Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shelley Tischkau | + 2190 word(s) | 2190 | 2021-10-19 05:19:52 | | | |
| 2 | Camila Xu | Meta information modification | 2190 | 2021-10-20 04:37:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tischkau, S. AhR in the Hallmarks of Brain Aging. Encyclopedia. Available online: https://encyclopedia.pub/entry/15156 (accessed on 07 February 2026).
Tischkau S. AhR in the Hallmarks of Brain Aging. Encyclopedia. Available at: https://encyclopedia.pub/entry/15156. Accessed February 07, 2026.
Tischkau, Shelley. "AhR in the Hallmarks of Brain Aging" Encyclopedia, https://encyclopedia.pub/entry/15156 (accessed February 07, 2026).
Tischkau, S. (2021, October 20). AhR in the Hallmarks of Brain Aging. In Encyclopedia. https://encyclopedia.pub/entry/15156
Tischkau, Shelley. "AhR in the Hallmarks of Brain Aging." Encyclopedia. Web. 20 October, 2021.
Copy Citation
AhR, a member of the basic helix-loop-helix (bHLH)-PAS superfamily, performs various functions within the brain. It is an ancient protein that possesses shared functions and structures across various species in the evolutionary tree. It is widely distributed in various regions of the brain, such as the hippocampus, the cortex, and the hypothalamus, and its expression changes during the course of brain development.
aryl hydrocarbon receptor
AhR endogenous/exogenous ligands
brain aging hallmarks
1. AhR Expression, Functions, and Signaling in the Brain
In neuronal progenitor cells, AhR interacts with its partners to direct differentiation into several neuronal subtypes, as well as to influence dendrite morphogenesis [1][2][3]. Although AhR expression decreases from the embryonic period into adult life [4][5], several physiological functions remain in the adult brain, which include the regulation of neurotransmitter levels, blood-brain barrier functions, and immune responses [6][7][8]. Furthermore, AhR contributes to glial cell and neuroendocrine system function [9][10]. AhR activation interacts at various levels in the neuroendocrine system, from the hypothalamus down to the target organ [9]. For example, the AhR agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts the secretion of several releasing hormones in the hypothalamus, such as corticotropin-releasing factor and vasopressin [11]. Furthermore, AhR activation in the brain leads to decreased estrogen receptors and estrogen levels [12][13]. Depending upon the ligand, AhR may act through different mechanisms to mediate its cellular and physiological functions [13]. AhR signaling is complex and broadly divided into canonical and non-canonical pathways. In the absence of ligands, AhR is predominantly found in a cytoplasmic complex with heat shock protein 90 (HSP90) dimers, HBV X-associated protein 2 (XAP-2), and p23 chaperone protein. However, in the canonical pathway, ligand activation of AhR leads to the dissociation of HBV X-associated protein 2 (XAP-2) from heat shock protein 90 (HSP90) in the cytoplasm; the activated AhR translocates into the nucleus, where it dimerizes with aryl hydrocarbon receptor nuclear translocator (ARNT) and binds to xenobiotic response elements (XREs) on the DNA, leading to the transcription of various cytochrome P450s (CYPs), and glutathione transferase (GST), which, among other events, feedback to metabolize the initial ligand. Some toxicological AhR ligands, such as TCDD and related compounds, are slowly metabolized following receptor induction, leading to persistent AhR activation [14]. Aryl hydrocarbon receptor repressor (AhRR), which is also an AhR target gene, helps mediate negative feedback through the sequestration of ARNT; ligand-activated AhR is subsequently degraded by the ubiquitin-proteasome system (Figure 1a). Apart from regulating phase 1 and phase 2 metabolic target genes for chemical defense, AhR also regulates several protein kinases, such as p21Cip1, and p27kip1, that are necessary for organ development [15]. Inflammatory genes, such as Interleukin (IL)-6 and IL-1beta, and energy homeostasis genes, such as TCDD-inducible poly-ADP-ribose polymerase (TiPARP/PARP7), are also direct targets of AhR [15]. Thus, the target genes for AhR are broad, and many are unrelated to the toxicological functions of AhR. Physiologically, AhR may form alternative partnerships with other transcription factors, such as nuclear factor kappa-light-chain-enhancers of activated B cells (NF-κB), proto-oncogene c-Maf, Krueppel-like factor 6 (KLF6), and others, in the cytoplasm. For example, AhR interacts with NF-κB, which is involved in inflammation, immune and stress responses [16]; the induction of antioxidant genes requires the presence of both AhR and NF-E2 p45-related factor (Nrf2) at the promoter [17][18]. AhR also interacts with circadian clock components and intracellular signaling, such as the mitogen-activated protein kinase (MAPK) cascade involved in apoptosis, inflammation and cell senescence [19][20] (Figure 1b). ARNT shares similar sequences with brain and muscle Arnt-like protein-1 (BMAL1), a clock component, which may contribute to AhR/circadian clock interactions [21]. In HT22 hippocampal neuronal cells, the activation of AhR by α-naphthoflavone (α-NF) induces the phosphorylation of MAPK, leading to cell death in an AhR-dependent manner [22]. ARNT-2, a neuronal transcription factor that also belongs to the bHLH-PAS superfamily, is mainly expressed in the central nervous system and has been shown to be involved in neuronal survival [23]. Although ARNT-2s have been shown to form dimers with AhR in vitro [24], the question of whether ARNT-2 can interact with AhR in vivo remains, and is of importance to the understanding of whether ARNT-2 dimerization with AhR also participates in the activation of gene transcription in a similar way to AhR/ARNT in the brain and other organs.
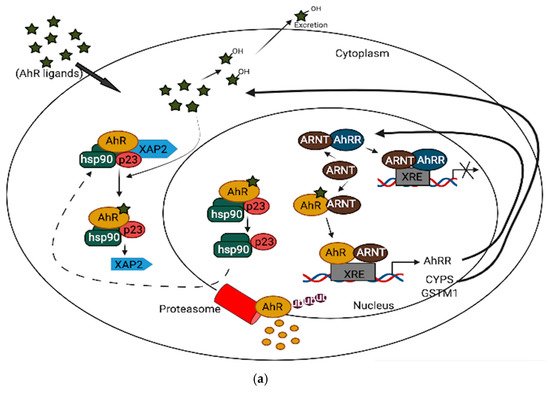
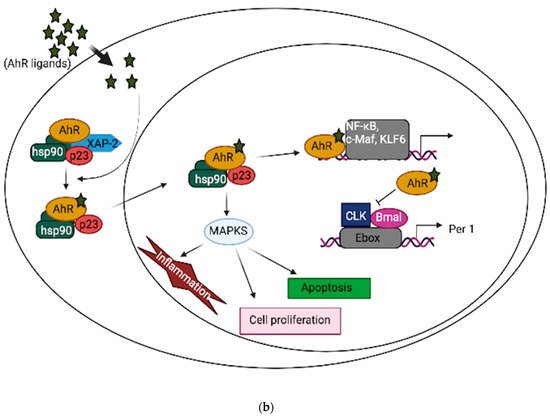
Figure 1. (a): AhR canonical pathway activation. (b): AhR non-canonical pathway activation.
Apart from xenobiotics, such as TCDD, and other polycyclic aromatic hydrocarbons (PAHs) that cross the blood-brain barrier (BBB) to mediate some of AhR’s effects in the brain, several endogenous tryptophan metabolites, such as kynurenine, serotonin, and 6-formylindolo [3,2-b] carbazole (FICZ), are implicated in AhR-related brain function and pathology [25][26]. Recently, attention has been drawn to the kynurenic pathway and microbial metabolites in the gut-brain axis, as well as central nervous system (CNS) development and diseases [26][27]. In the brain, L-tryptophan is primarily metabolized through kynurenic pathways, producing several ligands that bind to AhR [28]. AhR activation in glial cells by the microbial metabolism of dietary tryptophan interferes with the NF-κB inflammatory transcription program, thereby reducing neuroinflammation, which raises the possibility that this pathway could be targeted in neurodegenerative and autoimmune diseases in the CNS [29][30]. In addition to several gut microbiota metabolites, FICZ, an endogenous ligand of AhR, promotes neurogenesis in adult neurons, which is needed for hippocampal memory maintenance in mice. Several brain-related pathological conditions may also involve the non-canonical activation of AhR. For instance, in Alzheimer’s disease pathology, tryptophan derivatives (kynurenic acid and 5-hydroxyindole-acetic acid) can increase neprilysin expression, which is necessary for regulating amyloid beta clearance by proteolysis [31].
2. AhR and Aging Hallmarks in the Brain
2.1. Oxidative Stress
For years, the phenomenon of oxidative stress has been implicated in aging. Although several theories exist, the free radical theory of aging originally proposed by Denham Harman in the 1950s remains the most widely accepted, with modifications [32][33]. Aged tissues and senescent cells produce oxidative stress products, which lead to an imbalance between the oxidative and antioxidant defense network [34][35]. Besides, the exposure of cells to environmental oxidant generators, such as pesticides, heavy metals, and others, also contributes to this imbalance [36]. Just like other organs, a strong correlation exists between aging in the brain and increased reactive oxygen species (ROS) formation [37]; increased ROS can be attributed to mitochondrial dysfunction associated with aging [38][39]. Moreover, protein aggregation/modifications found in most aging-related brain diseases, including Alzheimer’s, have been attributed to increased ROS formation, which tends to impair proteasome and lysosome functions [40][41].
Aryl-hydrocarbon-receptor has been mechanistically shown to be involved in the generation of oxidative stress in the brain, as its activation by several ligands shifts the cellular redox balance towards favoring oxidative stress production [42][43][44]. The AhR agonist, TCDD, induces ROS production and oxidative DNA damage in astrocytes, leading to premature senescence, which is a hallmark of brain aging [45]. The generation of superoxide anions, the modulation of the CYP P450 system, mitochondrial dysfunction, and increased activation of arachidonic acid signaling are among the AhR-dependent pathways (Figure 2) that lead to increased ROS production in the brain [46][47]. Just like other organs in the body, the activation of AhR induces the expression of CYP1A1 and CYP1B1 in most brain regions, as well as the associated pituitary gland [48]; an increased expression of these xenobiotic metabolism enzymes can result in mitochondrial ROS production through an uncoupling process that leads to the release of superoxide and hydrogen peroxide (H2O2), which are believed to accelerate the aging process in the brain [49][50]. Increased production of ROS in mitochondria also regulates inflammasomes (NLRP3) by increasing the activation of inflammatory caspases in macrophages, which are necessary for cytokine synthesis, further contributing to brain inflammation [51]. In addition to the uncoupling process, arachidonic acid pathway activation by AhR leads to the increased generation of ROS through the metabolism of arachidonic acid by CYPs and other intracellular signaling processes [52][53].
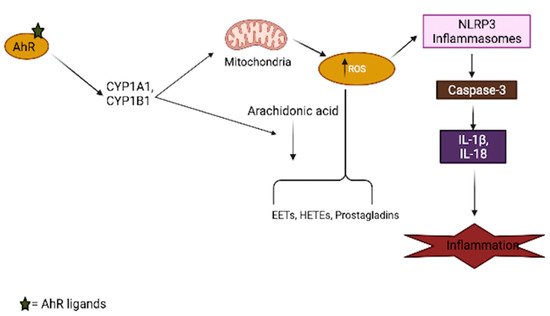
Figure 2. Involvement of AhR in oxidative stress generation. AhR activation by its ligands increases xenobiotic metabolism enzymes (CYPs), which results in mitochondrial toxicity, leading to the generation of reactive oxygen species (ROS). These enzymes also interact with the arachidonic acid pathway and increase the production of several arachidonic acid metabolites, such as EETs (epoxyeicosatrienoic acid), HETEs (hydroxyeicosatrienonic acid) and prostaglandins, which are sources of ROS in several tissues, including the brain. The generation of ROS in turn activates the inflammasome, which aids the secretion of inflammatory cytokines.
Although AhR has also been implicated in antioxidant responses through its cross-regulation with Nrf2 in various tissues [17][18], the evidence for this pathway in the brain is yet to be fully established. The activation of AhR with the agonist, β-Naphthoflavone (BNF), has no significant effect on Nrf2 mRNA levels or antioxidant enzymes, such as glutathione transferase, in the brain regions of pigs [54]. In mice, the absence of AhR helps reduce oxidative stress in the brain [55]. Therefore, it is reasonable to suggest that the antioxidant role of AhR is either cell-specific and absent in the brain, or that the oxidant response overwhelms the antioxidant response in the brain.
2.2. Stress Response
During stress, the body produces an adaptive response to reestablish the homeostasis that has been disrupted by the stressor [56]. Stress responses can either be cellular or generalized. The generalized stress response involves the release of glucocorticoids (stress hormone) via the neuroendocrine hypothalamic-pituitary axis. The cellular stress response involves various molecular changes, which may include the induction of heat shock proteins that are necessary for cell survival [57][58]. Brain aging can impose detrimental effects on both generalized and cellular stress responses, thus shifting away from an adaptive response towards a harmful effect. For instance, the age-related elevation of glucocorticoid levels contributes to hippocampal neuronal loss and cognitive impairment [58]. Postmortem cerebrospinal fluid in aged and Alzheimer’s patients contained elevated levels of cortisol [59], which suggests that the brain could be rejuvenated by inhibiting stress responses in the brain. Furthermore, organelle-specific stress response pathways and the ubiquitin proteasome system are also affected during aging [60]. Proteasome activities decline during aging, leading to increased protein modifications (a hallmark in various neurodegenerative diseases), which subsequently may reduce the effectiveness of the endoplasmic reticulum (ER) stress response [61]. Therefore, understanding stress response pathways during brain aging might provide relevant targets for therapeutic strategies in neurodegenerative diseases [62].
Aryl-hydrocarbon-receptor activation can modulate the neuroendocrine stress response system [9]. In the brain of rainbow trout, BNF acts through AhR signaling to downregulate steroidogenic acute regulatory protein, which is important for the biosynthesis of neurosteroids during stress. Furthermore, BNF suppressed pro-opiomelanocortin A (POMC-A), a precursor for adrenocorticotropic hormone (ACTH) that is necessary for the cortisol-induced stress response [63]. AhR also helps modulate the elevation in monoamine neurotransmitters that occurs during prolonged stress. For instance, AhR activation by PAHs and PAH-like compounds helps reduce cortisol and brain monoaminergic activities in rainbow trout after prolonged stress [64]. Cellular stress responses are also influenced by AhR activation [65][66], although these effects are yet to be explored specifically in the brain. Exploring AhR receptor involvement in glial cell cellular stress response mechanisms would be interesting, since these cells have been shown to be involved in brain stress responses [67][68].
2.3. Neurogenesis and Neuronal Plasticity
In the adult brain, neurogenesis appears to be important for the maintenance of the brain’s neuronal circuitry [69]. In the subgranular zone (SGZ) of the hippocampal dentate gyrus in young adult rats, newly generated neuronal cells tend to integrate with the pre-existing hippocampal circuit, which is necessary for learning and memory [70]. Neuronal stem/progenitor cells (NSC) are also found in the subependymal zones and olfactory bulbs of adult primates/humans [71][72]. Several neurodegenerative diseases, including Alzheimer’s disease, have been linked with aging-associated decline in neurogenesis and plasticity that occurs secondary to a loss in the proliferating potential of NSC [73][74]. Moreover, aged animals produce significantly fewer new neurons in the subventricular zone (SVZ) and SGZ of the hippocampus, which may contribute to a decline in cognitive functions that accompanies brain aging [75][76]. Aging also leads to the activation of glial cells and the subsequent secretion of pro-inflammatory cytokines, such as IL-1, which negatively impact NSC state and differentiation [76][77].
Aryl-hydrocarbon-receptor enhances neuronal proliferation during development; however, its role in adult neurogenesis is less well-investigated. AhR activation can regulate several genes involved in multiple aspects of synaptic plasticity and neurogenesis after brain development. A study using the Gene Ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis revealed that the administration of TCDD in the adult brain upregulates the genes required for synaptic plasticity and neuronal activities, including genes encoding for postsynaptic density 95 (PSD-95) protein and early growth response 1 (EGR1) [78]. The conditional deletion of AhR in adult mice also showed that AhR activation is necessary for SGZ neurogenesis by increasing the number of newborn granule cells in the DG of the hippocampus, which in turn improves hippocampus-dependent memory [79]. Similarly, AhR signaling helps restore neurogenesis after brain injury by enhancing ependymal glial cells to generate the new neurons necessary for repair in zebrafish [80]. Although several exogenous toxic AhR ligands have been studied for their neurotoxic effects targeting NSC in the adult brain, FICZ, an endogenous ligand of AhR, showed positive effects on the fate of NSCs by upregulating the ASCL1 and Ngn2 genes necessary for neuronal differentiation in the SGZ area of the adult mouse hippocampus [81]. Additionally, AhR activation by FICZ improves hippocampal-dependent memory and learning tasks, which was reversed following treatment with the AhR antagonist, CH22319 [81].
References
- Hongtao Qin; Jo Anne Powell-Coffman; The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Developmental Biology 2004, 270, 64-75, 10.1016/s0012-1606(04)00119-8.
- Kathrin Gassmann; Josef Abel; Hanno Bothe; Thomas Haarmann-Stemmann; Hans F. Merk; Kim N. Quasthoff; Thomas Dino Rockel; Timm Schreiber; Ellen Fritsche; Species-Specific Differential AhR Expression Protects Human Neural Progenitor Cells against Developmental Neurotoxicity of PAHs. Environmental Health Perspectives 2010, 118, 1571-1577, 10.1289/ehp.0901545.
- Sarah E. Latchney; Amy M. Hein; M. Kerry O'Banion; Emanuel DiCicco-Bloom; Lisa A. Opanashuk; Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. Journal of Neurochemistry 2012, 125, 430-445, 10.1111/jnc.12130.
- Eiki Kimura; Chiharu Tohyama; Embryonic and Postnatal Expression of Aryl Hydrocarbon Receptor mRNA in Mouse Brain. Frontiers in Neuroanatomy 2017, 11, 4, 10.3389/fnana.2017.00004.
- Barbara Abbott; Linda Birnbaum; G. H. Perdew; Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Developmental Dynamics 1995, 204, 133-143, 10.1002/aja.1002040204.
- James P. Byers; Karilane Masters; Jeffrey G. Sarver; Ezdihar A. Hassoun; Association between the levels of biogenic amines and superoxide anion production in brain regions of rats after subchronic exposure to TCDD. Toxicology 2006, 228, 291-298, 10.1016/j.tox.2006.09.009.
- XueQian Wang; Brian T. Hawkins; David S. Miller; Aryl hydrocarbon receptor‐mediated up‐regulation of ATP‐driven xenobiotic efflux transporters at the blood‐brain barrier. The FASEB Journal 2010, 25, 644-652, 10.1096/fj.10-169227.
- Wan-Ci Chen; Li-Hsin Chang; Shiang-Suo Huang; Yu-Jie Huang; Chun-Lien Chih; Hung-Chih Kuo; Yi-Hsuan Lee; I-Hui Lee; Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. Journal of Neuroinflammation 2019, 16, 1-13, 10.1186/s12974-019-1572-7.
- Ludmila Juricek; Xavier Coumoul; The Aryl Hydrocarbon Receptor and the Nervous System. International Journal of Molecular Sciences 2018, 19, 2504, 10.3390/ijms19092504.
- Yi-Hsuan Lee; Chun-Hua Lin; Pei-Chien Hsu; Yu-Yo Sun; Yu-Jie Huang; Jiun-Horng Zhuo; Chen-Yu Wang; Yu-Ling Gan; Chia-Chi Hung; Chia-Yi Kuan; et al.Feng-Shiun Shie Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia 2015, 63, 1138-1154, 10.1002/glia.22805.
- Bo-Hyun Moon; Chang Gwun Hong; Soo-Young Kim; Hyun-Ju Kim; Seung Keon Shin; Seungwoo Kang; Kuem-Ju Lee; Yong-Ku Kim; Min-Soo Lee; Kyung-Ho Shin; et al. A single administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin that produces reduced food and water intake induces long-lasting expression of corticotropin-releasing factor, arginine vasopressin, and proopiomelanocortin in rat brain. Toxicology and Applied Pharmacology 2008, 233, 314-322, 10.1016/j.taap.2008.09.001.
- Xin Gao; Kaori Mizuyachi; Paul F. Terranova; Karl K. Rozman; 2,3,7,8-Tetrachlorodibenzo-p-dioxin Decreases Responsiveness of the Hypothalamus to Estradiol as a Feedback Inducer of Preovulatory Gonadotropin Secretion in the Immature Gonadotropin-Primed Rat. Toxicology and Applied Pharmacology 2001, 170, 181-190, 10.1006/taap.2000.9099.
- D. Desaulniers; G.-H. Xiao; K. Leingartner; I. Chu; B. Musicki; B. K. Tsang; Comparisons of Brain, Uterus, and Liver mRNA Expression for Cytochrome P450s, DNA Methyltransferase-1, and Catechol-O-Methyltransferase in Prepubertal Female Sprague-Dawley Rats Exposed to a Mixture of Aryl Hydrocarbon Receptor Agonists. Toxicological Sciences 2005, 86, 175-184, 10.1093/toxsci/kfi178.
- Jay M. Wendling; Robert G. Orth; Hermann. Poiger; Determination of [3H]-2,3,7,8-tetrachlorodibenzo-p-dioxin in human feces to ascertain its relative metabolism in man. Analytical Chemistry 1990, 62, 796-800, 10.1021/ac00207a005.
- Karl Walter Bock; Aryl hydrocarbon receptor (AHR): From selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochemical Pharmacology 2019, 168, 65-70, 10.1016/j.bcp.2019.06.015.
- Christoph F. A. Vogel; Eric Sciullo; Wen Li; Pat Wong; Gwendal Lazennec; Fumio Matsumura; RelB, a New Partner of Aryl Hydrocarbon Receptor-Mediated Transcription. Molecular Endocrinology 2007, 21, 2941-2955, 10.1210/me.2007-0211.
- John D. Hayes; Albena Dinkova-Kostova; Michael McMahon; Cross-talk between Transcription Factors AhR and Nrf2: Lessons for Cancer Chemoprevention from Dioxin. Toxicological Sciences 2009, 111, 199-201, 10.1093/toxsci/kfp168.
- Ronnie L. Yeager; Scott A. Reisman; Lauren M. Aleksunes; Curtis D. Klaassen; Introducing the “TCDD-Inducible AhR-Nrf2 Gene Battery”. Toxicological Sciences 2009, 111, 238-246, 10.1093/toxsci/kfp115.
- Bozena Kaminska; Agata Gozdz; Malgorzata Zawadzka; Aleksandra Ellert-Miklaszewska; Maciej Lipko; MAPK Signal Transduction Underlying Brain Inflammation and Gliosis as Therapeutic Target. The Anatomical Record 2009, 292, 1902-1913, 10.1002/ar.21047.
- Yu Sun; Wen-Zhou Liu; Tao Liu; Xu Feng; Nuo Yang; Hua-Fu Zhou; Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. Journal of Receptors and Signal Transduction 2015, 35, 600-604, 10.3109/10799893.2015.1030412.
- Shelley A. Tischkau; Mechanisms of circadian clock interactions with aryl hydrocarbon receptor signalling. European Journal of Neuroscience 2019, 51, 379-395, 10.1111/ejn.14361.
- Ah-Ran Yu; Yeon Ju Jeong; Chi Yeon Hwang; Kyung-Sik Yoon; Wonchae Choe; Joohun Ha; Sung Soo Kim; Youngmi Kim Pak; Eui-Ju Yeo; Insug Kang; et al. Alpha-naphthoflavone induces apoptosis through endoplasmic reticulum stress via c-Src-, ROS-, MAPKs-, and arylhydrocarbon receptor-dependent pathways in HT22 hippocampal neuronal cells. NeuroToxicology 2018, 71, 39-51, 10.1016/j.neuro.2018.11.011.
- Guillaume Drutel; Anne Héron; Markus Kathmann; Claude Gros; Séverine Macé; Michel Plotkine; Jean‐Charles Schwartz; Jean‐Michel Arrang; ARNT2, a transcription factor for brain neuron survival?. European Journal of Neuroscience 1999, 11, 1545-1553, 10.1046/j.1460-9568.1999.00562.x.
- Edward J. Dougherty; Richard S. Pollenz; Analysis of Ah Receptor-ARNT and Ah Receptor-ARNT2 Complexes In Vitro and in Cell Culture. Toxicological Sciences 2007, 103, 191-206, 10.1093/toxsci/kfm300.
- María I. Cuartero; Iván Ballesteros; Juan de la Parra; Andrew L. Harkin; Aine Abautret-Daly; Eoin Sherwin; Pedro Fernández-Salguero; Ángel L. Corbí; Ignacio Lizasoain; María A. Moro; et al. L-Kynurenine/Aryl Hydrocarbon Receptor Pathway Mediates Brain Damage After Experimental Stroke. Circulation 2014, 130, 2040-2051, 10.1161/circulationaha.114.011394.
- Ning Ma; Ting He; Lee J. Johnston; Xi Ma; Host–microbiome interactions: the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes 2020, 11, 1203-1219, 10.1080/19490976.2020.1758008.
- Robert Schwarcz; Trevor Stone; The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2016, 112, 237-247, 10.1016/j.neuropharm.2016.08.003.
- Donald M. Dougherty; Dawn M. Marsh-Richard; Charles Mathias; Ashley Hood; Merideth A. Addicott; F. Gerard Moeller; Christopher J. Morgan; Abdulla A.-B. Badawy; Comparison of 50- and 100-g l-tryptophan depletion and loading formulations for altering 5-HT synthesis: pharmacokinetics, side effects, and mood states. Psychopharmacology 2008, 198, 431-445, 10.1007/s00213-008-1163-2.
- Veit Rothhammer; Ivan D. Mascanfroni; Lukas Bunse; Maisa C. Takenaka; Jessica Kenison; Lior Mayo; Chun-Cheih Chao; Bonny Patel; Raymond Yan; Manon Blain; et al.Jorge I. AlvarezHania KébirNiroshana AnandasabapathyGuillermo IzquierdoSteffen JungNikolaus ObholzerNathalie PochetClary B. ClishMarco PrinzAlexandre PratJack AntelFrancisco J. Quintana Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nature Medicine 2016, 22, 586-597, 10.1038/nm.4106.
- Veit Rothhammer; Davis M. Borucki; Emily C. Tjon; Maisa C. Takenaka; Chun-Cheih Chao; Alberto Ardura-Fabregat; Kalil Alves de Lima; Cristina Gutiérrez-Vázquez; Patrick Hewson; Ori Staszewski; et al.Manon BlainLuke HealyTradite NezirajMatilde BorioMichael WheelerLoic Lionel DraginDavid LaplaudJack AntelJorge Ivan AlvarezMarco PrinzFrancisco J. Quintana Microglial control of astrocytes in response to microbial metabolites. Nature Cell Biology 2018, 557, 724-728, 10.1038/s41586-018-0119-x.
- Michel Maitre; Christian Klein; Christine Patte-Mensah; Ayikoe-Guy Mensah-Nyagan; Tryptophan metabolites modify brain Aβ peptide degradation: A role in Alzheimer’s disease?. Progress in Neurobiology 2020, 190, 101800, 10.1016/j.pneurobio.2020.101800.
- D. Harman; Aging: A Theory Based on Free Radical and Radiation Chemistry. Journal of Gerontology 1956, 11, 298-300, 10.1093/geronj/11.3.298.
- Vadim N. Gladyshev; The Free Radical Theory of Aging Is Dead. Long Live the Damage Theory!. Antioxidants & Redox Signaling 2014, 20, 727-731, 10.1089/ars.2013.5228.
- Ilaria Liguori; Gennaro Russo; Francesco Curcio; Giulia Bulli; Luisa Aran; David DELLA Morte; Gaetano Gargiulo; Gianluca Testa; Francesco Cacciatore; Domenico Bonaduce; et al.Pasquale Abete Oxidative stress, aging, and diseases. Clinical Interventions in Aging 2018, ume 13, 757-772, 10.2147/cia.s158513.
- Bee Ling Tan; Mohd Esa Norhaizan; Winnie-Pui-Pui Liew; Heshu Rahman; Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Frontiers in Pharmacology 2018, 9, 1162, 10.3389/fphar.2018.01162.
- Fuli Zheng; Filipe Marques Gonçalves; Yumi Abiko; Huangyuan Li; Yoshito Kumagai; Michael Aschner; Redox toxicology of environmental chemicals causing oxidative stress. Redox Biology 2020, 34, 101475, 10.1016/j.redox.2020.101475.
- Carmelina Gemma; Jennifer Vila; Adam Bachstetter; Paula C. Bickford; Oxidative Stress and the Aging Brain: From Theory to Prevention. Brain Aging 2007, Chapter 15, 353-374, 10.1201/9781420005523-15.
- Giorgio Lenaz; Role of mitochondria in oxidative stress and ageing. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1998, 1366, 53-67, 10.1016/s0005-2728(98)00120-0.
- Dao-Fu Dai; Ying Ann Chiao; David J Marcinek; Hazel H Szeto; Peter S Rabinovitch; Mitochondrial oxidative stress in aging and healthspan. Longevity & Healthspan 2014, 3, 6-6, 10.1186/2046-2395-3-6.
- Julie Demers-Lamarche; Gérald Guillebaud; Mouna Tlili; Kiran Todkar; Noémie Bélanger; Martine Grondin; Angela P. Nguyen; Jennifer Michel; Marc Germain; Loss of Mitochondrial Function Impairs Lysosomes. Journal of Biological Chemistry 2016, 291, 10263-10276, 10.1074/jbc.m115.695825.
- Maria Lefaki; Nikoletta Papaevgeniou; Niki Chondrogianni; Redox regulation of proteasome function. Redox Biology 2017, 13, 452-458, 10.1016/j.redox.2017.07.005.
- Timothy P Dalton; Alvaro Puga; Howard G Shertzer; Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chemico-Biological Interactions 2002, 141, 77-95, 10.1016/s0009-2797(02)00067-4.
- Hongling Liu; Laihao Shi; John P. Giesy; Hongxia Yu; Polychlorinated diphenyl sulfides can induce ROS and genotoxicity via the AhR-CYP1A1 pathway. Chemosphere 2019, 223, 165-170, 10.1016/j.chemosphere.2019.01.169.
- Christoph F.A. Vogel; Laura S. Van Winkle; Charlotte Esser; Thomas Haarmann-Stemmann; The aryl hydrocarbon receptor as a target of environmental stressors – Implications for pollution mediated stress and inflammatory responses. Redox Biology 2020, 34, 101530, 10.1016/j.redox.2020.101530.
- Yang Zhang; Xiaoke Nie; Tao Tao; Wenbo Qian; Shengyang Jiang; Junkang Jiang; Aihong Li; Aisong Guo; Guangfei Xu; Qiyun Wu; et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin promotes astrocyte activation and the secretion of tumor necrosis factor-α via PKC/SSeCKS-dependent mechanisms. Journal of Neurochemistry 2014, 129, 839-849, 10.1111/jnc.12696.
- Ezdihar A. Hassoun; Feng Li; Ahmed Abushaban; Sidney J. Stohs; The relative abilities of TCDD and its congeners to induce oxidative stress in the hepatic and brain tissues of rats after subchronic exposure. Toxicology 2000, 145, 103-113, 10.1016/s0300-483x(99)00221-8.
- Jia Lin; Hua-Shan Zhao; Lei Qin; Xue-Nan Li; Cong Zhang; Jun Xia; Jin-Long Li; Atrazine Triggers Mitochondrial Dysfunction and Oxidative Stress in Quail (Coturnix C. coturnix) Cerebrum via Activating Xenobiotic-Sensing Nuclear Receptors and Modulating Cytochrome P450 Systems. Journal of Agricultural and Food Chemistry 2018, 66, 6402-6413, 10.1021/acs.jafc.8b01413.
- Ping Huangab; Agneta Rannugbc; Eva Ahlboma; Helen Håkansson; Sandra Ceccatelli; Effect of 2,3,7,8-Tetrachlorodibenzo-p-dioxin on the Expression of Cytochrome P450 1A1, the Aryl Hydrocarbon Receptor, and the Aryl Hydrocarbon Receptor Nuclear Translocator in Rat Brain and Pituitary. Toxicology and Applied Pharmacology 2000, 169, 159-167, 10.1006/taap.2000.9064.
- Seema Bansal; Adrian N. Leu; Frank J. Gonzalez; F. Peter Guengerich; Anindya Roy Chowdhury; Hindupur K. Anandatheerthavarada; Narayan G. Avadhani; Mitochondrial Targeting of Cytochrome P450 (CYP) 1B1 and Its Role in Polycyclic Aromatic Hydrocarbon-induced Mitochondrial Dysfunction. Journal of Biological Chemistry 2014, 289, 9936-9951, 10.1074/jbc.m113.525659.
- Matthew E. Albertolle; F. Peter Guengerich; The relationships between cytochromes P450 and H 2 O 2 : Production, reaction, and inhibition. Journal of Inorganic Biochemistry 2018, 186, 228-234, 10.1016/j.jinorgbio.2018.05.014.
- Rongbin Zhou; Aubry Tardivel; Bernard Thorens; Inpyo Choi; Jürg Tschopp; Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunology 2009, 11, 136-140, 10.1038/ni.1831.
- Lucie Larigot; Ludmila Juricek; Julien Dairou; Xavier Coumoul; AhR signaling pathways and regulatory functions. Biochimie Open 2018, 7, 1-9, 10.1016/j.biopen.2018.05.001.
- Daniel W. Nebert; Christopher L. Karp; Endogenous Functions of the Aryl Hydrocarbon Receptor (AHR): Intersection of Cytochrome P450 1 (CYP1)-metabolized Eicosanoids and AHR Biology. Journal of Biological Chemistry 2008, 283, 36061-36065, 10.1074/jbc.r800053200.
- Annalisa Nannelli; Francesco Rossignolo; Roberto Tolando; Paolo Rossato; Vincenzo Longo; P. Giovanni Gervasi; Effect of β-naphthoflavone on AhR-regulated genes (CYP1A1, 1A2, 1B1, 2S1, Nrf2, and GST) and antioxidant enzymes in various brain regions of pig. Toxicology 2009, 265, 69-79, 10.1016/j.tox.2009.09.010.
- Lucia García-Lara; Francisca Pérez-Severiano; Dinora González-Esquivel; Guillermo Elizondo; José Segovia; Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. Journal of Neuroscience Research 2015, 93, 1423-1433, 10.1002/jnr.23595.
- Dietmar Kültz; MOLECULAR AND EVOLUTIONARY BASIS OF THE CELLULAR STRESS RESPONSE. Annual Review of Physiology 2005, 67, 225-257, 10.1146/annurev.physiol.67.040403.103635.
- Helen M. Beere; Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. Journal of Clinical Investigation 2005, 115, 2633-2639, 10.1172/jci26471.
- David Miller; Patrice E. Fort; Heat Shock Proteins Regulatory Role in Neurodevelopment. Frontiers in Neuroscience 2018, 12, 821, 10.3389/fnins.2018.00821.
- Sonia J. Lupien; Mony de Leon; Susan De Santi; Antonio Convit; Chaim Y Tarshish; N. P. V. Nair; Mira Thakur; Bruce S. McEwen; Richard L. Hauger; Michael J. Meaney; et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience 1998, 1, 69-73, 10.1038/271.
- Nikos Kourtis; Nektarios Tavernarakis; Cellular stress response pathways and ageing: intricate molecular relationships. The EMBO Journal 2011, 30, 2520-2531, 10.1038/emboj.2011.162.
- Stuart K. Calderwood; Ayesha Murshid; Thomas Prince; The Shock of Aging: Molecular Chaperones and the Heat Shock Response in Longevity and Aging – A Mini-Review. Gerontology 2008, 55, 550-558, 10.1159/000225957.
- D Lindholm; H Wootz; L Korhonen; ER stress and neurodegenerative diseases. Cell Death & Differentiation 2006, 13, 385-392, 10.1038/sj.cdd.4401778.
- Neelakanteswar Aluru; Mathilakath M. Vijayan; Brain transcriptomics in response to β-naphthoflavone treatment in rainbow trout: The role of aryl hydrocarbon receptor signaling. Aquatic Toxicology 2008, 87, 1-12, 10.1016/j.aquatox.2007.12.012.
- Manuel Gesto; José L. Soengas; Jesús M. Míguez; Acute and prolonged stress responses of brain monoaminergic activity and plasma cortisol levels in rainbow trout are modified by PAHs (naphthalene, β-naphthoflavone and benzo(a)pyrene) treatment. Aquatic Toxicology 2008, 86, 341-351, 10.1016/j.aquatox.2007.11.014.
- Necola Guerrina; Noof Aloufi; Fangyi Shi; Kashmira Prasade; Caitlin Mehrotra; Hussein Traboulsi; Jason Matthews; David H. Eidelman; Qutayba Hamid; Carolyn J. Baglole; et al. The aryl hydrocarbon receptor reduces LC3II expression and controls endoplasmic reticulum stress. American Journal of Physiology-Lung Cellular and Molecular Physiology 2021, 320, L339-L355, 10.1152/ajplung.00122.2020.
- Hsueh-Chun Wang; Yufeng Zhou; Shau-Ku Huang; SHP-2 phosphatase controls aryl hydrocarbon receptor-mediated ER stress response in mast cells. Archives of Toxicology 2016, 91, 1739-1748, 10.1007/s00204-016-1861-1.
- Jiah Pearson-Leary; Danielle Maria Osborne; Ewan C. McNay; Role of Glia in Stress-Induced Enhancement and Impairment of Memory. Frontiers in Integrative Neuroscience 2016, 9, 63-63, 10.3389/fnint.2015.00063.
- Fernando Jauregui-Huerta; Yaveth Ruvalcaba-Delgadillo; Rocio Gonzalez-Castaneda; Joaquin Garcia-Estrada; Oscar Gonzalez-Perez; Sonia Luquin; Responses of glial cells to stress and glucocorticoids. Current Immunology Reviews 2010, 6, 195-204, 10.2174/157339510791823790.
- Fred H. Gage; Mammalian Neural Stem Cells. Science 2000, 287, 1433-1438, 10.1126/science.287.5457.1433.
- Tracey J. Shors; George Miesegaes; Anna Beylin; Mingrui Zhao; Tracy Rydel; Elizabeth Gould; Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001, 410, 372-376, 10.1038/35066584.
- Neeta S. Roy; Abdellatif Benraiss; Su Wang; Richard A.R. Fraser; Robert Goodman; William T. Couldwell; Maiken Nedergaard; Ayano Kawaguchi; Hideyuki Okano; Steven A. Goldman; et al. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. Journal of Neuroscience Research 2000, 59, 321-331, 10.1002/(sici)1097-4547(20000201)59:3<321::aid-jnr5>3.0.co;2-9.
- Maurice A. Curtis; Monica Kam; Ulf Nannmark; Michelle F. Anderson; Mathilda Zetterstrom Axell; Carsten Wikkelso; Stig Holtås; Willeke M. C. van Roon-Mom; Thomas Björk-Eriksson; Claes Nordborg; et al.Jonas FrisénMichael DragunowRichard L. M. FaullPeter S. Eriksson Human Neuroblasts Migrate to the Olfactory Bulb via a Lateral Ventricular Extension. Science 2007, 315, 1243-1249, 10.1126/science.1136281.
- Michael H. Donovan; Umar Yazdani; Rebekah D. Norris; Dora Games; Dwight C. German; Amelia J. Eisch; Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. The Journal of Comparative Neurology 2006, 495, 70-83, 10.1002/cne.20840.
- M. Bouab; G.N. Paliouras; A. Aumont; Karl Forest-Bérard; K.J.L. Fernandes; Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience 2010, 173, 135-149, 10.1016/j.neuroscience.2010.11.032.
- Owen Carmichael; Samuel Lockhart; The Role of Diffusion Tensor Imaging in the Study of Cognitive Aging. Neuroscience of Aggression 2011, 11, 289-320, 10.1007/7854_2011_176.
- Christopher S. Bjornsson; Maria Apostolopoulou; Yangzi Tian; Sally Temple; It Takes a Village: Constructing the Neurogenic Niche. Developmental Cell 2015, 32, 435-446, 10.1016/j.devcel.2015.01.010.
- Charise Garber; Michael Vasek; Lauren Vollmer; Tony Sun; Xiaoping Jiang; Robyn S. Klein; Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nature Immunology 2017, 19, 151-161, 10.1038/s41590-017-0021-y.
- Yangsheng Chen; Li Xu; Heidi Q.H. Xie; Tuan Xu; Hualing Fu; Songyan Zhang; Rui Sha; Yingjie Xia; Bin Zhao; Identification of differentially expressed genes response to TCDD in rat brain after long-term low-dose exposure. Journal of Environmental Sciences 2017, 62, 92-99, 10.1016/j.jes.2017.07.010.
- Juan De La Parra; Maribel Cuartero; Alberto Pérez-Ruiz; Alicia Garcia-Culebras; Ricardo Martín; José Sánchez-Prieto; Juan M. García-Segura; Ignacio Lizasoain; María A. Moro; AhR Deletion Promotes Aberrant Morphogenesis and Synaptic Activity of Adult-Generated Granule Neurons and Impairs Hippocampus-Dependent Memory. eneuro 2018, 5, -17.2018, 10.1523/eneuro.0370-17.2018.
- Rossella DI Giaimo; Tamara Durovic; Pablo Barquin; Anita Kociaj; Tjasa Lepko; Sven Aschenbroich; Christopher T. Breunig; Martin Irmler; Filippo Cernilogar; Gunnar Schotta; et al.Joana S. BarbosaDietrich TrümbachEmily Violette BaumgartAndrea M. NeunerJohannes BeckersWolfgang WurstStefan StrickerJovica Ninkovic The Aryl Hydrocarbon Receptor Pathway Defines the Time Frame for Restorative Neurogenesis. Cell Reports 2018, 25, 3241-3251.e5, 10.1016/j.celrep.2018.11.055.
- Majid Keshavarzi; Mohammad Javad Khoshnoud; Ali Ghaffarian Bahraman; Afshin Mohammadi-Bardbori; An Endogenous Ligand of Aryl Hydrocarbon Receptor 6-Formylindolo[3,2-b]Carbazole (FICZ) Is a Signaling Molecule in Neurogenesis of Adult Hippocampal Neurons. Journal of Molecular Neuroscience 2020, 70, 806-817, 10.1007/s12031-020-01506-x.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
20 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




