
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mirna Velki | + 2749 word(s) | 2749 | 2021-10-12 06:21:04 | | | |
| 2 | Catherine Yang | Meta information modification | 2749 | 2021-10-25 09:58:55 | | |
Video Upload Options
Vermicomposting is a process that has been intensively studied for years and has become significant and frequent topic. Numerous papers are describing its mechanism and performance, but each highlights different aspects. Although numerous studies have been conducted on the topic of vermicomposting and each of them has contributed to understanding the role of vermicompost, there are still unknowns that need to be additionally explored to maximize the potential of vermicompost and to recoup the process itself according to specific needs.
1. Introduction
Over the last few decades’ numerous human activities have led to an increased accumulation of waste materials. Therefore, waste management has become an important topic worldwide [1]. When waste materials are discussed, mostly solid waste (SW) is referred to. The overall objective SW management is to deal with waste in an environmentally and economically sustainable way [2]. According to the literature, about 2.01 billion metric tons of solid waste are produced annually, and it is estimated that this number will increase to 3.40 billion metric tons by 2050 [3]. SW includes organic and inorganic materials produced by different sources. There are numerous classifications of SW that are also complex, but research on domestic waste [4], municipal solid waste [5], sewage waste [6], ashes [7], manures [8] and many others in the literature can be found. Global waste, mostly industrial, can also be classified into hazardous and nonhazardous waste [9]. Since the highest percentage is nonhazardous waste, there has been an increasing interest to find an ecofriendly, rapid and financially favorable technique for efficient waste management that is an entry point to sustainable development [10][11].
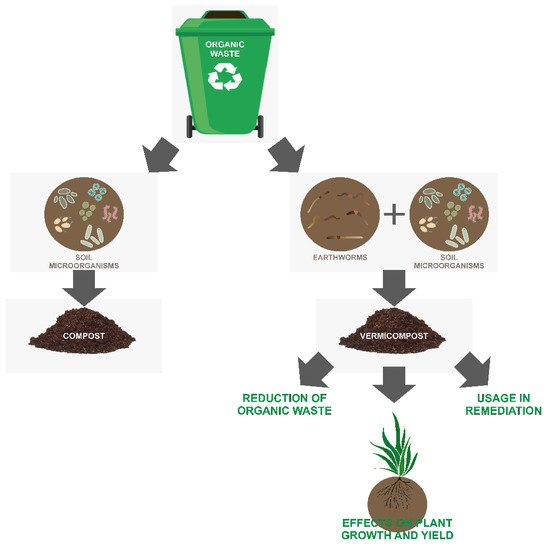
Vermicomposting is a biotechnological process of composting wide ranges of organic waste [12][13][14] that includes specific earthworms’ species that enhance the waste conversion into a very useful high-quality end product known as vermicompost [15][16]. Vermicomposting involves bio-oxidative processes and the stabilization of organic material just as in composting; except in vermicomposting, this includes interactions between earthworms and microorganisms. The role of microorganisms is the production of enzymes that cause the biochemical decomposition of organic matter, while earthworms contribute to a larger microbial population through fragmentation and ingestion of fresh organic material. Besides the above, earthworms also interact with other organisms in the soil and can affect various microflora and microfauna communities [17]. Although vermicomposting and composting have some similarities, there are many significant differences between them, which are highlighted in numerous reviews [18][19][20]. These differences include the lack of the thermophilic phase in vermicomposting during which pathogens are reduced [6], different requirements of moisture content that are higher for vermicomposting and differences of end-product quality where vermicomposting shows more positive effects on the physicochemical properties of the soil and on plant growth [21][22]. The conversion of industrial waste into vermicompost is important for pollution monitoring and controlling, since vermicompost has potential application in remediation and can be used for the reduction of the waste ( Figure 1 ) [23]. Additionally, vermicompost has many beneficial effects on plants including induction of plant growth and yield ( Figure 1 ). Therefore, it is also important for agriculture and horticulture purposes because it is used as fertilizing material [24] but also in terms of sustainable development.
At least 4400 species of earthworms are classified on the basis of their deeding and burrowing strategies into three ecological niches: epigeic, anecic and endogeic. Epigeic species are pigmented, live superficially in the litter layer, form no permanent burrows and feed on decaying organic matter and litter materials; endogeic species live in horizontal burrows at approximately 10–15 cm depth and feed on the organic matter in the soil; and anecic species are relatively large and live in vertical burrows from which they collect dead organic matter on the surface at night [25]. Epigeic species are the most suitable for vermicomposting due to a high affinity for the organic substrate, high rates of consumption and digestion, owing to its tolerance for changes in environmental conditions, short life cycles, high reproductive rates and easy culturing [26]. According to mentioned characteristics, few species such as Eisenia fetida (Bouché), Eisenia andrei (Savigny) and Perionyx excavates (Perrier) have been used extensively in vermicomposting. Among the above and according to the literature reviewed, E. fetida is used more often than others in various studies that include the effect of vermicompost on plant growth, bacterial community size and structure and soil physicochemical properties [27][28][29][30][31]. Domínguez et al. [32] and Gómez-Brandón et al. [33] used E. andrei in elucidating the impact of vermicomposting on changes in the composition and function of bacterial communities. Furthermore, Perionyx excavates is also used extensively in vermicomposting of different materials such as domestic waste [4][14], urban green waste [34] and agriculture waste [14]. Unlike Eisenia fetida and Eisenia andrei which are temperate, Perionyx excavates is the tropical epigeic earthworm.
2. Bacterial Community Structure in Vermicompost
Although earthworms are key players in the vermicomposting process, microorganisms perform the actual biochemical decomposition of organic matter, whether those bacteria are from the soil or the earthworm’s gut. The dependent relationship and synergistic actions between earthworms and microorganisms are unquestionable. Namely, due to earthworms’ physical activities of substrate aeration, mixing and grinding, they increase the available area for a habitation of microorganisms and affect their structure, composition, activity, abundance and growth rate [29][32]. The end product of vermicomposting is rich in diverse microbial communities such as phosphate solubilizers, N 2 fixers [35], enzyme-producing and plant growth-promoting bacteria [36]. In general, bacterial communities in the soil and their activities affect soil properties and other soil organisms and contribute to the nutrients cycling in nature such as carbon, nitrogen and phosphorus.
Even though there are a lot of data about bacterial succession during vermicomposting [37][38], little is known about bacterial communities. Few studies have contributed to clarifying this topic and characterizing the temporal changes in bacterial communities throughout the process [30][32][33][39]. According to Domínguez et al. [32], during vermicomposting of Scotch broom ( Cytisus scoparius ), bacterial communities can be classified in three groups—bacteria present in freshly cut Scotch broom (day 0); bacteria that have recently passed through the intestines of earthworms and been excreted (day 14); and bacteria associated with the cast aging process (days 42 and 91). Their results showed that bacterial composition was split between phylum Proteobacteria, Bacteroidetes, Actinobacteria, Firmicutes and Verrucimicrobia. Proteobacteria were most abundant at the beginning of the process, while after the 14th day, their abundance decreased but still remained significant. On the 14th day, other phyla appeared, but their abundance also differed depending the phase of vermicomposting. Similar results showed by Kolbe et al. [39] in vermicomposting of grape marc for 91 days. Significant changes in bacterial community composition were observed at day 7 until day 91, where taxonomic, phylogenetic and functional diversity increased through experiment. In fresh grape marc, compared with results for a fresh Scotch broom, besides Proteobacteria, Kolbe et al. [39] found a high abundance of Firmicutes. When it comes to bacterial diversity and dynamics of bacterial succession, both the starting substrate and the used earthworm species are crucial [32][40]. In both Scotch broom and grape marc, bacterial diversity in the starting material was relatively low. Even though bacterial diversity is generally low in starting substrates and during the first phase of vermicomposting, it significantly increases during the process [32]. Except for bacterial diversity, earthworms can also have a dual impact on microbial abundance, which also depends on a starting material. Comparing Scotch broom and grape marc with other types of substrate such as manure or sewage, differences in bacterial diversity and abundance are expected. Namely, manure or sewage are substrates that are firstly processed by animals, and that kind of already-diverse substrate has a greater bacterial diversity and higher abundance, while the process of vermicomposting can reduce both [41]. Considering bacterial phylum composition during vermicomposting and its detailed analysis, a clear link can be made between specific bacteria and their respective roles which may explain the beneficial properties of vermicompost. Chitrapriya et al. [35] showed that vermicompost produced from cow dung and saw dust contained Bacillus (Firmicutes), Streptomyces (Actinobacteria) and Pseudomonas sp. (Proteobacteria) as phosphate solubilizers and Azobacter (Proteobacteria) as nitrogen-fixing bacteria. Domínguez et al. [32] detected the genus Devosia (Proteobacteria), which can contribute nitrogen fixation and release plant growth-promoting substances, family Cellulomonodaceae (Actinobacteria) and genus Achromobacter (Proteobacteria), which produce plant cell degrading enzymes.
In most of the abovementioned studies, the same bacterial phylum appears in vermicompost of different substrates with some exceptions in total phylum number, time of their appearance and dominance of the specific phylum. All these differences can be driven by various factors, not just the type of initial substrate and earthworm species used. Changes in microbial communities are also correlated with changes in the organic carbon source, pH value, which can affect nutrient availability, and changes in the physical properties of the substrate, which can promote the growth of aerobic bacteria [30][33].
3. Effect of Vermicompost on Plant Growth and Yield
When generally speaking about improved plant growth and development, in substrate enriched with vermicompost, it is primarily due to the presence of humic acids (HAs) [42][43][44] and different micro- and macro-nutrients [45], which are converted during vermicomposting into more plant-available forms.
Macronutrients such as nitrogen and phosphorus are more available to plants due to N 2 fixers and phosphate solubilizing bacteria [35]. Considering that phosphorus is often one of the prime limiting factors for plant growth and the least mobile and therefore mostly unavailable to plants compared to other nutrients, phosphate-solubilizing bacteria play an important role in supplying phosphate to plants. The utilization of these bacteria for direct application in agriculture is reviewed by Khan et al. (2007) as a promising strategy with great potential for use in sustainable agriculture.
Vermicompost enriched with HAs plays an important role in stimulating plant growth and development. Namely, Gholami et al. [46] determined the effects of HA at 0, 0.3, 0.6 and 0.9 kg ha −1 and vermicompost at 0, 5, 7.5 and 10 t ha −1 on mineral elements N, P, K, Fe, Zn, Mn and Cu uptake and photosynthetic pigment concentrations of chicory. Due to the presence of HAs, the activity of microorganisms in the soil was improved that finally increased N, P and K content in plants. This is an example of ‘indirect action’ of HAs on plants, while there is another ‘direct action’ that includes plant hormones [42]. As plant growth hormones are found in an aqueous solution of vermicompost, Arancon et al. [42] hypothesized that hormones such as auxins (indole-3-acetic acid-IAA), which are water-soluble, may adsorb on to humates and become more persistent in soil and thus extend the period of action on the plants.
According to most of the known literature, different types of vermicompost induce higher germination rate, plant growth and yield in many plant species such as tomato [27], lettuce [47], cucumber [48], petunia [8], pine trees [49], thyme [50], begonia, sugarcane and mint [31]. However, according to some data, one cannot generalize and speak exclusively about the positive effects of vermicompost [50][51]. Amoogaghaie and Golmohammadi [50] investigated the effect of various cow manure vermicompost (25, 50, and 75%) on the germination, growth and development of thyme. Their results showed that only 25% vermicompost substitution promoted seedling emergence, while other substitutions did not have a beneficial effect. Moreover, in 50% vermicompost substitution the maximum length, fresh and dry weight and photosynthetic efficiency were observed. Similar results observed Atiyeh et al. [27] who showed that vermicompost increased seed germination and growth only to a certain amount of vermicompost substitution, while higher amounts (100%) had negative effects, which were evident in shorter seedlings, fewer leaves and decreased germination. Ievinsh [51] reported that cow manure vermicompost substitution inhibited seed germination or did not have any effect which depended on the concentration of vermicompost (10–100%) and the plant species he used. All negative effects of higher vermicompost concentrations could be due to the induced stress by the high-soluble salt concentration or phenolic compounds from vermicompost [50].
4. Disease and Pest Control by Vermicompost
The rapid growth of the world’s population requires much higher agricultural production to meet basic human needs. On the other hand, world agriculture is facing many problems in crop production, among which are plant diseases and pests [52]. The application of chemicals such as pesticides gives positive results in regard to the control of pests, but they also cause several negative side effects such as environmental pollution, disruption of the soil’s natural fertility and the destruction of beneficial organisms [53][54]. To overcome problems of harmful organisms and diseases, in recent years, vermicompost has been mentioned as a key alternative in the fight against plant diseases, pests and pathogens [55]. With the various indirect beneficial effects of vermicompost on plants, the suppression of plant diseases and pests is one of the most significant. It is important to emphasize that vermicomposting contributes not only to the reduction of plant but also human and animal pathogens.
Namely, organic wastes, such as animal byproducts that can be vermicomposted and used as fertilizers, may contain pathogenic microorganisms [56][57][58]. Roubalova et al. [56] observed the reduction of pathogens such as Escherichia coli , Enterococcus spp. , and thermotolerant coliform bacteria in grape marc during vermicomposting. There are several possible ways by which earthworms contribute to the reduction of pathogens including bacteria, fungi and many others. They include a reduced-oxygen environment inside the gut and the presence of intestinal enzymes and coelomic fluids, which kill the parasites present in the waste [59][56]. Monroy et al. [60] reported a decrease in the number of nematodes in a pig slurry after the passage through the earthworm’s gut. The decrease occurred due to the digestion of nematodes by the proteolytic activity of enzymes present in the earthworms’ gut. When it comes to coelomic fluids, it is well known that they possess antimicrobial, proteolytic, hemolytic and antifungal effects [59][61]. Plavšin et al. [59] showed that coelomic fluid extracts of two earthworm species, Dendrobaena veneta and Eisenia fetida , negatively affected phytopathogenic fungi Fusarium oxysporum in vitro conditions. They concluded that earthworms might negatively affect fungal growth by ingestion and by contact as well. Although some plant pathogens are removed during earthworm digestion, vermicompost, as a final product of vermicomposting, is a true modulator not only of plant growth but also of disease and pest suppression [62]. The application of vermicompost for the suppression of different soil-borne phytopathogens has grown significantly in recent years [63][64][65].
Because bacterial communities change greatly during vermicomposting, vermicompost has a significantly different bacterial structure than the initial material. Vermicompost contains beneficial microorganisms such as bacteria, fungi and actinomycetes, which can improve overall plant growth, but also antagonistic microorganisms, which mediate the control of diseases and pests [66][67]. Liu et al. [66] isolated 374 bacterial strains from vermicompost made from fresh cow dung of which 28 strains showed antagonistic activity against Fusarium oxysporum f. sp. cucumerinum (FOC). FOC is a fungal pathogen that causes enormous damage to cucumbers worldwide [66]. Similarly, suppressions of Fusarium oxysporum and Phytophthora infestans have also been reported by vermicompost treatment [32]. It is important to emphasize that the influence of vermicompost on pathogens depends a lot on the type of initial substrate [68]. Szczech and Smolinska [68] showed that vermicompost from animal manure reduced the infection of tomato seedlings by Phytophthora nicotianae, while vermicompost from sewage sludge did not protect seedlings from infection. The influence of vermicompost on various pathogens also depends on the type of earthworms, i.e., it depends on the morphological and physiological characteristics of the digestive system of earthworms [9]. Regarding the suppression of fungal diseases, they include the effect of vermicompost on reduced sporulation, reduced growth of pathogenic fungi and, generally, reduced infection [17]. Except for the suppression of fungal diseases, vermicompost can also suppress bacterial diseases and pests. Dominguez et al. [15] observed an increase in salicylic acid and streptomycin synthesis after vermicomposting. Salicylic acid can induce plant pathogen resistance mechanisms and antibiotic streptomycin has been shown to control bacterial diseases of fruits, vegetables and crops [16]. Furthermore, vermicompost can manage pests such as mites ( Tetranychus urticae ), mealy bugs ( Pseudococcus sp.) , aphids ( Myzus persicae ) [69], corn earworm ( Helicoverpa zea ) [70], nematode ( Meloidogyne incognita ) [71], chili pest ( Polyphagotarsonemus latus ) [63], etc. Arancon et al. [69] tested the capacity of food waste vermicompost on reduction of three arthropod pests populations and damage to cucumbers, tomatoes, bush beans, eggplants and cabbage plants. Besides noticing the reduction in arthropod populations, pest damage and reproduction, they also noticed that vermicompost made the plants less attractive to the pests. Jangra et al. [63] also recorded a reduction in population, and a number of chili pest eggs after the vermicompost was applied in a rate of 5 t/ha. They hypothesized that a possible reason for the suppression of pests was due to soluble micro- and macro-nutrients in vermicompost. It is correlated with the conclusion of Arancon et al. [69]. Possible mechanisms can also include the production of phenolic compounds by the plants after applications of vermicomposts, making the tissues unpalatable or even the presence of chitinase enzyme in vermicompost that helps in controlling arthropods [72][73].
References
- Demirbas, A. Waste management, waste resource facilities and waste conversion processes. Energy Convers. Manag. 2011, 52, 1280–1287.
- Sabbas, T.; Polettini, A.; Pomi, R.; Astrup, T.; Hjelmar, O.; Mostbauer, P.; Cappai, G.; Magel, G.; Salhofer, S.; Speiser, C.; et al. Management of municipal solid waste incineration residues. Waste Manag. 2003, 23, 61–88.
- Hoornweg, D.; Bhata-Tata, P. What a Waste: A Global Review of Solid Waste Menagement; World Bank: Washington, DC, USA, 2012; ISBN 9781409406877.
- Suthar, S.; Singh, S. Vermicomposting of domestic waste by using two epigeic earthworms. J. Environ. Sci. Technol. 2008, 5, 99–106.
- Kaviraj; Sharma, S. Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour. Technol. 2003, 90, 169–173.
- Dumontet, S.; Dinel, H.; Baloda, S.B. Pathogen Reduction in Sewage Sludge by Composting and Other Biological Treatments: A Review. Biol. Agric. Hortic. 1999, 16, 409–430.
- Usmani, Z.; Kumar, V.; Gupta, P.; Gupta, G.; Rani, R.; Chandra, A. Enhanced soil fertility, plant growth promotion and microbial enzymatic activities of vermicomposted fly ash. Sci. Rep. 2019, 9, 10455.
- Arancon, N.Q.; Edwards, C.A.; Babenko, A.; Cannon, J.; Galvis, P.; Metzger, J.D. Influences of vermicomposts, produced by earthworms and microorganisms from cattle manure, food waste and paper waste, on the germination, growth and flowering of petunias in the greenhouse. Appl. Soil Ecol. 2008, 39, 91–99.
- Bhat, S.A.; Singh, S.; Singh, J.; Kumar, S.; Bhawana; Vig, A.P. Bioremediation and detoxification of industrial wastes by earthworms: Vermicompost as powerful crop nutrient in sustainable agriculture. Bioresour. Technol. 2018, 252, 172–179.
- Samal, K.; Raj Mohan, A.; Chaudhary, N.; Moulick, S. Application of vermitechnology in waste management: A review on mechanism and performance. J. Environ. Chem. Eng. 2019, 7, 103392.
- Ahmad, A.; Aslam, Z.; Bellitürk, K.; Iqbal, N.; Naeem, S.; Idrees, M.; Kaleem, Z.; Nawaz, M.Y.; Nawaz, M.; Sajjad, M.; et al. Vermicomposting Methods from Different Wastes: An Environment Friendly, Economically Viable and Socially Acceptable Approach for Crop Nutrition: A Review. Int. J. Food Sci. Agric. 2021, 5, 58–68.
- Elvira, C.; Goicoechea, M.; Sampedro, L.; Mato, S.; Nogales, R. Bioconversion of solid paper-pulp mill sludge by earthworms. Bioresour. Technol. 1996, 57, 173–177.
- Nogales, R.; Cifuentes, C.; Benítez, E. Vermicomposting of winery wastes: A laboratory study. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2005, 40, 659–673.
- Suthar, S. Vermicomposting potential of Perionyx sansibaricus (Perrier) in different waste materials. Bioresour. Technol. 2007, 98, 1231–1237.
- Chattopadhyay, G.N. Use of vermicomposting biotechnology for recycling organic wastes in agriculture. Int. J. Recycl. Org. Waste Agric. 2012, 1, 8.
- Bhat, S.A.; Singh, J.; Vig, A.P. Earthworms as Organic Waste Managers and Biofertilizer Producers. Waste Biomass Valorization 2018, 9, 1073–1086.
- Lores, M.; Gómez-Brandón, M.; Pérez-Díaz, D.; Domínguez, J. Using FAME profiles for the characterization of animal wastes and vermicomposts. Soil Biol. Biochem. 2006, 38, 2993–2996.
- Mahboub Khomami, A.; Padasht, M.N.; Ajili Lahiji, A.; Shirinfekr, A. The effect of sawdust vermicompost extract on Syngonium podophyllum growth and nutrition. J. Plant. Nutr. 2019, 42, 410–416.
- Thakur, A.; Kumar, A.; Kumar, C.V.; Kiran, B.S.; Kumar, S.; Athokpam, V. A review on vermicomposting: By-products and its importance. Plant. Cell Biotechnol. Mol. Biol. 2021, 22, 156–164.
- Barthod, J.; Rumpel, C.; Dignac, M.F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17.
- Tognetti, C.; Laos, F.; Mazzarino, M.J.; Hernández, M.T. Composting vs. vermicomposting: A comparison of end product quality. Compos. Sci. Util. 2005, 13, 6–13.
- Guo, L.; Wu, G.; Li, C.; Liu, W.; Yu, X.; Cheng, D.; Jiang, G. Vermicomposting with maize increases agricultural benefits by 304%. Agron. Sustain. Dev. 2015, 35, 1149–1155.
- Shi, Z.; Liu, J.; Tang, Z.; Zhao, Y.; Wang, C. Vermiremediation of organically contaminated soils: Concepts, current status, and future perspectives. Appl. Soil Ecol. 2019, 147, 103377.
- Bhat, S.A.; Singh, J.; Vig, A.P. Potential utilization of bagasse as feed material for earthworm Eisenia fetida and production of vermicompost. Springerplus 2015, 4, 11.
- Bouché, M.B. PU-43-Stratégies-Lombriciennes; Lohm, U., Persson, T., Eds.; Swedish Natural Science Research Council: Stockholm, Sweden, 1977; pp. 122–132.
- Gajalakshmi, S.; Abbasi, S.A. Earthworms and vermicomposting. Indian J. Biotechnol. 2004, 3, 486–494.
- Atiyeh, R.M.; Lee, S.; Edwards, C.A.; Arancon, N.Q.; Metzger, J.D. The influence of humic acids derived from earthworm. Bioresour. Technol. 2002, 84, 7–14.
- Vivas, A.; Moreno, B.; Garcia-Rodriguez, S.; Benitez, E. Assessing the impact of composting and vermicomposting on bacterial community size and structure, and microbial functional diversity of an olive-mill waste. Bioresour. Technol. 2009, 100, 1319–1326.
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 2017, 110, 97–108.
- Lv, B.; Xing, M.; Yang, J. Exploring the effects of earthworms on bacterial profiles during vermicomposting process of sewage sludge and cattle dung with high-throughput sequencing. Environ. Sci. Pollut. Res. 2018, 25, 12528–12537.
- Arancon, N.; Van Cleave, J.; Hamasaki, R.; Nagata, K.; Felts, J. The influence of vermicompost water extracts on growth of plants propagated by cuttings. J. Plant. Nutr. 2020, 43, 176–185.
- Domínguez, J.; Aira, M.; Kolbe, A.R.; Gómez-Brandón, M.; Pérez-Losada, M. Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci. Rep. 2019, 9, 9657.
- Brandón, M.G.; Aira, M.; Kolbe, A.R.; de Andrade, N.; Pérez-Losada, M.; Domínguez, J. Rapid bacterial community changes during vermicomposting of grape marc derived from red winemaking. Microorganisms 2019, 7, 473.
- Pattnaik, S.; Reddy, M.V. Nutrient Status of Vermicompost of Urban Green Waste Processed by Three Earthworm Species—Eisenia fetida, Eudrilus eugeniae, and Perionyx excavatus. Appl. Environ. Soil Sci. 2010, 2010, 967526.
- Chitrapriya, K.; Asokan, S.; Nagarajan, R. Estimating the Level of Phosphate Solubilising Bacteria and Azotobacter in the Vermicompost of Eudrilus Eugeniae and Perionyx Excavatus with Various Combinations of Cow- Dung and Saw-Dust. Int. J. Sci. Res. 2013, 3, 1–6.
- Sinha, R.K.; Agarwal, S.; Chauhan, K.; Valani, D. The wonders of earthworms & its vermicompost in farm production: Charles Darwin’s ‘friends of farmers’, with potential to replace destructive chemical fertilizers. Agric. Sci. 2010, 1, 76–94.
- Gómez-Brandón, M.; Aira, M.; Lores, M.; Domínguez, J. Changes in microbial community structure and function during vermicomposting of pig slurry. Bioresour. Technol. 2011, 102, 4171–4178.
- Gómez-Brandón, M.; Lores, M.; Domínguez, J. Species-specific effects of epigeic earthworms on microbial community structure during first stages of decomposition of organic matter. PLoS ONE 2012, 7, e31895.
- Kolbe, A.R.; Aira, M.; Gómez-Brandón, M.; Pérez-Losada, M.; Domínguez, J. Bacterial succession and functional diversity during vermicomposting of the white grape marc Vitis vinifera v. Albariño. Sci. Rep. 2019, 9, 7472.
- Gopal, M.; Bhute, S.S.; Gupta, A.; Prabhu, S.R.; Thomas, G.V.; Whitman, W.B.; Jangid, K. Changes in structure and function of bacterial communities during coconut leaf vermicomposting. Antonie Van Leeuwenhoek 2017, 110, 1339–1355.
- Monroy, F.; Aira, M.; Domínguez, J. Reduction of total coliform numbers during vermicomposting is caused by short-term direct effects of earthworms on microorganisms and depends on the dose of application of pig slurry. Sci. Total Environ. 2009, 407, 5411–5416.
- Arancon, N.Q.; Lee, S.; Edwards, C.A.; Atiyeh, R. Effects of humic acids derived from cattle, food and paper-waste vermicomposts on growth of greenhouse plants. Pedobiologia 2003, 47, 741–744.
- Arancon, N.Q.; Edwards, C.A.; Lee, S.; Byrne, R. Effects of humic acids from vermicomposts on plant growth. Eur. J. Soil Biol. 2006, 42, 65–69.
- Hernandez, O.L.; Calderín, A.; Huelva, R.; Martínez-Balmori, D.; Guridi, F.; Aguiar, N.O.; Olivares, F.L.; Canellas, L.P. Humic substances from vermicompost enhance urban lettuce production. Agron. Sustain. Dev. 2015, 35, 225–232.
- Ramnarain, Y.I.; Ori, L.; Ansari, A.A. Evaluation of the use of vermicompost on the crop production of two varieties of Pak choi (Brassica rapa var. chinensis) and on the soil structure in Suriname. Asian J. Agric. 2017, 1, 73–79.
- Gholami, H.; Ghani, A.; Raouf Fard, F.; Saharkhiz, M.J.; Hazrati, H. Changes in photosynthetic pigments and uptake of some soil elements by chicory supplied with organic fertilizers. Acta Ecol. Sin. 2019, 39, 250–256.
- Lončarić, Z.; Engler, M.; Karalić, K.; Bukvić, G.; Lončarić, R.; Kralik, D. Ocjena kvalitete vermikompostiranog goveđeg stajskog gnoja. Poljoprivreda 2005, 11, 57–63.
- Sallaku, G.; Babaj, I.; Kaciu, S.; Balliu, A. The influence of vermicompost on plant growth characteristics of cucumber (Cucumis sativus L.) seedlings under saline conditions. J. Food Agric. Environ. 2009, 7, 869–872.
- Lazcano, C.; Sampedro, L.; Zas, R.; Domínguez, J. Vermicompost enhances germination of the maritime pine (Pinus pinaster Ait.). New For. 2010, 39, 387–400.
- Amooaghaie, R.; Golmohammadi, S. Effect of Vermicompost on Growth, Essential Oil, and Health of Thymus Vulgaris. Compos. Sci. Util. 2017, 25, 166–177.
- Ievinsh, G. Vermicompost treatment differentially affects seed germination, seedling growth and physiological status of vegetable crop species. Plant. Growth Regul. 2011, 65, 169–181.
- Fried, G.; Chauvel, B.; Reynaud, P.; Sache, I. Decreases in Crop Production by Non-native Weeds, Pests, and Pathogens. In Impact of Biological Invasions on Ecosystem Services; Springer: Cham, Switzerland, 2017.
- Fernandes, M.E.S.; Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and sublethal effects of seven insecticides on three beneficial insects in laboratory assays and field trials. Chemosphere 2016, 156, 45–55.
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12.
- Yatoo, A.M.; Ali, M.N.; Baba, Z.A.; Hassan, B. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agron. Sustain. Dev. 2021, 41, 1–26.
- Roubalová, R.; Procházková, P.; Hanč, A.; Dvořák, J.; Bilej, M. Mutual interactions of E. andrei earthworm and pathogens during the process of vermicomposting. Environ. Sci. Pollut. Res. 2020, 27, 33429–33437.
- Rodríguez-Canché, L.G.; Cardoso Vigueros, L.; Maldonado-Montiel, T.; Martínez-Sanmiguel, M. Pathogen reduction in septic tank sludge through vermicomposting using Eisenia fetida. Bioresour. Technol. 2010, 101, 3548–3553.
- Pachepsky, Y.A.; Sadeghi, A.M.; Bradford, S.A.; Shelton, D.R.; Guber, A.K.; Dao, T. Transport and fate of manure-borne pathogens: Modeling perspective. Agric. Water Manag. 2006, 86, 81–92.
- Plavšin, I.; Velki, M.; Ečimović, S.; Vrandečić, K.; Ćosić, J. Inhibitory effect of earthworm coelomic fluid on growth of the plant parasitic fungus Fusarium oxysporum. Eur. J. Soil Biol. 2017, 78, 1–6.
- Monroy, F.; Aira, M.; Domínguez, J. Changes in density of nematodes, protozoa and total coliforms after transit through the gut of four epigeic earthworms (Oligochaeta). Appl. Soil Ecol. 2008, 39, 127–132.
- Dales, R.P.; Kalaç, Y. Phagocytic defence by the earthworm Eisenia foetida against certain pathogenic bacteria. Comp. Biochem. Physiol. Part. A Physiol. 1992, 101, 487–490.
- Sarma, B.K.; Singh, P.; Susheel, P.; Harikesh, S. Vermicompost as Modulator of Plant Growth and Disease Suppression. Glob. Sci. Books 2010, 4, 58–66.
- Jangra, M.; Sindhu, S.; Gulati, R.; Batra, V.K. Studies on efficacy of vermicompost for the management of Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) infesting chilli (Capsicum annuum L.) in Haryana. Pharm. Innovat. J. 2019, 8, 86–89.
- You, X.; Kimura, N.; Okura, T.; Murakami, S.; Okano, R.; Shimogami, Y.; Matsumura, A.; Tokumoto, H.; Ogata, Y.; Tojo, M. Suppressive effects of vermicomposted-bamboo powder on cucumber damping-off. Jpn. Agric. Res. Q. 2019, 53, 13–19.
- Basco, M.J.; Bisen, K.; Keswani, C.; Singh, H.B. Biological management of Fusarium wilt of tomato using biofortified vermicompost. Mycosphere 2017, 8, 467–483.
- Liu, D.; Liu, D.; Liu, L.; Wang, Y.; Zhang, Y. Screening and identification of antagonistic bacteria from vermicompost against Fusarium oxysporum f. sp. cucumerinum. Acta Agric. Scand. Sect. B Soil Plant. Sci. 2021, 71, 266–272.
- Simsek Ersahin, Y.; Haktanir, K.; Yanar, Y. Vermicompost suppresses Rhizoctonia solani Kühn in cucumber seedlings. J. Plant Dis. Prot. 2009, 116, 182–188.
- Szczech, M.; Smolinska, U. Comparison of suppressiveness of vermicomposts produced from animal manures and sewage sludge against phytophthora nicotianae breda de haan var. nicotianae. J. Phytopathol. 2001, 149, 77–82.
- Arancon, N.Q.; Edwards, C.A.; Yardim, E.N.; Oliver, T.J.; Byrne, R.J.; Keeney, G. Suppression of two-spotted spider mite (Tetranychus urticae), mealy bug (Pseudococcus sp.) and aphid (Myzus persicae) populations and damage by vermicomposts. Crop. Prot. 2007, 26, 29–39.
- Cardoza, Y.J.; Buhler, W.G. Soil organic amendment impacts on corn resistance to Helicoverpa zea: Constitutive or induced? Pedobiologia 2012, 55, 343–347.
- Xiao, Z.; Liu, M.; Jiang, L.; Chen, X.; Griffiths, B.S.; Li, H.; Hu, F. Vermicompost increases defense against root-knot nematode (Meloidogyne incognita) in tomato plants. Appl. Soil Ecol. 2016, 105, 177–186.
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20.
- Bavaresco, L.; Pezzutto, S.; Gatti, M.; Mattivi, F. Role of the variety and some environmental factors on grape stilbenes. Vitis J. Grapevine Res. 2007, 46, 57–61.




