
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmed Safwat Abbass Mahdy Hedar | + 1232 word(s) | 1232 | 2021-10-14 10:37:05 | | | |
| 2 | Catherine Yang | Meta information modification | 1232 | 2021-10-15 03:27:26 | | |
Video Upload Options
Atherosclerosis can be classified into primary simple atherosclerosis, which occurs with age, and secondary autoimmune atherosclerosis, which was also coined as accelerated atherosclerosis. Atherosclerosis is the main cause of cardiovascular diseases in autoimmune rheumatic diseases.
1. Introduction
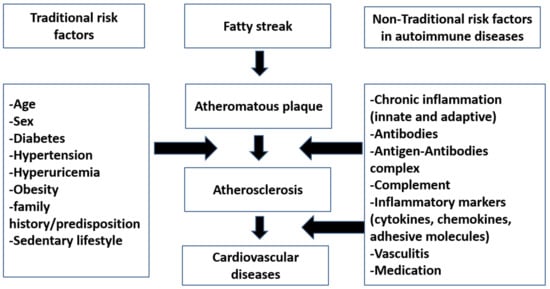
Until now, no evidence about the true mechanism of the atherosclerosis process in autoimmune rheumatic diseases (AIRDs) is known [1]. Current research findings are often contradictory. Whether traditional risk factor like age, gender, smoking, or hypertension contribute solely to the atherosclerosis, or whether it is a consequence of a nontraditional states like chronic inflammation or changes in cytokines, antibodies that accompany atherosclerosis, or a combination of both aspects is not yet clear and is debated [1]. Moreover, it is currently not known which factors play a major role in process of development of premature atherosclerosis in autoimmune rheumatic disease patients.
Atherosclerosis can be classified into primary simple atherosclerosis, which occurs with age, and secondary autoimmune atherosclerosis, which was also coined as accelerated atherosclerosis [2]. Atherosclerosis is the main cause of cardiovascular diseases in autoimmune rheumatic diseases.
2. Traditional Risk Factors in Atherosclerosis
3. Nontraditional Risk Factors in Atherosclerosis
References
- Frostegård, J. Atherosclerosis in Patients with Autoimmune Disorders. Arter. Thromb. Vasc. Biol. 2005, 25, 1776–1785.
- Sima, P.; Vannucci, L.; Vetvicka, V. Atherosclerosis as autoimmune disease. Ann. Transl. Med. 2018, 6, 116.
- Giannelou, M.; Mavragani, C.P. Cardiovascular disease in systemic lupus erythematosus: A comprehensive update. J. Autoimmun. 2017, 82, 1–12.
- Amaya-Amaya, J.; Montoya-Sánchez, L.; Rojas-Villarraga, A. Cardiovascular Involvement in Autoimmune Diseases. BioMed Res. Int. 2014, 2014, 367359.
- Teixeira, V.; Tam, L.-S. Novel Insights in Systemic Lupus Erythematosus and Atherosclerosis. Front. Med. 2018, 4, 262.
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109–114.
- Masuko, K. Rheumatoid Cachexia Revisited: A Metabolic Co-Morbidity in Rheumatoid Arthritis. Front. Nutr. 2014, 1, 20.
- Pérez-Baos, S.; Prieto-Potin, I.; Roman-Blas, J.A.; Pernaute, O.S.; Largo, R.; Herrero-Beaumont, G. Mediators and Patterns of Muscle Loss in Chronic Systemic Inflammation. Front. Physiol. 2018, 9, 409.
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142.
- Klaasen, R.; Wijbrandts, C.A.; Gerlag, D.M.; Tak, P.P. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum. 2010, 63, 359–364.
- García-Poma, A.; Segami, M.I.; Mora, C.S.; Ugarte-Gil, M.F.; Terrazas, H.N.; Rhor, E.A.; García, E.; Ramos, M.P.; Alva, M.; Castañeda, I.; et al. Obesity is independently associated with impaired quality of life in patients with rheumatoid arthritis. Clin. Rheumatol. 2007, 26, 1831–1835.
- de Resende Guimarães, M.F.B.; Rodrigues, C.E.M.; Gomes, K.W.P.; Machado, C.; Brenol, C.V.; Krampe, S.F.; De Andrade, N.P.B.; Kakehasi, A.M. High prevalence of obesity in rheumatoid arthritis patients: Association with disease activity, hypertension, dyslipidemia and diabetes, a multi-center study. Adv. Rheumatol. 2019, 59, 44–49.
- van der Helm-van Mil, A.H.M.; Van Der Kooij, S.M.; Allaart, C.F.; Toes, R.; Huizinga, T.W.J. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 769–774.
- Kerekes, G.; Nurmohamed, M.T.; González-Gay, M.A.; Seres, I.; Paragh, G.; Kardos, Z.; Baráth, Z.; Tamási, L.; Soltész, P.; Szekanecz, Z. Rheumatoid arthritis and metabolic syndrome. Nat. Rev. Rheumatol. 2014, 10, 691–696.
- Zhu, J.; Nelson, K.; Toth, J.; Muscat, J.E. Nicotine dependence as an independent risk factor for atherosclerosis in the National Lung Screening Trial. BMC Public Health 2019, 19, 103.
- Mahmoudi, M.; Aslani, S.; Fadaei, R.; Jamshidi, A.R. New insights to the mechanisms underlying atherosclerosis in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 287–297.
- Spinelli, F.R.; Pecani, A.; Ciciarello, F.; Colasanti, T.; Di Franco, M.; Miranda, F.; Conti, F.; Valesini, G.; Alessandri, C. Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2017, 18, 214.
- Tinelli, C.; DI Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies. Front. Nutr. 2019, 6, 49.
- Lazzerini, P.E.; Capecchi, P.L.; Selvi, E.; Lorenzini, S.; Bisogno, S.; Galeazzi, M.; Pasini, F.L. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun. Rev. 2007, 6, 503–509.
- Mok, C.C. Metabolic syndrome and systemic lupus erythematosus: The connection. Expert Rev. Clin. Immunol. 2019, 15, 765–775.
- Meek, I.L.; Vonkeman, H.E.; Van De Laar, M.A. Hyperuricaemia: A marker of increased cardiovascular risk in rheumatic patients: Analysis of the ACT-CVD cohort. BMC Musculoskelet. Disord. 2014, 15, 174.
- Skeoch, S.; Bruce, I.N. Atherosclerosis in rheumatoid arthritis: Is it all about inflammation? Nat. Rev. Rheumatol. 2015, 11, 390–400.
- Sherer, Y.; Shoenfeld, Y. Mechanisms of Disease: Atherosclerosis in autoimmune diseases. Nat. Clin. Pr. Rheumatol. 2006, 2, 99–106.
- Kobezda, T.; Ghassemi-Nejad, S.; Mikecz, K.; Glant, T.T.; Szekanecz, Z. Of mice and men: How animal models advance our understanding of T-cell function in RA. Nat. Rev. Rheumatol. 2014, 10, 160–170.
- Szekanecz, Z.; Kerekes, G.; Végh, E.; Kardos, Z.; Baráth, Z.; Tamási, L.; Shoenfeld, Y. Autoimmune atherosclerosis in 3D: How it develops, how to diagnose and what to do. Autoimmun. Rev. 2016, 15, 756–769.
- Mengya, Z.; Hanyou, M.; Dong, L.; Xiaohong, L.; Lihua, Z. Th17/Treg imbalance induced by increased incidence of atherosclerosis in patients with Systemic Lupus Erythematosus (SLE). Clin. Rheumatol. 2013, 32, 1045–1052.
- Li, P.; Zheng, Y.; Chen, X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front. Pharmacol. 2017, 8, 460.
- Kerekes, G.; Szekanecz, Z.; Dér, H.; Sándor, Z.; Lakos, G.; Muszbek, L.; Csipö, I.; Sipka, S.; Seres, I.; Paragh, G.; et al. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: A multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J. Rheumatol. 2008, 35, 398–406.
- Matsuura, E.; Atzeni, F.; Sarzi-Puttini, P.; Turiel, M.; Lopez, L.R.; Nurmohamed, M.T. Is atherosclerosis an autoimmune disease? BMC Med. 2014, 12, 47.
- Sanjadi, M.; Sichanie, Z.R.; Totonchi, H.; Karami, J.; Rezaei, R.; Aslani, S. Atherosclerosis and autoimmunity: A growing relationship. Int. J. Rheum. Dis. 2018, 21, 908–921.
- Bugatti, S.; Manzo, A.; Montecucco, C.; Caporali, R.F. The Clinical Value of Autoantibodies in Rheumatoid Arthritis. Front. Med. 2018, 5, 339.
- Westerlind, H.; Rönnelid, J.; Hansson, M.; Alfredsson, L.; Mathsson-Alm, L.; Serre, G.; Cornillet, M.; Holmdahl, R.; Jakobsson, P.; Skriner, K.; et al. Anti–Citrullinated Protein Antibody Specificities, Rheumatoid Factor Isotypes, and Incident Cardiovascular Events in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 1658–1667.
- Majka, D.S.; Vu, T.-H.T.; Pope, R.M.; Teodorescu, M.; Karlson, E.W.; Liu, K.; Chang, R.W. Association of Rheumatoid Factors With Subclinical and Clinical Atherosclerosis in African American Women: The Multiethnic Study of Atherosclerosis. Arthritis Rheum. 2016, 69, 166–174.
- Pertovaara, M.; Kähönen, M.; Juonala, M.; Laitinen, T.; Taittonen, L.; Lehtimäki, T.; Viikari, J.S.A.; Raitakari, O.T.; Hurme, M. Autoimmunity and atherosclerosis: The presence of antinuclear antibodies is associated with decreased carotid elasticity in young women. The Cardiovascular Risk in Young Finns Study. Rheumatology 2009, 48, 1553–1556.
- Didier, K.; Bolko, L.; Giusti, D.; Toquet, S.; Robbins, A.; Antonicelli, F.; Servettaz, A. Autoantibodies Associated With Connective Tissue Diseases: What Meaning for Clinicians? Front. Immunol. 2018, 9, 541.
- Ames, P.R.J.; Alves, J.D.; Lopez, L.R.; Gentile, F.; Margarita, A.; Pizzella, L.; Batuca, J.; Scenna, G.; Brancaccio, V.; Matsuura, E. Antibodies Against β2-Glycoprotein I Complexed With an Oxidised Lipoprotein Relate to Intima Thickening of Carotid Arteries in Primary Antiphospholipid Syndrome. Clin. Dev. Immunol. 2006, 13, 1–9.
- Cinoku, I.; Mavragani, C.P.; Tellis, C.C.; Nezos, A.; Tselepis, A.D.; Moutsopoulos, H.M. Autoantibodies to ox-LDL in Sjögren’s syndrome: Are they atheroprotective? Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 61–67.
- Lopez, L.R.; Salazar-Paramo, M.; Palafox-Sanchez, C.; Hurley, B.L.; Matsuura, E.; La Torre, I.G.-D. Oxidized low-density lipoprotein and β2-glycoprotein I in patients with systemic lupus erythematosus and increased carotid intima-media thickness: Implications in autoimmune-mediated atherosclerosis. Lupus 2006, 15, 80–86.
- Sciascia, S.; Amigo, M.-C.; Roccatello, D.; Khamashta, M. Diagnosing antiphospholipid syndrome: ’extra-criteria’ manifestations and technical advances. Nat. Rev. Rheumatol. 2017, 13, 548–560.
- Napodano, C.; Gulli, F.; Rapaccini, G.L.; Marino, M.; Basile, U. Cryoglobulins: Identification, classification, and novel biomarkers of mysterious proteins. Adv. Clin. Chem. 2021, 104, 299–340.
- Ragab, G.; Hussein, M.A. Vasculitic syndromes in hepatitis C virus: A review. J. Adv. Res. 2017, 8, 99–111.
- Filer, A.; Gardner-Medwin, J.M.; Thambyrajah, J.; Raza, K.; Carruthers, D.M.; Stevens, R.J.; Liu, L.; Lowe, S.E.; Townend, J.; Bacon, P.A. Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann. Rheum. Dis. 2003, 62, 162–167.
- Miranda-Carús, M.-E.; Askanase, A.D.; Clancy, R.M.; Di Donato, F.; Chou, T.-M.; Libera, M.R.; Chan, E.K.L.; Buyon, J.P. Anti-SSA/Ro and Anti-SSB/La Autoantibodies Bind the Surface of Apoptotic Fetal Cardiocytes and Promote Secretion of TNF-α by Macrophages. J. Immunol. 2000, 165, 5345–5351.
- Jobling, K.; Rajabally, H.; Ng, W.-F. Anti-Ro antibodies and complete heart block in adults with Sjögren’s syndrome. Eur. J. Rheumatol. 2018, 5, 194–196.
- Sung, M.J.; Park, S.-H.; Kim, S.-K.; Lee, Y.-S.; Park, C.-Y.; Choe, J.-Y. Complete Atrioventricular Block in Adult Sjögren’s Syndrome with Anti-Ro Autoantibody. Korean J. Intern. Med. 2011, 26, 213–215.




