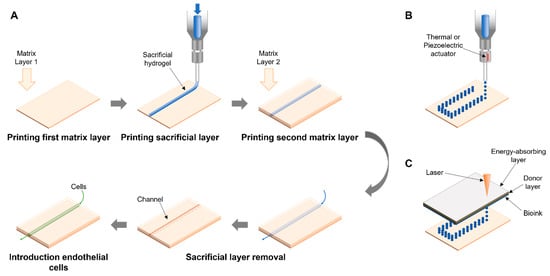
Extrusion-Assisted Techniques
2.2. Droplet-Based Bioprinting
Indirect Bioprinting Using DBB
2.3. Light-Based Techniques
Laser-Induced Forward Transfer
2.4. Four-Dimensional (4D) Bioprinting
References
- Levick, J.R. An Introduction to Cardiovascular Physiology; Butterworth-Heinemann: London, UK, 1991; pp. 1–12.
- Pugsleya, M.K.; Tabrizchib, R. The vascular system—An overview of structure and function. J. Pharmacol. Toxicol. Methods 2000, 44, 333–340.
- Berillis, P. The Role of Collagen in the Aorta’s Structure. Open Circ. Vasc. J. 2013, 6, 1–8.
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, Blood Vessels. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Okada, H.; Takemura, G.; Suzuki, K.; Oda, K.; Takada, C.; Hotta, Y.; Nagisa, M.; Akiko, T.; Isamu, M.; Yoshiaki, A.; et al. Three-dimensional ultrastructure of capillary endothelial glycocalyx under normal and experimental endotoxemic conditions. Crit. Care 2017, 21, 261.
- Zamani, Y.; Mohammadi, J.; Amoabediny, G.; Helder, M.N.; Zandhie-Doulabi, B.; Klein-Nulend, J. Bioprinting of Alginate-Encapsulated Pre-osteoblasts in PLGA/β-TCP Scaffolds Enhances Cell Retention but Impairs Osteogenic Differentiation Compared to Cell Seeding after 3D-Printing. Regen. Eng. Transl. Med. 2020.
- Zhang, Y.S.; Oklu, R.; Dokmeci, M.R.; Khademhosseini, A. Three-Dimensional Bioprinting Strategies for Tissue Engineering. Cold Spring Harb. Perspect. Med. 2018, 8, a025718.
- Thomas, A.; Orellano, I.; Lam, T.; Noichl, B.; Geiger, M.A.; Amler, A.K.; Kreuder, A.E.; Palmer, C.; Duda, G.; Lauster, R.; et al. Vascular bioprinting with enzymatically degradable bioinks via multi-material projection-based stereolithography. Acta Biomater. 2020, 117, 121–132.
- Burdick, J.A.; Chung, C.; Jia, X.; Randolph, M.A.; Langer, R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 2005, 6, 386–391.
- Liao, C.Y.; Wu, W.J.; Hsieh, C.T.; Yang, H.C.; Tseng, C.S.; Hsu, S.H. Water/ice as sprayable sacrificial materials in low-temperature 3D printing for biomedical applications. Mater. Des. 2018, 60, 624–635.
- Zhou, Y. The Application of Ultrasound in 3D Bio-Printing. Molecules 2016, 21, 590.
- Rasheed, A.; Azizi, L.; Turkki, P.; Janka, M.; Hytönen, V.P.; Tuukkanen, S. Extrusion-Based Bioprinting of Multilayered Nanocellulose Constructs for Cell Cultivation Using In SituFreezing and Preprint CaCl2 Cross-Linking. ACS Omega 2020, 6, 569–578.
- Fisch, P.; Holub, M.; Zenobi-Wong, M. Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion. Biofabrication 2021, 13, 015012.
- Justino Netto, J.M.; Idogava, H.T.; Frezzatto Santos, L.E.; de Castro Silveira, Z.; Romio, P.; Lino Alves, J. Screw-assisted 3D printing with granulated materials: A systematic review. Int. J. Adv. Manuf. Technol. 2021, 115, 2711–2727.
- Cooke, M.E.; Rosenzweig, D.H. The Rheology of Direct and Suspended Extrusion Bioprinting. APL Bioeng. 2021, 5, 011502.
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344.
- Leucht, A.; Volz, A.-C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced Gelatin-Based Vascularization Bioinks for Extrusion-Based Bioprinting of Vascularized Bone Equivalents. Sci. Rep. 2020, 10, 5330.
- Freeman, S.; Ramos, R.; Alexis Chando, P.; Zhou, L.; Reeser, K.; Jin, S.; Soman, P.; Ye, K. A Bioink Blend for Rotary 3D Bioprinting Tissue Engineered Small-Diameter Vascular Constructs. Acta Biomater. 2019, 95, 152–164.
- Naghieh, S.; Sarker, M.D.; Abelseth, E.; Chen, X. Indirect 3D Bioprinting and Characterization of Alginate Scaffolds for Potential Nerve Tissue Engineering Applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193.
- Lee, J.-Y.; Choi, B.; Wu, B.; Lee, M. Customized Biomimetic Scaffolds Created by Indirect Three-Dimensional Printing for Tissue Engineering. Biofabrication 2013, 5, 045003.
- Contessi Negrini, N.; Celikkin, N.; Tarsini, P.; Farè, S.; Święszkowski, W. Three-Dimensional Printing of Chemically Crosslinked Gelatin Hydrogels for Adipose Tissue Engineering. Biofabrication 2020, 12, 025001.
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel Bioprinted Microchannel Networks for Vascularization of Tissue Engineering Constructs. Lab Chip 2014, 14, 2202–2211.
- Mae, H.J.; Delgadillo, L.; Wan, J. On-demand modulation of 3D-printed elastomers using programmable droplet inclusions. Proc. Natl. Acad. Sci. USA 2020, 117, 14790–14797.
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42.
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.-S.; Vincent, P.A.; Dai, G. Creating Perfused Functional Vascular Channels Using 3D Bio-Printing Technology. Biomaterials 2014, 35, 8092–8102.
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112.
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-Assisted Cell Printing: Principle, Physical Parameters versus Cell Fate and Perspectives in Tissue Engineering. Nanomedicine 2010, 5, 507–515.
- Wu, P.K.; Ringeisen, B.R. Development of Human Umbilical Vein Endothelial Cell (HUVEC) and Human Umbilical Vein Smooth Muscle Cell (HUVSMC) Branch/Stem Structures on Hydrogel Layers via Biological Laser Printing (BioLP). Biofabrication 2010, 2, 014111.
- Morouço, P.; Azimi, B.; Milazzo, M.; Mokhtari, F.; Fernandes, C.; Reis, D.; Danti, S. Four-Dimensional (Bio-)Printing: A Review on Stimuli-Responsive Mechanisms and Their Biomedical Suitability. Appl. Sci. 2020, 10, 9143.
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D Bioprinting: The next-Generation Technology for Biofabrication Enabled by Stimuli-Responsive Materials. Biofabrication 2016, 9, 012001.
- Kirillova, A.; Maxson, R.; Stoychev, G.; Gomillion, C.T.; Ionov, L. 4D Biofabrication Using Shape-Morphing Hydrogels. Adv. Mater. 2017, 29, 1703443.
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Principles of the Kenzan Method for Robotic Cell Spheroid-Based Three-Dimensional Bioprinting. Tissue Eng. Part B Rev. 2017, 23, 237–244.
- Itoh, M.; Nakayama, K.; Noguchi, R.; Kamohara, K.; Furukawa, K.; Uchihashi, K.; Toda, S.; Oyama, J.-I.; Node, K.; Morita, S. Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization When Implanted in Rat Aortae. PLoS ONE 2015, 10, e0136681.




