Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andreas Nussler | + 2137 word(s) | 2137 | 2021-11-05 05:21:11 | | | |
| 2 | Catherine Yang | Meta information modification | 2137 | 2021-11-08 02:14:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nussler, A. Intervertebral Disc Nucleus Repair. Encyclopedia. Available online: https://encyclopedia.pub/entry/15750 (accessed on 07 February 2026).
Nussler A. Intervertebral Disc Nucleus Repair. Encyclopedia. Available at: https://encyclopedia.pub/entry/15750. Accessed February 07, 2026.
Nussler, Andreas. "Intervertebral Disc Nucleus Repair" Encyclopedia, https://encyclopedia.pub/entry/15750 (accessed February 07, 2026).
Nussler, A. (2021, November 05). Intervertebral Disc Nucleus Repair. In Encyclopedia. https://encyclopedia.pub/entry/15750
Nussler, Andreas. "Intervertebral Disc Nucleus Repair." Encyclopedia. Web. 05 November, 2021.
Copy Citation
Degenerative disc disease (DDD) is a common clinical condition that causes chronic back pain. Lower back pain is one of the leading causes of disability and thus places a high burden on healthcare systems worldwide; yet, it is not among the top 10 disorders receiving research funding.
intervertebral disc
Scaffold
Tissue Engineering
1. IVD Degeneration
Intervertebral discs (IVDs) are cartilaginous structures between the vertebral bodies that mainly provide flexibility and elasticity [1] and have a wide range of movement to the spine as a whole. In addition, IVDs strongly provide pressure and tensile resistance while transmitting mechanical load through the spinal column [2] and therefore support a variety of loads during daily activities. A healthy IVD is comprised of a proteoglycan-rich gelatinous center called the NP, which is peripherally enclosed by the collagen-rich annulus fibrosus (AF) and the cartilaginous endplate (CEP), which limit the peripheral rim of the disc superiorly and inferiorly [3]. The primary components of IVDs are water, cells (mainly chondrocyte-like cells and fibroblasts), proteoglycan, collagen, and other matrix components [4]. Fibrillar collagens, aggrecan, and water are the three main structural components of the IVD, all together contributing to around 90–95% of the volume of a healthy IVD [5], although their percentages vary across the disc [2].
Several etiological factors such as aging, smoking, infection, abnormal biomechanical loading, and nutrition insufficiency are thought to be involved in the pathogenesis of IVD degeneration [5][6]. Among these factors, genetic heritability is estimated to account for up to 74% [7]. As the degeneration process is highly correlated with aging, its pathologic changes occur starting from the second decade of life [8][9]. Substantial changes in biochemical composition and progressive loss of structural integrity are hallmarks of IVD degeneration [3] (as illustrated in Figure 1, where curved arrows define the transition from normal disc structure to later degenerative disc), which occurs mostly in adults aged over 30 years in one or more discs or during trauma and injury. Loss of proteoglycans and a decrease in the ratio of proteoglycan to collagen [5] consequently results in the loss of hydrostatic properties, which induces structural wear of the IVD [10] and thus progresses towards a fibrotic nature. Dehydration of NP and gradual disappearance of the NP–AF border contributes to the loss of normal architecture. Stress distribution over the NP tends to reduce at the center and accumulates more pressure around the periphery, effectively disabling the NP’s load transfer function [11]. Due to a lack of intradiscal pressure, the load absorption and transmission in such dehydrated discs is significantly altered and subsequently, it results in disc-height reduction, osteophyte formation, facet joint arthritis, and deformation of vertebral bodies [12]. With continuing degeneration, the structural deficit is accompanied by leakage of the central NP material through cracks in the AF into the periphery. This results in immune cell activation, thereby evoking chronic back pain [13][14]. Since biochemical changes within IVDs have not yet been directly associated with chronic back pain, it is difficult to determine if the observed changes are due to aging or pathology [15].
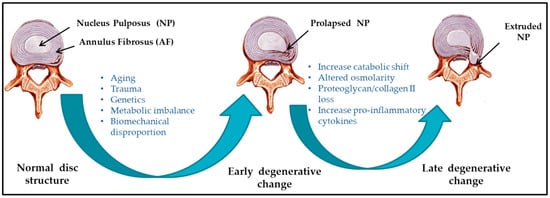
Figure 1. Schematic illustration of intervertebral disc (IVD) pathophysiology during degeneration.
IVD degeneration often results in lower back pain but is not always the only causative factor. Location of the affected disc, degree of nerve damage, and amount of pressure on the spinal column contribute to define the degree of degeneration. For example, some patients may not feel pain, while others with similar degrees and extents of IVD damage may experience chronic back pain. Therefore, the degree and extent of degeneration does not correlate with the degree of pain. IVD degeneration is the most common cause of lower back pain [16]. The worldwide prevalence of chronic back pain is approximate 60%, with the majority seen in the elderly [17].
2. Nucleus pulposus (NP) Repair
Tissue engineering approaches over the past few years have been addressing the objective to restore functional and structural features of the healthy IVD. Reparative treatment mainly targets intervention at early stages of IVD degeneration to restore extracellular matrix (ECM) homeostasis, control inflammation, and prevent angiogenesis [18]. Current surgical procedures mainly focus on alleviating symptoms associated with IVD degeneration but fail to promote tissue remodeling. Tissue engineering offers an alternative to design biomaterials by encompassing cells and growth factors that will aid IVD tissue regeneration. Thereby, it offers multiple strategies to prevent and possibly cure IVD degeneration by encouraging disc repair. The exact mechanisms of IVD regeneration are still not known, however several studies have focused on the effect of segmental distraction in IVD disease [19][20][21]. Synthetic and / or natural material based scaffolds for IVD tissue engineering were regarded as the prominent method over the past decades [22][23]. In spite of considerable progress, some issues related to scaffold integration and tissue repair still remained unsolved [24]. Alternatively, scaffold free tissue engineering (refer Table 1) is an emerging field, where cells, growth factors, or peptide delivery are mainly responsible for regaining the tissue integrity upon the application [25][26]. Stimulatory factors together with cells either unaided or together with biomaterials have aimed to provide suitable repair site to ensure maximum cell differentiation or deposition of appropriate ECM. Nonetheless, selection of biomaterials, cells and appropriate stimulatory factors is crucial as the ideal combination is yet to be established.
Table 1. Advantages and disadvantages of scaffold-free IVD tissue engineering.
| Methods | Categories | Advantages | Disadvantages/Limitations | References |
|---|---|---|---|---|
| Cell therapy | NP cells |
|
|
[27][28][29][30] |
| MSCs |
|
|
[31][32][33][34][35][36][37][38][39] | |
| Growth factors | TGF-β | Enhances cartilage formation and extracellular matrix production |
|
[40][41] |
| BMP2 | Enhances ECM production and phenotypic characteristics of NP cells | Induces apoptosis, Col I accumulation, and aggrecan-production hindrance | [42][43][44][45][46][47] | |
| BMP7 | Promotes proliferation and accelerates chondrogenesis | Short half-life time and biodegradation in vivo | [48][49][50][51][52][53][54][55] | |
| GDF-5 | Induces NP-like differentiation of MSCs | Possible association between GDF-5 gene polymorphisms and IDD | [56][57][58] | |
| IGF-1 | Enhances the ECM production and proliferation of IVD cells | Enhances glucose consumption and lactate concentration | [59][60] | |
| Injectable hydrogel | Cell-free hydrogel | Physiological swelling and greasing | Limited payload | [61][62] |
| Cell-seeded hydrogel |
|
No direct cell contact | [63][64][65][66] |
MSC: mesenchymal stem cell; BMP: bone morphogenetic protein; GDF: growth and differentiation factor; IGF: insulin-like growth factor; ECM: extracellular matrix; IDD: intervertebral disc degeneration.
3. Cell-Based Therapies
Cell based therapy for IVD nucleus repair mainly aims for NP like cells injections, addressing the imbalance of biochemical environment (proteoglycan synthesis and water content). Stimulation of the residing cells is insufficient to achieve tissue repair; hence, diseased phenotype properties of the native NP cells certainly limit their use. Thus, injecting functional cells aimed to overcome this problem by compensating cell death and disc shrinkage [61][67]. Concomitantly, Abbott et al. proposed NP cells do possess regeneration potential even in severe status of degeneration [68]. Several cell-based strategies have been investigated to retard NP degeneration, using different IVD model system [27][28][29][30], with NP and/or AF cells, articular chondrocytes, or mesenchymal stem/stromal cells (MSCs). Feng et al. summarized GDF-5 a suitable growth factor for inducing NP-like cells based on its positive effect on the differentiation of MSCs towards an NP-like phenotype [69]. The effect of growth factors such as TGF-β, IGF-1, FGF-2, PDGF and GDF-5 on the differentiation of MSCs into NP-like cells have been investigated [69]. Fundamentally, the transplanted MSCs are expected to differentiate, maintain and enhance the function of existing NP cells to reverse the IDD. Thus, preclinical studies will be required to confirm the functional potency of the MSC-based therapy for IDD. Good accessibility and the ability of MSCs (bone marrow, adipose and synovial tissue derived) to differentiate into different cell types including chondrocyte like cells, promoted MSCs as prime source for cell therapies for several diseases and in IVD regenerative treatment [32][33]. Intradiscal delivery of bone marrow and adipose derived MSCs has demonstrated to promote regeneration by maintaining cell viability and proliferation, obtaining IVD-like phenotypes, and providing expression of typical chondrocyte markers, in several studies using rabbit, rat, dog, and goat models [70][34][35][36][37]. Nevertheless, one common problem affecting the healing satisfaction is the tendency of hypertrophic differentiation of MSCs [38][39]. How to achieve an ideal IVD repair mainly relies on manipulation of hypertrophic chondrogenesis of the injected and/or implanted MSCs.
In parallel, the use of notochordal cells is also being considered, no matter from allogenic, autologous or xenogenic origin [71][72][73]. Risbud et al. has previously proposed that morphology and size variation correlates to different stages of maturation and/or function of NP cells derived from notochordal precursors [74]. Moreover, it has been speculated that degeneration is due to the selective loss of the notochordal cells fraction while considering overall reduction in the structural and functional activity of IVD cells. While there is considerable agreement, Bach et al. demonstrated the species (human, canine and porcine) specific regenerative effects of notochordal cell conditioned medium on human NP cells. It further confirmed the canine and porcine secreted factors exerted regenerative effect on human NP cells [75]. The fact that NP cells with diseased phenotype possess regeneration potential, allows autologous NP cells to be expanded using conditioned medium that these cells can be used as a source for cell therapy during nucleus repair. Likewise, stem cells can be differentiated towards NP like cell type. van Uden et al. has already addressed [76] the importance of hypoxia during NP repair. This key factor may restrict the success of the stem cell based therapy, as stem cells tend to die due to lack of oxygen. Serigano et al. demonstrated the effect of cell number on mesenchymal stem cell transplantation in canine disc degeneration model, where they showed high possibility of apoptosis, low cell viability, while maintaining microenvironment during stem cell transplantation [77]. Altogether, it affected the regeneration capability. However, in order to advance clinical translation of cell therapy, assessment of in vivo integration (in terms of functional and mechanical repair) in large animals is necessary. Therefore, a comparative analysis of cell types and sources of cells using large animal models is essential to enlighten the suitable strategy.
4. Injectable Hydrogels
Synthetic or biologically based injectable materials are largely focused on NP replacement, to stabilize and restore the function and structure of the discs and AF. Moreover, the biomaterial must satisfy the biomechanical strength with no migration and displacement, and durability with high wear resistance provoking low immune response. Injectable hydrogel over the solid-state scaffold opens up novel approaches in musculoskeletal application. Hydrogels mainly composed of natural polysaccharides (chitosan, chondroitin sulfate, hyaluronic acid), proteins (silk, resilin), or synthetic polymers (polyvinyl alcohol (PVA), polyacrylic acid, acrylamide), are emergent matrix substitutes in cartilage and IVD regeneration [78]. They are hydrophilic in nature, and have a water retention capacity between 20% and 99% by weight when placed in aqueous conditions. Therefore, these water soluble polymers are often used to build scaffolds by three-dimensional crosslinking either by covalent or physical methods. Hydrogels (depending on the physical structure and chemical composition) that mimic the mechanical stability and matrix composition of native IVD ECM, are a potential promising choice for IVD repair. Cell-free hydrogels and cell-seeded hydrogels are the two broad subtypes of injectable scaffolds found in literature [62][63].
Theoretically, biodegradability of some of the scaffolds allows remodeling of the scaffold in the regeneration process, while other scaffolds mechanically support and resist the compressive load for longer duration. For example, alginate and lyophilized chitosan gelatin scaffolds showed cytocompatibility towards the NP like cells, supporting cell growth. Li et al. stimulated NP cells using BMP-2 and BMP-7 heterodimer combined with fibrinhyaluronan hydrogel [64]. Their in vitro as well as ex vivo study demonstrated to produce aggrecan and collagen II by NP cells upon delivery, simulating in vivo conditions [64]. Moreover, cell numbers were found to be increased in alginate compare to lyophilized chitosan-gelatin based scaffolds, after 21 days of cell culture [61]. Hydrogels on the other hand resemble NP material, mainly due to their resilient and hydrophilic properties. Recently, several studies [30][62][63][65][66] have investigated the combination of cells and hydrogels to catalyze the tissue repair. Homogenous distribution of cells within defect size prior to gelation tends to support tissue repair [61]. To allow the sustainable effect of the growth factor Yan et al. administered the injection of poly (lactic-co-glycolic acid) (PLGA) microspheres loaded with recombinant human GDF-5 into a rat caudal disc degeneration model induced by needle puncture [79]. Sustained release of active GDF-5 for more than 42 days confirmed its therapeutic efficacy. Further Frith et al. [66] conducted a study examining the composite of injectable hydrogels (polyethylene glycol, hyaluronic acid, and pentosanpolysulphate) coupled with MSCs. In vivo examination of this scaffold composite together MSCs in rats, certainly supported the cartilage like tissue formation, thereby confirming the deposition of collagen II. However, there is a need to identify relevant combination of biomolecules and hydrogel material that may direct NP cell survival in vivo, in the challenging mechanical and physical microenvironment of the NP. While doing so, the biomaterial should withstand the mechanical load which ultimately contribute to the longevity of the scaffold.
References
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44.
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of intervertebral disc cells: Phenotype and function. J. Anat. 2012, 221, 480–496.
- Walter, B.A.; Torre, O.M.; Laudier, D.; Naidich, T.P.; Hecht, A.C.; Iatridis, J.C. Form and function of the intervertebral disc in health and disease: A morphological and stain comparison study. J. Anat. 2015, 227, 707–716.
- Singh, K.; Masuda, K.; Thonar, E.J.; An, H.S.; Cs-Szabo, G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine 2009, 34, 10–16.
- Urban, J.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130.
- Roberts, S.; Evans, H.; Trivedi, J.; Menage, J. Histology and pathology of the human intervertebral disc. J. Bone Jt. Surg. Am. 2006, 88, 10–14.
- MacGregor, A.J.; Andrew, T.; Sambrook, P.N.; Spector, T.D. Structural, psychological, and genetic influences on low back and neck pain: A study of adult female twins. Arthritis Rheum. 2004, 51, 160–167.
- Eskola, P.J.; Lemmelä, S.; Kjaer, P.; Solovieva, S.; Männikkö, M.; Tommerup, N.; Lind-Thomsen, A.; Husgafvel-Pursiainen, K.; Cheung, K.M.; Chan, D.; et al. Genetic association studies in lumbar disc degeneration: A systematic review. PLoS ONE 2012, 7, e49995.
- Walker, M.H.; Anderson, D.G. Molecular basis of intervertebral disc degeneration. Spine J. 2004, 4, 158S–166S.
- Noble, P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002, 21, 25–29.
- Wang, Y.; Yi, X.-D. The influence of artificial nucleus pulposus replacement on stress distribution in the cartilaginous endplate in a 3-dimensional finite element model of the lumbar intervertebral disc. Medicine 2017, 96, e9149.
- Freemont, A. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology 2009, 48, 5–10.
- Vaday, G.G.; Lider, O. Extracellular matrix moieties, cytokines, and enzymes: Dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 2000, 67, 149–159.
- Bao, Q.B.; McCullen, G.M.; Higham, P.A.; Dumbleton, J.H.; Yuan, H.A. The artificial disc: Theory, design and materials. Biomaterials 1996, 17, 1157–1167.
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am. J. Neuroradiol. 2015, 36, 811–816.
- Lim, T.K.Y.; Anderson, K.M.; Hari, P.; Di Falco, M.; Reihsen, T.E.; Wilcox, G.L.; Belani, K.G.; LaBoissiere, S.; Pinto, M.R.; Beebe, D.S.; et al. Evidence for a role of nerve injury in painful intervertebral disc degeneration: A cross-sectional proteomic analysis of human cerebrospinal fluid. J. Pain 2017, 18, 1253–1269.
- Masuda, K.; Lotz, J.C. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng. Part B Rev. 2010, 16, 147–158.
- Iatridis, J.C.; Kang, J.; Kandel, R.; Risbud, M.V. New horizons in spine research: Intervertebral disc repair and regeneration. J. Orthop. Res. 2017, 35, 5–7.
- Fontana, G.; See, E.; Pandit, A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv. Drug Deliv. Rev. 2015, 84, 146–158.
- Guterl, C.C.; See, E.Y.; Blanquer, S.B.; Pandit, A.; Ferguson, S.J.; Benneker, L.M.; Grijpma, D.W.; Sakai, D.; Eglin, D.; Alini, M.; et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur. Cell Mater. 2013, 25, 1–21.
- Buric, J.; Pulidori, M. Long-term reduction in pain and disability after surgery with the interspinous device for intervertebral assisted motion (diam) spinal stabilization system in patients with low back pain: 4-year follow-up from a longitudinal prospective case series. Eur. Spine J. 2011, 20, 1304–1311.
- Liu, Y.; Zhou, G.; Cao, Y. Recent progress in cartilage tissue engineering—our experience and future directions. Engineering 2017, 3, 28–35.
- Cao, Z.; Dou, C.; Dong, S. Scaffolding biomaterials for cartilage regeneration. J. Nanomater. 2014, 2014, 1–8.
- Liu, W.; Cao, Y. Application of scaffold materials in tissue reconstruction in immunocompetent mammals: Our experience and future requirements. Biomaterials 2007, 28, 5078–5086.
- Sart, S.; Tsai, A.C.; Li, Y.; Ma, T. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng. Part B Rev. 2014, 20, 365–380.
- Fujie, H.; Nansai, R.; Ando, W.; Shimomura, K.; Moriguchi, Y.; Hart, D.A.; Nakamura, N. Zone-specific integrated cartilage repair using a scaffold-free tissue engineered construct derived from allogenic synovial mesenchymal stem cells: Biomechanical and histological assessments. J. Biomech. 2015, 48, 4101–4108.
- Coric, D.; Pettine, K.; Sumich, A.; Boltes, M.O. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 joint spine section meeting. J. Neurosurg. Spine 2013, 18, 85–95.
- Peroglio, M.; Douma, L.; Caprez, T.; Janki, M.; Benneker, L.; Alini, M.; Grad, S. Intervertebral disc response to stem cell treatment is conditioned by disc state and cell carrier: An ex vivo study. J. Orthop. Transl. 2017, 9, 43–51.
- Watanabe, T.; Sakai, D.; Yamamoto, Y.; Iwashina, T.; Serigano, K.; Tamura, F.; Mochida, J. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J. Orthop. Res. 2010, 28, 623–630.
- Zhou, X.; Wang, J.; Fang, W.; Tao, Y.; Zhao, T.; Xia, K.; Liang, C.; Hua, J.; Li, F.; Chen, Q. Genipin cross-linked type ii collagen/chondroitin sulfate composite hydrogel-like cell delivery system induces differentiation of adipose-derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018, 71, 496–509.
- Vedicherla, S.; Buckley, C.T. Cell-based therapies for intervertebral disc and cartilage regeneration—Current concepts, parallels, and perspectives. J. Orthop. Res. 2017, 35, 8–22.
- Sakai, D.; Schol, J. Cell therapy for intervertebral disc repair: Clinical perspective. J. Orthop. Transl. 2017, 9, 8–18.
- Chakravarthy, K.; Chen, Y.; He, C.; Christo, P.J. Stem cell therapy for chronic pain management: Review of uses, advances, and adverse effects. Pain Physician 2017, 20, 293–305.
- Sakai, D.; Mochida, J.; Iwashina, T.; Watanabe, T.; Nakai, T.; Ando, K.; Hotta, T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: Potential and limitations for stem cell therapy in disc regeneration. Spine 2005, 30, 2379–2387.
- Zhang, Y.; Drapeau, S.; An, H.S.; Thonar, E.J.M.A.; Anderson, D.G. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc-injury model. Spine 2011, 36, 372.
- Ganey, T.; Hutton, W.C.; Moseley, T.; Hedrick, M.; Meisel, H.-J. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: Experiments in a canine model. Spine 2009, 34, 2297–2304.
- Chen, X.; Zhu, L.; Wu, G.; Liang, Z.; Yang, L.; Du, Z. A comparison between nucleus pulposus-derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model. Int. J. Surg. 2016, 28, 77–82.
- Murdoch, A.D.; Grady, L.M.; Ablett, M.P.; Katopodi, T.; Meadows, R.S.; Hardingham, T.E. Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: Generation of scaffold-free cartilage. Stem Cells 2007, 25, 2786–2796.
- Fernandez-Moure, J.; Moore, C.A.; Kim, K.; Karim, A.; Smith, K.; Barbosa, Z.; Van Eps, J.; Rameshwar, P.; Weiner, B. Novel therapeutic strategies for degenerative disc disease: Review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018, 6, 2050312118761674.
- Fei, S.L.; Yu, Y.L.; Tang, C.L.; Lv, F.Z. Effects of tgf-β1 and il-1β on expression of adamts enzymes and timp-3 in human intervertebral disc degeneration. Exp. Ther. Med. 2013, 6, 1522–1526.
- Walsh, A.J.; Bradford, D.S.; Lotz, J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine 2004, 29, 156–163.
- Lee, K.I.; Moon, S.H.; Kim, H.; Kwon, U.H.; Kim, H.J.; Park, S.N.; Suh, H.; Lee, H.M.; Kim, H.S.; Chun, H.J.; et al. Tissue engineering of the intervertebral disc with cultured nucleus pulposus cells using atelocollagen scaffold and growth factors. Spine 2012, 37, 452–458.
- Gilbertson, L.; Ahn, S.H.; Teng, P.N.; Studer, R.K.; Niyibizi, C.; Kang, J.D. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008, 8, 449–456.
- Leung, V.; Zhou, L.; Tam, W.; Sun, Y.; Lv, F.; Zhou, G.; Cheung, K. Bone morphogenetic protein-2 and-7 mediate the anabolic function of nucleus pulposus cells with discrete mechanisms. Connect. Tissue Res. 2017, 58, 573–585.
- Fei, Q.; Jiang, X.; Chen, T.; Li, J.; Murakami, H.; Tsai, K.; Hutton, W. Changes with age and the effect of recombinant human bmp-2 on proteoglycan and collagen gene expression in rabbit anulus fibrosus cells. Acta Biochim. Et Biophys. Sin. 2006, 38, 773–779.
- Kong, M.; Do, D.; Miyazaki, M.; Wei, F.; Yoon, S.; Wang, J. Rabbit model for in vivo study of intervertebral disc degeneration and regeneration. J. Korean Neurosurg. Soc. 2008, 44, 327–333.
- Huang, K.; Yan, J.; Hsieh, C.; Chang, M.; Lin, R. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc—An animal experiment. Spine 2007, 32, 1174–1180.
- Kawakami, M.; Matsumoto, T.; Hashizume, H.; Kuribayashi, K.; Chubinskaya, S.; Yoshida, M. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine 2005, 30, 1933–1939.
- Wei, A.; Brisby, H.; Chung, S.A.; Diwan, A.D. Bone morphogenetic protein-7 protects human intervertebral disc cells in vitro from apoptosis. Spine J. 2008, 8, 466–474.
- Takegami, K.; Thonar, E.; An, H.; Kamada, H.; Masuda, K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine 2002, 27, 1318–1324.
- An, H.; Takegami, K.; Kamada, H.; Nguyen, C.; Thonar, E.; Singh, K.; Andersson, G.; Masuda, K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 2005, 30, 25–31.
- Masuda, K.; Imai, Y.; Okuma, M.; Muehleman, C.; Nakagawa, K.; Akeda, K.; Thonar, E.; Andersson, G.; An, H. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine 2006, 31, 742–754.
- Chubinskaya, S.; Kawakami, M.; Rappoport, L.; Matsumoto, T.; Migita, N.; Rueger, D. Anti-catabolic effect of op-1 in chronically compressed intervertebral discs. J. Orthop. Res. 2007, 25, 517–530.
- Willems, N.; Bach, F.C.; Plomp, S.G.M.; van Rijen, M.H.P.; Wolfswinkel, J.; Grinwis, G.C.M.; Bos, C.; Strijkers, G.J.; Dhert, W.J.A.; Meij, B.P. Intradiscal application of rhbmp-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res. Ther. 2015, 17, 137.
- Van Dijk, B.G.M.; Potier, E.; van Dijk, M.; Creemers, L.B.; Ito, K. Osteogenic protein 1 does not stimulate a regenerative effect in cultured human degenerated nucleus pulposus tissue. J. Tissue Eng. Regen. Med. 2017, 11, 2127–2135.
- Chujo, T.; An, H.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of growth differentiation factor-5 on the intervertebral disc - in vitro bovine study and in vivo rabbit disc degeneration model study. Spine 2006, 31, 2909–2917.
- Feng, C.; Liu, H.; Yang, Y.; Huang, B.; Zhou, Y. Growth and differentiation factor-5 contributes to the structural and functional maintenance of the intervertebral disc. Cell. Physiol. Biochem. 2015, 35, 1–16.
- Bach, F.C.; Willems, N.; Penning, L.C.; Ito, K.; Meij, B.P.; Tryfonidou, M.A. Potential regenerative treatment strategies for intervertebral disc degeneration in dogs. BMC Vet. Res. 2014, 10, 3.
- Kim, J.; Ellman, M.; An, H.; van Wijnen, A.; Borgia, J.; Im, H. Insulin-like growth factor 1 synergizes with bone morphogenetic protein 7-mediated anabolism in bovine intervertebral disc cells. Arthritis Rheum. 2010, 62, 3706–3715.
- Pratsinis, H.; Kletsas, D. Organotypic cultures of intervertebral disc cells: Responses to growth factors and signaling pathways involved. BioMed Res. Int. 2015, 2015, 427138.
- Priyadarshani, P.; Li, Y.; Yao, L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthr. Cartil. 2016, 24, 206–212.
- Chuang, E.-Y.; Chiang, C.-W.; Wong, P.-C.; Chen, C.-H. Hydrogels for the application of articular cartilage tissue engineering: A review of hydrogels. Adv. Mater. Sci. Eng. 2018, 2018, 1–13.
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25.
- Li, Z.; Lang, G.; Karfeld-Sulzer, L.S.; Mader, K.T.; Richards, R.G.; Weber, F.E.; Sammon, C.; Sacks, H.; Yayon, A.; Alini, M.; et al. Heterodimeric bmp-2/7 for nucleus pulposus regeneration-in vitro and ex vivo studies. J. Orthop. Res. 2017, 35, 51–60.
- Nesti, L.J.; Li, W.J.; Shanti, R.M.; Jiang, Y.J.; Jackson, W.; Freedman, B.A.; Kuklo, T.R.; Giuliani, J.R.; Tuan, R.S. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (hanfs) amalgam. Tissue Eng. Part A 2008, 14, 1527–1537.
- Frith, J.E.; Cameron, A.R.; Menzies, D.J.; Ghosh, P.; Whitehead, D.L.; Gronthos, S.; Zannettino, A.C.W.; Cooper-White, J.J. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 2013, 34, 9430–9440.
- Benneker, L.M.; Andersson, G.; Iatridis, J.C.; Sakai, D.; Härtl, R.; Ito, K.; Grad, S. Cell therapy for intervertebral disc repair: Advancing cell therapy from bench to clinics. Eur. Cell Mater. 2014, 27, 5–11.
- Abbott, R.D.; Purmessur, D.; Monsey, R.D.; Iatridis, J.C. Regenerative potential of tgfbeta3 + dex and notochordal cell conditioned media on degenerated human intervertebral disc cells. J. Orthop. Res. 2012, 30, 482–488.
- Risbud, M.V.; Albert, T.J.; Guttapalli, A.; Vresilovic, E.J.; Hillibrand, A.S.; Vaccaro, A.R.; Shapiro, I.M. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: Implications for cell-based transplantation therapy. Spine 2004, 29, 2627–2632.
- Cunha, C.; Almeida, C.R.; Almeida, M.I.; Silva, A.M.; Molinos, M.; Lamas, S.; Pereira, C.L.; Teixeira, G.Q.; Monteiro, A.T.; Santos, S.G.; et al. Systemic delivery of bone marrow mesenchymal stem cells for in situ intervertebral disc regeneration. Stem Cells Transl. Med. 2017, 6, 1029–1039.
- Arkesteijn, I.T.M.; Potier, E.; Ito, K. The regenerative potential of notochordal cells in a nucleus pulposus explant. Glob. Spine J. 2017, 7, 14–20.
- Gantenbein-Ritter, B.; Chan, S.C. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: An experimental 3-d co-culture study. Eur. Spine J. 2012, 21 (Suppl. 6), S819–S825.
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular therapy for degenerative disc disease: Clues from secretome analysis of the notochordal cell-rich nucleus pulposus. Sci. Rep. 2017, 7, 45623.
- Risbud, M.V.; Shapiro, I.M. Notochordal cells in the adult intervertebral disc: New perspective on an old question. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 29–41.
- Bach, F.C.; de Vries, S.A.; Krouwels, A.; Creemers, L.B.; Ito, K.; Meij, B.P.; Tryfonidou, M.A. The species-specific regenerative effects of notochordal cell-conditioned medium on chondrocyte-like cells derived from degenerated human intervertebral discs. Eur. Cell Mater. 2015, 30, 132–147.
- Van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017, 21, 22.
- Serigano, K.; Sakai, D.; Hiyama, A.; Tamura, F.; Tanaka, M.; Mochida, J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J. Orthop. Res. 2010, 28, 1267–1275.
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360.
- Yan, J.; Yang, S.; Sun, H.; Guo, D.; Wu, B.; Ji, F.; Zhou, D. Effects of releasing recombinant human growth and differentiation factor-5 from poly(lactic-co-glycolic acid) microspheres for repair of the rat degenerated intervertebral disc. J. Biomater. Appl. 2014, 29, 72–80.
More
Information
Subjects:
Medicine, Research & Experimental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
724
Revisions:
2 times
(View History)
Update Date:
08 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




