Gastric cancer(GC) is the fourth most common cause of cancer-related mortalities worldwide. The incidence rate of GC rises progressively with age, the median age at diagnosis is 70 years. GC is a multifactorial disease, and both environmental and genetic factors have a role in its aetiology. Regarding the age of diagnosis, it is divided into early-onset gastric carcinoma (EOGC; 45 years or younger) and conventional GC (CGC; older than 45). EOGC relies mostly on genetic factors and therefore provides a unique model to study genetic alterations in GCs.
- gastric cancer
- early-onset gastric cancer
- conventional gastric cancer
1. Classification Systems in Gastric Cancer
Classification Systems in Gastric Cancer
In 1965 the Lauren classification of GC was established, and nowadays it is the most frequently used, compared to other available GC classifications [1]. According to the Lauren division, two histological subtypes of GC can be distinguished—intestinal and diffuse; later the indeterminate type was also included to characterize infrequent histology. Signet ring cell carcinoma is assigned to the diffuse subtype. Multiple studies have shown that the intestinal type is the most common, the second is diffuse and ending with the indeterminate type [2]. Intestinal carcinoma is characterized by visible glands and cohesion between tumour cells. The diffuse subtype encompasses poorly cohesive cells, diffusely infiltrating the gastric wall with little or no gland formation. The cells are usually small and round, also with a signet ring cell formation. There is evidence that the intestinal subtype is associated with intestinal metaplasia of the gastric mucosa and the occurrence of
H. pylori infection. Some studies also revealed that the incidence of the diffuse GC subtype is higher among females and younger patients, and that this type of GC originates from the normal gastric mucosa [2].
infection. Some studies also revealed that the incidence of the diffuse GC subtype is higher among females and younger patients, and that this type of GC originates from the normal gastric mucosa [2].
The World Health Organization (WHO) classification issued in 2010 is perceived to be the most detailed among all classification systems. The WHO classification, apart from stomach adenocarcinomas, also describes other types of gastric tumors with decreased attendance [3]. The gastric adenocarcinoma type includes multiple subgroups, like tubular, mucinous, papillary and mixed carcinoma, which are similar to the indeterminate type according to the Lauren classification system. The poorly cohesive carcinoma type contains the signet ring cell carcinoma. The remainder of the classified gastric adenocarcinomas are described as uncommon, mainly because of their low clinical importance. Following the WHO classification, the most common GC subtype is tubular adenocarcinoma, then the papillary and mucinous types. The signet ring cell carcinoma encompasses around 10% of GCs and is described by the occurrence of signet ring cells in over 50% of the tumor [2,3 ,4 ,5].
The World Health Organization (WHO) classification issued in 2010 is perceived to be the most detailed among all classification systems. The WHO classification, apart from stomach adenocarcinomas, also describes other types of gastric tumors with decreased attendance [3]. The gastric adenocarcinoma type includes multiple subgroups, like tubular, mucinous, papillary and mixed carcinoma, which are similar to the indeterminate type according to the Lauren classification system. The poorly cohesive carcinoma type contains the signet ring cell carcinoma. The remainder of the classified gastric adenocarcinomas are described as uncommon, mainly because of their low clinical importance. Following the WHO classification, the most common GC subtype is tubular adenocarcinoma, then the papillary and mucinous types. The signet ring cell carcinoma encompasses around 10% of GCs and is described by the occurrence of signet ring cells in over 50% of the tumor [2][3][4][5].
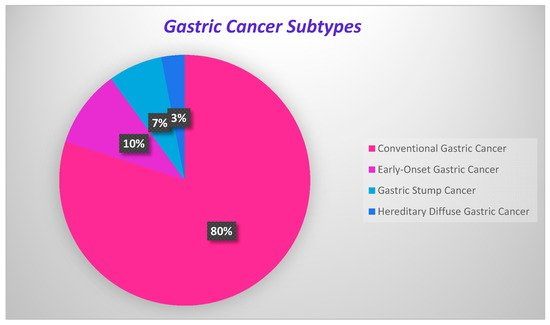
GC development onsets are present in Figure 1, where the percentage of each carcinoma is displayed.
Figure 1. Onsets of gastric cancer development.
Onsets of gastric cancer development.
2. Conventional Gastric Cancer
Conventional Gastric Cancer
Gastric carcinomas that appear intermittently mostly occur among the older population, at over 45 years of age, and are so-called “conventional gastric cancers”. The genetic factors that cause cancer development are less important in this type of cancer, where environmental agents are prevalent [6]. Patients are diagnosed between 60 and 80 years of age. These gastric carcinomas affect mostly men, who are two times more likely to develop them than women [7][8].
Gastric carcinomas that appear intermittently mostly occur among the older population, at over 45 years of age, and are so-called “conventional gastric cancers”. The genetic factors that cause cancer development are less important in this type of cancer, where environmental agents are prevalent [6]. Patients are diagnosed between 60 and 80 years of age. These gastric carcinomas affect mostly men, who are two times more likely to develop them than women [7,8].
3. Early-Onset Gastric Cancer
Early-Onset Gastric Cancer
Early-onset gastric cancer (EOGC) is described as a GC occurring at the age of 45 years or younger. Around 10% of GCs are categorized as EOGCs, however rates differ between 2.7% and 15%, depending on the performed cohort studies [9]. In the young population, diffuse lesions are more frequent and they are related to the background of the histologically “normal” gastric mucosa. Young patients are less exposed to environmental carcinogens, therefore, an EOGC is a good model to study genetic alterations in the gastric carcinogenesis process [10].
H. pylori
infection is important for the development of tumors in EOGC patients, however, there is no statistically significant difference in the distribution of
IL1β
polymorphisms between young and old patients [11].
EBV
infection is observed to be importantly decreased or absent in EOGCs [12]. It is postulated that around 10% of EOGCs have a positive family history [13]. It has been revealed that the early-onset type has different clinicopathological characteristics compared to the conventional subtype, which suggests that they display separate models within gastric carcinogenesis, and molecular patterns support this [14].
4. Gastric Stump Cancer
Gastric Stump Cancer
Gastric stump cancer (GSC) is described as a carcinoma in the gastric remnant after partial gastric resection, usually due to peptic ulcer disease (PUD). The incidence of GSC varies from 1% to 8% [15]. The major pathogenesis of GSC is biliary pancreatic reflux, provoking chronic inflammation of the mucosa, followed by atrophic gastritis, intestinal metaplasia and dysplasia. Other possible causes are achlorhydria and bacteria overgrowth, and
H. pylori
seem to be the main agent included in the etiopathogenesis of the GSC [16]. The surveillance of these patients with endoscopy and biopsies might allow for the early diagnosis of these patients, however, the benefit to cost ratio is still to be considered. Viste et al. (1986), made a comparison of GSC patients with other GC patients and discovered relevant differences in gender, age, staging, resectability rates and operative procedures, however, the postoperative mortality and survival rates were approximate [17].
5. Hereditary Diffuse Gastric Cancer
Hereditary Diffuse Gastric Cancer
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant susceptibility for diffuse GC, a weakly differentiated adenocarcinoma that penetrates into the stomach wall leading to the thickening of the wall, usually without producing an explicit mass. The median age of HDGC onset is around 38 years, with a range of 14–69 years [18]. HDGC should be considered for screening with several important symptoms, like two or more documented cases of diffuse GC in first- or second-degree relatives, with at least one diagnosed before the age of 50, or three or more cases of documented diffuse gastric cancer in first/second-degree relatives, independent of the age of onset [19]. When clinical characteristics and family history are insufficient, the identification of a heterozygous germline
CDH1 pathogenic variant using screening with available genetic tests checks out the diagnosis and enables for family research [20][21].
pathogenic variant using screening with available genetic tests checks out the diagnosis and enables for family research [20, 21].
Among
CDH1
mutation-negative patients within HDGC families, there were displayed candidate mutations within genes of high and moderate penetrance, like:
BRCA2
,
STK11
,
ATM
,
SDHB
,
PRSS1
,
MSR1
,
CTNNA1
and
PALB2
[22]. Therefore, in HDGC families, with no detected alterations in the
CDH1
gene, the clinical importance of other tumor suppressor genes, like
CTNNA1
, should be considered
. CTNNA1
is concerned in intercellular adhesion and is a questionable tumor suppressor gene for HDGC. The group discovered a novel variant (
N1287fs
) in the
BRCA2
gene, which is the first report of the occurrence of a truncating
BRCA2
variant among HDGC families. That is why it is important to consider HDGC syndrome as associated to
CDH1 mutations and closely related genes, then consider the clinical criteria of families with heterogeneous susceptibility profiles.
mutations and closely related genes, then consider the clinical criteria of families with heterogeneous susceptibility profiles.
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand.1965, 64, 31–49. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. Gastrointest. Oncol.2012, 3, 251–261. [Google Scholar] [PubMed]
- Fléjou, J.F. WHO Classification of digestive tumors: The fourth edition. Pathol.2011, 31, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Sarbia, M. Pathology of upper gastrointestinal malignancies. Oncol.2004, 31, 465–475. [Google Scholar] [CrossRef]
- Werner, M.; Becker, K.; Keller, G.; Höfler, H. Gastric adenocarcinoma: Pathomorphology and molecular pathology. Cancer Res. Clin. Oncol.2001, 127, 207–216. [Google Scholar] [CrossRef]
- Kikuchi, S.; Nakajima, T.; Nishi, T.; Kobayashi, O.; Konishi, T.; Inaba, Y.; Wada, O.; Satou, H.; Ishibashi, T.; Ichikawa, S.; et al. Association between Family History and Gastric Carcinoma among Young Adults. J. Cancer Res.1996, 87, 332–336. [Google Scholar] [CrossRef]
- Forman, D.; Burley, V. Gastric cancer: Global pattern of the disease and an overview of environmental risk factors. Best Pract. Res. Clin. Gastroenterol.2006, 20, 633–649. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res.1992, 52, 6735–6740. [Google Scholar]
- Milne, A.N.; Offerhaus., G.J.A. Early-onset gastric cancer: Learning lessons from the young. World J. Gastrointest. Oncol.2010, 2, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Skierucha, M.; Milne, A.N.; Offerhaus, G.J.A.; Polkowski, W.P.; Maciejewski, R.; Sitarz, R. Molecular alterations in gastric cancer with special reference to the early-onset subtype. World J. Gastroenterol.2016, 22, 2460–2474. [Google Scholar] [CrossRef]
- Milne, A.N.; Sitarz, R.; Carvalho, R.; Carneiro, F.; Offerhaus, G.J.A. Early onset gastric cancer: On the road to unraveling gastric carcinogenesis. Mol. Med.2007, 7, 15–28. [Google Scholar] [CrossRef]
- Carvalho, R.; Milne, A.N.; Van Rees, B.P.; Caspers, E.; Cirnes, L.; Figueiredo, C.; Offerhaus, G.J.A.; Weterman, M.A. Early-onset gastric carcinomas display molecular characteristics distinct from gastric carcinomas occurring at a later age. Pathol.2004, 204, 75–83. [Google Scholar] [CrossRef]
- Kokkola, A.; Sipponen, P. Gastric carcinoma in young adults. Hepatogastroenterology2002, 48, 1552–1555. [Google Scholar]
- Milne, A.N.; Carvalho, R.; Morsink, F.M.; Musler, A.R.; De Leng, W.W.; Ristimäki, A.; Offerhaus, G.J.A. Early-onset gastric cancers have a different molecular expression profile than conventional gastric cancers. Pathol.2006, 19, 564–572. [Google Scholar] [CrossRef]
- Toftgaard, C. Gastric Cancer after Peptic Ulcer Surgery. Surg.1989, 210, 159–164. [Google Scholar] [CrossRef]
- Sinning, C.; Schaefer, N.; Standop, J.; Hirner, A.; Wolff, M. Gastric stump carcinoma—Epidemiology and current concepts in pathogenesisand treatment. J. Surg. Oncol.2007, 33, 133–139. [Google Scholar] [CrossRef]
- Viste, A.; Bjørnestad, E.; Opheim, P.; Skarstein, A.; Thunold, J.; Hartveit, F.; Eide, G.E.; Eide, T.J.; Søreide, O. Risk of carcinoma following gastric operations for benign disease. A historical cohort study of 3470 patients. Lancet1986, 2, 502–505. [Google Scholar] [CrossRef]
- Luo, W.; Fedda, F.; Lynch, P.; Tan, D. CDH1 Gene and Hereditary Diffuse Gastric Cancer Syndrome: Molecular and Histological Alterations and Implications for Diagnosis and Treatment. Pharmacol.2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Negri, E.; Gentile, A.; Franceschi, S. Family history and the risk of stomach and colorectal cancer. Cancer1992, 70, 50–55. [Google Scholar] [CrossRef]
- Kaurah, P.; Huntsman, D.G. Hereditary Diffuse Gastric Cancer. In GeneReviews [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2019. [Google Scholar]
- Guilford, P.; Blair, V.; More, H.; Humar, B. A short guide to hereditary diffuse gastric cancer. Cancer Clin. Pr.2007, 5, 183–194. [Google Scholar] [CrossRef]
- Hansford, S.; Kaurah, P.; Li-Chang, H.; Woo, M.; Senz, J.; Pinheiro, H.; Schrader, K.A.; Schaeffer, D.F.; Shumansky, K.; Zogopoulos, G.; et al. Hereditary Diffuse Gastric Cancer Syndrome. JAMA Oncol.2015, 1, 23–32. [Google Scholar] [CrossRef]