| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robert Sitarz | + 1707 word(s) | 1707 | 2020-06-08 08:13:42 | | | |
| 2 | Rita Xu | -631 word(s) | 1076 | 2020-06-22 10:29:44 | | | | |
| 3 | Rita Xu | -7 word(s) | 1069 | 2020-10-27 04:33:58 | | |
Video Upload Options
Gastric cancer(GC) is the fourth most common cause of cancer-related mortalities worldwide. The incidence rate of GC rises progressively with age, the median age at diagnosis is 70 years. GC is a multifactorial disease, and both environmental and genetic factors have a role in its aetiology. Regarding the age of diagnosis, it is divided into early-onset gastric carcinoma (EOGC; 45 years or younger) and conventional GC (CGC; older than 45). EOGC relies mostly on genetic factors and therefore provides a unique model to study genetic alterations in GCs.
1. Classification Systems in Gastric Cancer
In 1965 the Lauren classification of GC was established, and nowadays it is the most frequently used, compared to other available GC classifications [1]. According to the Lauren division, two histological subtypes of GC can be distinguished—intestinal and diffuse; later the indeterminate type was also included to characterize infrequent histology. Signet ring cell carcinoma is assigned to the diffuse subtype. Multiple studies have shown that the intestinal type is the most common, the second is diffuse and ending with the indeterminate type [2]. Intestinal carcinoma is characterized by visible glands and cohesion between tumour cells. The diffuse subtype encompasses poorly cohesive cells, diffusely infiltrating the gastric wall with little or no gland formation. The cells are usually small and round, also with a signet ring cell formation. There is evidence that the intestinal subtype is associated with intestinal metaplasia of the gastric mucosa and the occurrence of H. pylori infection. Some studies also revealed that the incidence of the diffuse GC subtype is higher among females and younger patients, and that this type of GC originates from the normal gastric mucosa [2].
The World Health Organization (WHO) classification issued in 2010 is perceived to be the most detailed among all classification systems. The WHO classification, apart from stomach adenocarcinomas, also describes other types of gastric tumors with decreased attendance [3]. The gastric adenocarcinoma type includes multiple subgroups, like tubular, mucinous, papillary and mixed carcinoma, which are similar to the indeterminate type according to the Lauren classification system. The poorly cohesive carcinoma type contains the signet ring cell carcinoma. The remainder of the classified gastric adenocarcinomas are described as uncommon, mainly because of their low clinical importance. Following the WHO classification, the most common GC subtype is tubular adenocarcinoma, then the papillary and mucinous types. The signet ring cell carcinoma encompasses around 10% of GCs and is described by the occurrence of signet ring cells in over 50% of the tumor [2][3][4][5].
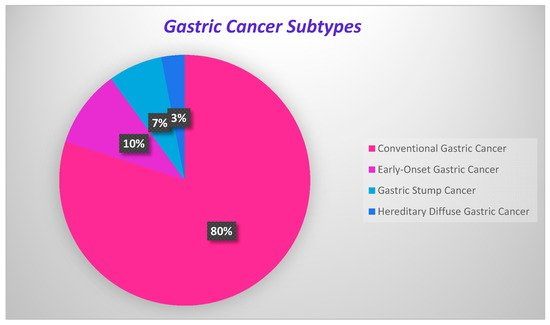
GC development onsets are present in Figure 1, where the percentage of each carcinoma is displayed.
Figure 1. Onsets of gastric cancer development.
2. Conventional Gastric Cancer
Gastric carcinomas that appear intermittently mostly occur among the older population, at over 45 years of age, and are so-called “conventional gastric cancers”. The genetic factors that cause cancer development are less important in this type of cancer, where environmental agents are prevalent [6]. Patients are diagnosed between 60 and 80 years of age. These gastric carcinomas affect mostly men, who are two times more likely to develop them than women [7][8].
3. Early-Onset Gastric Cancer
Early-onset gastric cancer (EOGC) is described as a GC occurring at the age of 45 years or younger. Around 10% of GCs are categorized as EOGCs, however rates differ between 2.7% and 15%, depending on the performed cohort studies [9]. In the young population, diffuse lesions are more frequent and they are related to the background of the histologically “normal” gastric mucosa. Young patients are less exposed to environmental carcinogens, therefore, an EOGC is a good model to study genetic alterations in the gastric carcinogenesis process [10]. H. pylori infection is important for the development of tumors in EOGC patients, however, there is no statistically significant difference in the distribution of IL1β polymorphisms between young and old patients [11]. EBV infection is observed to be importantly decreased or absent in EOGCs [12]. It is postulated that around 10% of EOGCs have a positive family history [13]. It has been revealed that the early-onset type has different clinicopathological characteristics compared to the conventional subtype, which suggests that they display separate models within gastric carcinogenesis, and molecular patterns support this [14].
4. Gastric Stump Cancer
Gastric stump cancer (GSC) is described as a carcinoma in the gastric remnant after partial gastric resection, usually due to peptic ulcer disease (PUD). The incidence of GSC varies from 1% to 8% [15]. The major pathogenesis of GSC is biliary pancreatic reflux, provoking chronic inflammation of the mucosa, followed by atrophic gastritis, intestinal metaplasia and dysplasia. Other possible causes are achlorhydria and bacteria overgrowth, and H. pylori seem to be the main agent included in the etiopathogenesis of the GSC [16]. The surveillance of these patients with endoscopy and biopsies might allow for the early diagnosis of these patients, however, the benefit to cost ratio is still to be considered. Viste et al. (1986), made a comparison of GSC patients with other GC patients and discovered relevant differences in gender, age, staging, resectability rates and operative procedures, however, the postoperative mortality and survival rates were approximate [17].
5. Hereditary Diffuse Gastric Cancer
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant susceptibility for diffuse GC, a weakly differentiated adenocarcinoma that penetrates into the stomach wall leading to the thickening of the wall, usually without producing an explicit mass. The median age of HDGC onset is around 38 years, with a range of 14–69 years [18]. HDGC should be considered for screening with several important symptoms, like two or more documented cases of diffuse GC in first- or second-degree relatives, with at least one diagnosed before the age of 50, or three or more cases of documented diffuse gastric cancer in first/second-degree relatives, independent of the age of onset [19]. When clinical characteristics and family history are insufficient, the identification of a heterozygous germline CDH1 pathogenic variant using screening with available genetic tests checks out the diagnosis and enables for family research [20][21].
Among CDH1 mutation-negative patients within HDGC families, there were displayed candidate mutations within genes of high and moderate penetrance, like: BRCA2, STK11, ATM, SDHB, PRSS1, MSR1, CTNNA1 and PALB2 [22]. Therefore, in HDGC families, with no detected alterations in the CDH1 gene, the clinical importance of other tumor suppressor genes, like CTNNA1, should be considered. CTNNA1 is concerned in intercellular adhesion and is a questionable tumor suppressor gene for HDGC. The group discovered a novel variant (N1287fs) in the BRCA2 gene, which is the first report of the occurrence of a truncating BRCA2 variant among HDGC families. That is why it is important to consider HDGC syndrome as associated to CDH1 mutations and closely related genes, then consider the clinical criteria of families with heterogeneous susceptibility profiles.
References
- P Lauren; THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathologica Microbiologica Scandinavica 1965, 64, 31–49.
- Bing Hu; Nassim El Hajj; Scott Sittler; Nancy Lammert; Robert Barnes; Aurelia Meloni-Ehrig; Gastric cancer: Classification, histology and application of molecular pathology. Journal of Gastrointestinal Oncology 2012, 3, 251-261, 10.3978/j.issn.2078-6891.2012.021.
- Fléjou, J.F; WHO Classification of digestive tumors: The fourth edition. Pathol. 2011, 31, S27–S31.
- Mario Sarbia; Pathology of upper gastrointestinal malignancies. Seminars in Oncology 2004, 31, 465-475, 10.1053/j.seminoncol.2004.04.020.
- Martin Werner; Karl Becker; Gisela Keller; Heinz Höfler; Gastric adenocarcinoma: pathomorphology and molecular pathology. Journal of Cancer Research and Clinical Oncology 2001, 127, 207-216, 10.1007/s004320000195.
- Kikuchi, S.; Nakajima, T.; Nishi, T.; Kobayashi, O.; Konishi, T.; Inaba, Y.; Wada, O.; Satou, H.; Ishibashi, T.; Ichikawa, S.; et al.et al Association between Family History and Gastric Carcinoma among Young Adults. J. Cancer Res. 1996, 87, 332–336.
- D. Forman; Victoria Burley; Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Practice & Research Clinical Gastroenterology 2006, 20, 633-649, 10.1016/j.bpg.2006.04.008.
- P Correa; Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Research 1992, 52, 6735–6740.
- Anya N Milne; G Johan A Offerhaus.; Early-onset gastric cancer: Learning lessons from the young. World Journal of Gastrointestinal Oncology 2010, 2, 59-64, 10.4251/wjgo.v2.i2.59.
- Małgorzata Skierucha; Anya Na Milne; G Johan A Offerhaus; Wojciech P Polkowski; Ryszard Maciejewski; Robert Sitarz; Molecular alterations in gastric cancer with special reference to the early-onset subtype. World Journal of Gastroenterology 2016, 22, 2460-2474, 10.3748/wjg.v22.i8.2460.
- Anya N Milne; Robert Sitarz; Ralph Carvalho; Fatima Carneiro; G. Johan A. Offerhaus; Early onset gastric cancer: on the road to unraveling gastric carcinogenesis. Current Molecular Medicine 2007, 7, 15-28, 10.2174/156652407779940503.
- Ralph Carvalho; Anya Na Milne; Bastiaan P Van Rees; Eric Caspers; Luís Cirnes; Ceu Figueiredo; G Johan A Offerhaus; Marian Aj Weterman; Early-onset gastric carcinomas display molecular characteristics distinct from gastric carcinomas occurring at a later age. The Journal of Pathology 2004, 204, 75-83, 10.1002/path.1602.
- Kokkola, A.; Sipponen, P; Gastric carcinoma in young adults. Hepatogastroenterology 2002, 48, 1552–1555.
- Anya N A Milne; Ralph Carvalho; Folkert M Morsink; Alex R Musler; Wendy W J De Leng; Ari Ristimäki; G Johan A Offerhaus; Early-onset gastric cancers have a different molecular expression profile than conventional gastric cancers. Modern Pathology 2006, 19, 564-572, 10.1038/modpathol.3800563.
- C. Toftgaard; Gastric Cancer After Peptic Ulcer Surgery. Annals of Surgery 1989, 210, 159-164, 10.1097/00000658-198908000-00004.
- Sinning, C.; Schaefer, N.; Standop, J.; Hirner, A.; Wolff, M; Gastric stump carcinoma—Epidemiology and current concepts in pathogenesisand treatment. J. Surg. Oncol. 2007, 33, 133–139.
- A Viste; E Bjørnestad; P Opheim; A Skarstein; J Thunold; F Hartveit; G E Eide; T J Eide; O Søreide; Risk of carcinoma following gastric operations for benign disease. A historical cohort study of 3470 patients. The Lancet 1986, 2, 502–505.
- Wenyi Luo; Faysal Fedda; Patrick Lynch; Dongfeng Tan; CDH1 Gene and Hereditary Diffuse Gastric Cancer Syndrome: Molecular and Histological Alterations and Implications for Diagnosis And Treatment. Frontiers in Pharmacology 2018, 9, 1421, 10.3389/fphar.2018.01421.
- Carlo La Vecchia; Eva Negri; Antonella Gentile; Silvia Franceschi; Family history and the risk of stomach and colorectal cancer. Cancer 1992, 70, 50-55, 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i.
- Kaurah, P.; Huntsman, D.G. Hereditary Diffuse Gastric Cancer. In GeneReviews [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2019.
- Parry Guilford; Vanessa Blair; Helen More; Bostjan Humar; A short guide to hereditary diffuse gastric cancer. Hereditary Cancer in Clinical Practice 2007, 5, 183-194, 10.1186/1897-4287-5-4-183.
- Hansford, S.; Kaurah, P.; Li-Chang, H.; Woo, M.; Senz, J.; Pinheiro, H.; Schrader, K.A.; Schaeffer, D.F.; Shumansky, K.; Zogopoulos, G.; et al.et al Hereditary Diffuse Gastric Cancer Syndrome. JAMA Oncol. 2015, 1, 23–32.