Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Andreas Nussler and Version 2 by Catherine Yang.
Degenerative disc disease (DDD) is a common clinical condition that causes chronic back pain. Lower back pain is one of the leading causes of disability and thus places a high burden on healthcare systems worldwide; yet, it is not among the top 10 disorders receiving research funding.
- intervertebral disc
- Scaffold
- Tissue Engineering
1. IVD Degeneration
Intervertebral discs (IVDs) are cartilaginous structures between the vertebral bodies that mainly provide flexibility and elasticity [1][13] and have a wide range of movement to the spine as a whole. In addition, IVDs strongly provide pressure and tensile resistance while transmitting mechanical load through the spinal column [2][14] and therefore support a variety of loads during daily activities. A healthy IVD is comprised of a proteoglycan-rich gelatinous center called the NP, which is peripherally enclosed by the collagen-rich annulus fibrosus (AF) and the cartilaginous endplate (CEP), which limit the peripheral rim of the disc superiorly and inferiorly [3][15]. The primary components of IVDs are water, cells (mainly chondrocyte-like cells and fibroblasts), proteoglycan, collagen, and other matrix components [4][16]. Fibrillar collagens, aggrecan, and water are the three main structural components of the IVD, all together contributing to around 90–95% of the volume of a healthy IVD [5][17], although their percentages vary across the disc [2][14].
Several etiological factors such as aging, smoking, infection, abnormal biomechanical loading, and nutrition insufficiency are thought to be involved in the pathogenesis of IVD degeneration [5][6][17,18]. Among these factors, genetic heritability is estimated to account for up to 74% [7][19]. As the degeneration process is highly correlated with aging, its pathologic changes occur starting from the second decade of life [8][9][6,20]. Substantial changes in biochemical composition and progressive loss of structural integrity are hallmarks of IVD degeneration [3][15] (as illustrated in Figure 1, where curved arrows define the transition from normal disc structure to later degenerative disc), which occurs mostly in adults aged over 30 years in one or more discs or during trauma and injury. Loss of proteoglycans and a decrease in the ratio of proteoglycan to collagen [5][17] consequently results in the loss of hydrostatic properties, which induces structural wear of the IVD [10][21] and thus progresses towards a fibrotic nature. Dehydration of NP and gradual disappearance of the NP–AF border contributes to the loss of normal architecture. Stress distribution over the NP tends to reduce at the center and accumulates more pressure around the periphery, effectively disabling the NP’s load transfer function [11][22]. Due to a lack of intradiscal pressure, the load absorption and transmission in such dehydrated discs is significantly altered and subsequently, it results in disc-height reduction, osteophyte formation, facet joint arthritis, and deformation of vertebral bodies [12][23]. With continuing degeneration, the structural deficit is accompanied by leakage of the central NP material through cracks in the AF into the periphery. This results in immune cell activation, thereby evoking chronic back pain [13][14][24,25]. Since biochemical changes within IVDs have not yet been directly associated with chronic back pain, it is difficult to determine if the observed changes are due to aging or pathology [15][26].
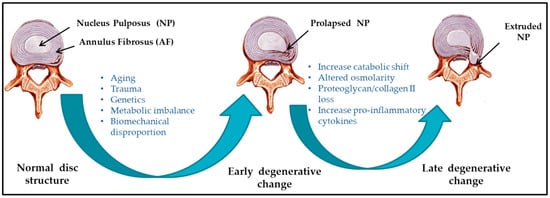
Figure 1.
Schematic illustration of intervertebral disc (IVD) pathophysiology during degeneration.
IVD degeneration often results in lower back pain but is not always the only causative factor. Location of the affected disc, degree of nerve damage, and amount of pressure on the spinal column contribute to define the degree of degeneration. For example, some patients may not feel pain, while others with similar degrees and extents of IVD damage may experience chronic back pain. Therefore, the degree and extent of degeneration does not correlate with the degree of pain. IVD degeneration is the most common cause of lower back pain [16][27]. The worldwide prevalence of chronic back pain is approximate 60%, with the majority seen in the elderly [17][28].
2. Nucleus pulposus (NP) Repair
Tissue engineering approaches over the past few years have been addressing the objective to restore functional and structural features of the healthy IVD. Reparative treatment mainly targets intervention at early stages of IVD degeneration to restore extracellular matrix (ECM) homeostasis, control inflammation, and prevent angiogenesis [18][2]. Current surgical procedures mainly focus on alleviating symptoms associated with IVD degeneration but fail to promote tissue remodeling. Tissue engineering offers an alternative to design biomaterials by encompassing cells and growth factors that will aid IVD tissue regeneration. Thereby, it offers multiple strategies to prevent and possibly cure IVD degeneration by encouraging disc repair. The exact mechanisms of IVD regeneration are still not known, however several studies have focused on the effect of segmental distraction in IVD disease [19][20][21][11,12,52]. Synthetic and / or natural material based scaffolds for IVD tissue engineering were regarded as the prominent method over the past decades [22][23][53,54]. In spite of considerable progress, some issues related to scaffold integration and tissue repair still remained unsolved [24][55]. Alternatively, scaffold free tissue engineering (refer Table 1) is an emerging field, where cells, growth factors, or peptide delivery are mainly responsible for regaining the tissue integrity upon the application [25][26][56,57]. Stimulatory factors together with cells either unaided or together with biomaterials have aimed to provide suitable repair site to ensure maximum cell differentiation or deposition of appropriate ECM. Nonetheless, selection of biomaterials, cells and appropriate stimulatory factors is crucial as the ideal combination is yet to be established.Table 1. Advantages and disadvantages of scaffold-free IVD tissue engineering.
| Methods | Categories | Advantages | Disadvantages/Limitations | References |
|---|---|---|---|---|
| Cell therapy | NP cells |
|
|
[27][28][29][30][88,89,90,91] |
| MSCs |
|
|
[31][32][33][34][35][36][37][38][39][94,95,96,97,98,99,100,101,102] | |
| Growth factors | TGF-β | Enhances cartilage formation and extracellular matrix production |
|
[40][41][58,77] |
| BMP2 | Enhances ECM production and phenotypic characteristics of NP cells | Induces apoptosis, Col I accumulation, and aggrecan-production hindrance | [42][43][44][45][46][47][60,61,62,63,64,65] | |
| BMP7 | Promotes proliferation and accelerates chondrogenesis | Short half-life time and biodegradation in vivo | [48][49][50][51][52][53][54][55][66,67,68,69,70,71,72,73] | |
| GDF-5 | Induces NP-like differentiation of MSCs | Possible association between GDF-5 gene polymorphisms and IDD | [56][57][58][74,75,76] | |
| IGF-1 | Enhances the ECM production and proliferation of IVD cells | Enhances glucose consumption and lactate concentration | [59][60][78,79] | |
| Injectable hydrogel | Cell-free hydrogel | Physiological swelling and greasing | Limited payload | [61][62][51,110] |
| Cell-seeded hydrogel |
|
No direct cell contact | [63][64][65][66][111,112,113,114] |
MSC: mesenchymal stem cell; BMP: bone morphogenetic protein; GDF: growth and differentiation factor; IGF: insulin-like growth factor; ECM: extracellular matrix; IDD: intervertebral disc degeneration.
3. Cell-Based Therapies
Cell based therapy for IVD nucleus repair mainly aims for NP like cells injections, addressing the imbalance of biochemical environment (proteoglycan synthesis and water content). Stimulation of the residing cells is insufficient to achieve tissue repair; hence, diseased phenotype properties of the native NP cells certainly limit their use. Thus, injecting functional cells aimed to overcome this problem by compensating cell death and disc shrinkage [61][67][51,86]. Concomitantly, Abbott et al. proposed NP cells do possess regeneration potential even in severe status of degeneration [68][87]. Several cell-based strategies have been investigated to retard NP degeneration, using different IVD model system [27][28][29][30][88,89,90,91], with NP and/or AF cells, articular chondrocytes, or mesenchymal stem/stromal cells (MSCs). Feng et al. summarized GDF-5 a suitable growth factor for inducing NP-like cells based on its positive effect on the differentiation of MSCs towards an NP-like phenotype [69][92]. The effect of growth factors such as TGF-β, IGF-1, FGF-2, PDGF and GDF-5 on the differentiation of MSCs into NP-like cells have been investigated [69][92]. Fundamentally, the transplanted MSCs are expected to differentiate, maintain and enhance the function of existing NP cells to reverse the IDD. Thus, preclinical studies will be required to confirm the functional potency of the MSC-based therapy for IDD. Good accessibility and the ability of MSCs (bone marrow, adipose and synovial tissue derived) to differentiate into different cell types including chondrocyte like cells, promoted MSCs as prime source for cell therapies for several diseases and in IVD regenerative treatment [32][33][95,96]. Intradiscal delivery of bone marrow and adipose derived MSCs has demonstrated to promote regeneration by maintaining cell viability and proliferation, obtaining IVD-like phenotypes, and providing expression of typical chondrocyte markers, in several studies using rabbit, rat, dog, and goat models [70][34][35][36][37][9,97,98,99,100]. Nevertheless, one common problem affecting the healing satisfaction is the tendency of hypertrophic differentiation of MSCs [38][39][101,102]. How to achieve an ideal IVD repair mainly relies on manipulation of hypertrophic chondrogenesis of the injected and/or implanted MSCs.
In parallel, the use of notochordal cells is also being considered, no matter from allogenic, autologous or xenogenic origin [71][72][73][103,104,105]. Risbud et al. has previously proposed that morphology and size variation correlates to different stages of maturation and/or function of NP cells derived from notochordal precursors [74][106]. Moreover, it has been speculated that degeneration is due to the selective loss of the notochordal cells fraction while considering overall reduction in the structural and functional activity of IVD cells. While there is considerable agreement, Bach et al. demonstrated the species (human, canine and porcine) specific regenerative effects of notochordal cell conditioned medium on human NP cells. It further confirmed the canine and porcine secreted factors exerted regenerative effect on human NP cells [75][107]. The fact that NP cells with diseased phenotype possess regeneration potential, allows autologous NP cells to be expanded using conditioned medium that these cells can be used as a source for cell therapy during nucleus repair. Likewise, stem cells can be differentiated towards NP like cell type. van Uden et al. has already addressed [76][48] the importance of hypoxia during NP repair. This key factor may restrict the success of the stem cell based therapy, as stem cells tend to die due to lack of oxygen. Serigano et al. demonstrated the effect of cell number on mesenchymal stem cell transplantation in canine disc degeneration model, where they showed high possibility of apoptosis, low cell viability, while maintaining microenvironment during stem cell transplantation [77][108]. Altogether, it affected the regeneration capability. However, in order to advance clinical translation of cell therapy, assessment of in vivo integration (in terms of functional and mechanical repair) in large animals is necessary. Therefore, a comparative analysis of cell types and sources of cells using large animal models is essential to enlighten the suitable strategy.
4. Injectable Hydrogels
Synthetic or biologically based injectable materials are largely focused on NP replacement, to stabilize and restore the function and structure of the discs and AF. Moreover, the biomaterial must satisfy the biomechanical strength with no migration and displacement, and durability with high wear resistance provoking low immune response. Injectable hydrogel over the solid-state scaffold opens up novel approaches in musculoskeletal application. Hydrogels mainly composed of natural polysaccharides (chitosan, chondroitin sulfate, hyaluronic acid), proteins (silk, resilin), or synthetic polymers (polyvinyl alcohol (PVA), polyacrylic acid, acrylamide), are emergent matrix substitutes in cartilage and IVD regeneration [78][109]. They are hydrophilic in nature, and have a water retention capacity between 20% and 99% by weight when placed in aqueous conditions. Therefore, these water soluble polymers are often used to build scaffolds by three-dimensional crosslinking either by covalent or physical methods. Hydrogels (depending on the physical structure and chemical composition) that mimic the mechanical stability and matrix composition of native IVD ECM, are a potential promising choice for IVD repair. Cell-free hydrogels and cell-seeded hydrogels are the two broad subtypes of injectable scaffolds found in literature [62][63][110,111].
Theoretically, biodegradability of some of the scaffolds allows remodeling of the scaffold in the regeneration process, while other scaffolds mechanically support and resist the compressive load for longer duration. For example, alginate and lyophilized chitosan gelatin scaffolds showed cytocompatibility towards the NP like cells, supporting cell growth. Li et al. stimulated NP cells using BMP-2 and BMP-7 heterodimer combined with fibrinhyaluronan hydrogel [64][112]. Their in vitro as well as ex vivo study demonstrated to produce aggrecan and collagen II by NP cells upon delivery, simulating in vivo conditions [64][112]. Moreover, cell numbers were found to be increased in alginate compare to lyophilized chitosan-gelatin based scaffolds, after 21 days of cell culture [61][51]. Hydrogels on the other hand resemble NP material, mainly due to their resilient and hydrophilic properties. Recently, several studies [30][62][63][65][66][91,110,111,113,114] have investigated the combination of cells and hydrogels to catalyze the tissue repair. Homogenous distribution of cells within defect size prior to gelation tends to support tissue repair [61][51]. To allow the sustainable effect of the growth factor Yan et al. administered the injection of poly (lactic-co-glycolic acid) (PLGA) microspheres loaded with recombinant human GDF-5 into a rat caudal disc degeneration model induced by needle puncture [79][115]. Sustained release of active GDF-5 for more than 42 days confirmed its therapeutic efficacy. Further Frith et al. [66][114] conducted a study examining the composite of injectable hydrogels (polyethylene glycol, hyaluronic acid, and pentosanpolysulphate) coupled with MSCs. In vivo examination of this scaffold composite together MSCs in rats, certainly supported the cartilage like tissue formation, thereby confirming the deposition of collagen II. However, there is a need to identify relevant combination of biomolecules and hydrogel material that may direct NP cell survival in vivo, in the challenging mechanical and physical microenvironment of the NP. While doing so, the biomaterial should withstand the mechanical load which ultimately contribute to the longevity of the scaffold.
