
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefan Ioan Voicu | + 2434 word(s) | 2434 | 2020-06-10 10:54:47 | | | |
| 2 | Catherine Yang | Meta information modification | 2434 | 2020-06-11 07:36:44 | | | | |
| 3 | Catherine Yang | -5 word(s) | 2429 | 2020-10-27 11:05:13 | | |
Video Upload Options
Cellulose-based materials are a viable alternative to synthetic polymers due to their favorable physico-chemical and biological characteristics. They are also an appropriate organic matrix for the incorporation of hydroxyapatite particles, inter and intramolecular hydrogen bonds, as well as electrostatic interactions being formed between the functional groups on the polymeric chains surface and the inorganic filler. Considering the versatility of hydroxyapatite particles, the hybrid materials offer favorable prospects for applications in water purification, tissue engineering, drug delivery, and hemodialysis. The preparation technique and the chemical composition have a big influence on the final membrane properties. The well-established membrane fabrication methods such as phase inversion, electrospinning, or gradual electrostatic assembly are discussed, together with the various strategies employed to obtain a homogenous dispersion of the inorganic particles in the polymeric matrix. Finally, the main conclusions and the future directions regarding the preparation and applications of cellulose derivatives/hydroxyapatite composite membranes are presented.
1. Introduction
Among the functional materials currently known, membranes possess a unique characteristic and that is selectivity [1]. Another aspect is represented by the fact that they were the first functional materials known on earth—the membrane of the first unicellular organism [2]. Polymeric membranes were manufactured on a wide scale as filtering materials after the Second World War from the practical necessity of obtaining drinkable water from affected or contaminated natural sources. The first polymer applied industrially to obtain these membranes was cellulose nitrate, an explosive powder used to fabricate bombs and as a consequence, available in large quantities. Membrane technology was constituted in an individual scientific field starting with the research conducted by Loeb and Sourirajan that explained the formation mechanism of asymmetric membranes and the coagulation phenomenon of a polymer from concentrated solution in the presence of a non-solvent [3]. Once the formation mechanism of polymeric membranes was established and understood, more and more materials were developed for different practical applications such as gas separation [4][5][6], protein concentration [7][8][9], heavy metals separation [10], and removal of environmental pollutants [11][12][13]. As time passed, beyond their primary role as filtering materials used to obtain drinking water, membranes were employed in more and more advanced applications, the field with the most advanced performance demands being biomedical engineering. To fulfill the requests of such applications, composite membranes were developed by incorporating nanostructured inorganic fillers in the polymeric matrix thus resulting in a synergistic performance by combining the advantages of organic and inorganic materials [14]. One of the first niche domains targeted the obtainment of hemodialysis membranes [15]. Hemodialysis is the membrane process designed for patients with chronic kidney disease [16] and it is used to substitute kidney functions once every two days. The main chemical species that are separated during this process are urea, uric acid, and excess of creatinine and salts from the human body [17]. Other biomedical applications based on the extracorporeal blood circulation is the artificial lung used especially during open-heart surgeries [18][19] or experimental studies for an artificial liver based on composite membranes functionalized with porcine hepatocytes [20].
2. Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite
Due to the porosity and semi-permeable properties, polymeric membranes are also used in the development of controlled drug delivery devices [21], in an attempt to avoid the toxic effects of high quantities of pharmaceutically active substances on the human body. Another increasingly researched field of application is the one of osseointegration membranes that are placed at the interface between a metallic implant and the bone, with the purpose of favoring the pre-osteoblasts growth and spreading, in order to integrate the implant into the host bone tissue [22][23][24]. These latter membranes are based especially on composites with hydroxyapatite. Hydroxyapatite is one of the natural bone components, the so called “soft component”. It can be used as such, to obtain composites [25][26] or with the addition of other elements, like silver, to obtain hydroxyapatite with antibacterial properties [27]. Hydroxyapatite also has a high synthesis versatility because it can be of animal [28][29] or synthetic origin [30]. The present review desires to offer the reader an overview on the recent progress made in the domain of composite membranes based on cellulose derivatives and hydroxyapatite. Why these two components? They both present the great advantage of originating from natural sources, which is associated with biocompatibility of the resulting composites. More than that, once inserted into the human body, in the case of tissue engineering scaffolds, cellulose presents the remarkable property of bioresorbability, with only glucose molecules resulting after its hydrolysis and degradation. Cellulose derivatives-based membranes for various applications such as water purification, tissue engineering, osseointegration, drug delivery, and hemodialysis will be presented, all having in common hydroxyapatite as a filler agent.
Pure cellulose is frequently turned into cellulose derivatives to overcome the drawbacks related to its poor solubility in common organic solvents [31]. Due to the low cost and widespread availability of cellulose [32], cellulose acetate (CA) and carboxymethylcellulose (CMC) particularly are among the most used cellulose-based matrices for the incorporation of inorganic hydroxyapatite particles in order to obtain hybrid composite membranes with improved physico-chemical and biological characteristics.
Cellulose acetate is a cellulose ester formed by partial or full acetylation of the free hydroxyl groups in the anhydroglucose unit. Depending on the acetyl content that usually ranges between 29% and 48%, mono-, di-, and triacetate can be differentiated [[33]. The ability of facile processing by various techniques and its broad range of applications make cellulose acetate the most commonly synthesized cellulose derivative worldwide, the global production of CA from biomass being projected by Global Industry Analysis to be 751.1 thousand metric tons until 2024 [34]. The classic cellulose acetylation process is based on the reaction between wood or cotton pulp with acetic anhydride as the acetylation agent and sulfuric acid as catalyst in an acetic acid reaction media [35]. Currently, this approach is used industrially but more and more research is being conducted on the use of agro-industrial residues and environmentally friendly synthesis routes that involve replacing the sulfuric and acetic acids by eco-friendly reagents [36]. Cellulose acetate is employed for the production of a variety of consumer goods including textiles, photographic films, personal hygiene products, and cigarette filters [37] but its resistance to the action of chemical agents, good thermal stability, flexibility, and mechanical strength [38], coupled with low fouling susceptibility and a hydrophilic nature [39], recommend this polymer especially for the production of membranes, with applications in industrial and biomedical processes (e.g., adsorption, separation, catalysis, biosensing, drug delivery, or tissue regeneration). Cellulose acetate was used in the purification process of contaminated resources of natural gas starting with the mid 1980s, when several companies applied dried CA membranes for CO2/CH4 natural gas separations. The hydrophilic nature of cellulose acetate makes the membranes suitable for assisting chemical and biochemical reactions as well as for the removal of polar compounds or specific organic–organic separations using pervaporation. The separation of helium particularly is of great interest in the natural gas purification process, due to its high added value. Asymmetric CA membranes presented an acceptable permeability for He and a good He/N2 and He/CH4 selectivity, being considered economically feasible for usage in three stage membrane processes with recycle streams [40]. Carboxymethyl cellulose, one of the most important cellulose ethers, is synthesized by treating alkali cellulose with monochloroacetic acid or sodium monochloro-acetate in an aqueous sodium hydroxide (NaOH) medium [41]. Wood residues, cotton linters, paper sludge, and agricultural waste biomass such as orange peels, corncobs, sugarcane bagasse, rice, or corn husks were used so far as cellulose sources for the preparation of carboxymethylcellulose [42]. As the reaction takes place, the hydroxyl groups in the cellulose backbone are replaced with carboxymethyl groups in the C6 > C3 > C2 order [43]. The chemical structure obtained following etherification is responsible for the unique properties of carboxymethylcellulose, such as water solubility, non-toxicity, biodegradability, transparency, and good film forming ability [44]. Owing to these characteristics, carboxymethylcellulose is already applied in the food, pharmaceutical, and daily-use chemical industries as an emulsifier, thickener, and a flocculating or chelating agent [45][46]. Currently, carboxymethylcellulose based materials are investigated for biomedical applications such as tissue engineering [47] and drug delivery [48] mainly in the shape of hydrogels, membranes, and nanoparticles [49].
Some of the most popular membrane manufacturing techniques include electrospinning, gradual electrostatic assembly, and phase inversion by immersion precipitation or solvent evaporation.
Phase inversion is a popular method for the preparation of cellulose acetate membranes. The first step of this process consists in the dissolution of the polymer in an appropriate solvent, such as hexafluoro-2-propanol [50], formic acid [51], N, N-dimethylformamide [52], acetone [53][54][55], or a mixed solvent system of the latter two [56], to obtain a homogenous polymeric solution. Afterwards, the obtained solution is cast on a glass plate and submerged in a coagulation bath containing a non-solvent, usually distilled water. The penetration of the solvent into the non-solvent and non-solvent into the polymeric solution cause demixing and polymer precipitation with the formation of a membrane with an asymmetric structure, composed in most cases of a thin film top layer also called “skin”, a support porous substructure, and a bottom layer [54][57].
Gradual electrostatic assembly is based on the spontaneous interactions between two oppositely charged polysaccharides, mixed in an aqueous solution. The electrostatic interactions are followed by polymeric chain entanglement and hydrogen bond formation, this resulting in a polyelectrolyte complex membrane. Due to its anionic nature, carboxymethylcellulose was used in combination with cationic polymers such as chitosan (CS) to prepare such composites [58].
Cellulose acetate was among the first electrospun polymers [59]; it is considered suitable for electrospinning because it can be easily dissolved in common organic solvents and maintains high mechanical strength during membrane fabrication [60]. To date, there are no studies that report the effective electrospinning of carboxymethylcellulose without the addition of another polymer; nonetheless, blends of CMC with polyvinylpyrrolidone (PVP) [61], polyvinyl alcohol (PVA) [62], or polyethylene oxide (PEO) [63][64] were successfully electrospun into nanofibrous membranes with applications especially in the biomedical area. The electrospinning experiments usually take place at room temperature under normal atmospheric conditions. The device consists of three major parts: high voltage power supply, feeding nozzle or spinneret, and a grounded collecting plate (metal plate or rotating drum). The polymeric solution is inserted into a capillary tube connected to a feeding nozzle. A high voltage source is used to inject a certain polarity charge into the polymeric solution or melt. When the electric field reaches a critical value the repulsive electrical forces surpass the surface tension forces at the tip of the nozzle and the solution is accelerated towards the opposite polarity collector. The solvent is evaporated and polymeric fibers are formed [65]. Solution viscosity, polymer concentration, and molecular weight are important factors that influence the electrospinning process and an optimal balance between them must be achieved in order to generate uniform fibers. Due to their unique properties, electrospun fibers have been successfully applied in various domains such as environmental engineering, pharmaceutics, optoelectronics, biomedicine, and biotechnology [66].
Lately, an increased research interest was directed towards the utilization of cellulose derivatives/hydroxyapatite composite membranes in environmental or biomedical engineering (e.g., water purification, bone tissue regeneration, wound healing, controlled drug delivery). It was found that there is a good compatibility between cellulose-based matrices and hydroxyapatite, the inorganic particles interacting with the organic component by inter and intramolecular hydrogen bonds, and also by ion-dipole forces formed between the calcium ions of HA and the functional groups on the cellulose derivatives surface [60]. For example, in the case of CA, the positively charged calcium ions bind with the negatively charged carboxylate groups and hydrogen bonds are formed between the hydroxyl groups of cellulose acetate and hydroxyapatite (Figure 1).
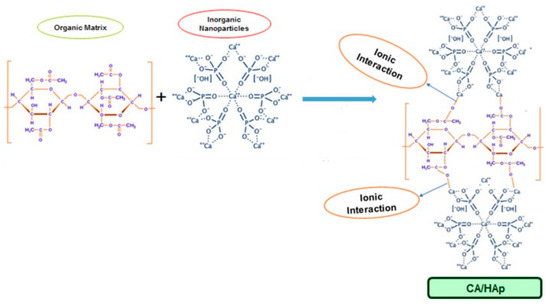
Figure 1. Proposed formation mechanism of cellulose acetate/hydroxyapatite hybrid composites (reproduced with permission from Ref. [60]).
It is demonstrated that hydroxyapatite (HA) particles are good mineral adsorbers and have the capacity to bind divalent heavy metal ions, hence they were researched for the removal of harmful substances in drinking water [53]. Hydroxyapatite particles can also be used as additives for cellulose acetate to improve the morphology and properties of the membranes, this resulting in a better separation performance and higher water flux values [54]. More than that they could act as a catalyst for the precipitation process involved in phase inversion, by increasing the viscosity of the solution [39][57]. The most common synthesis methods for membranes are phase inversion with two possible variants—precipitation in a non-solvent and solvent evaporation. The most versatile is the precipitation of the membrane in a non-solvent. The non-solvent flow through solution polymer film will determine the porosity and morphology depending on several parameters—viscosity, temperature, and miscibility with the polymer solvent. These properties directly influence the speed of membrane formation—the higher the formation speed, the smaller the pore diameter with a high distribution of pores at the surface. A slow process of membrane coagulation will lead to pores with a large diameter and a lower distribution of pores on the surface. Each possibility is preferred depending on the application of the synthesized membrane. The presence of hydroxyapatite in a polymer solution acts in two ways—firstly it will increase the viscosity of the solution and second it will influence the speed of non-solvent through the polymer solution film. The first influence is always higher, so from a more viscous initial solution, membranes with a decreased diameter of pores will be obtained. This can be translated into a more efficient separation. More than that, hydroxyapatite particles itself, in the structure of the membrane, participate in the separation process due to their porosity and ability to retain small chemical species like cations, pesticides, and dyes.
An arising issue is represented by the aggregation tendency of the inorganic particles but various strategies such as ultrasound-assisted mixing [52], surfactants addition [54], or modification of the hydroxyapatite surface [56] are investigated for an improved dispersion. The aggregation tendency of hydroxyapatite particles in aqueous media was successfully overcome by using electrospinning as membrane fabrication technique. Electrospun membranes have some advantages over phase inversion membranes in terms of volume and aspect ratio, specific area, and porosity, but, according to a recent study, HA concentrations higher than 3 wt/v% generated a “beads on a string” morphology in the case of CA solutions (15 wt/v%) in a mixed solvent system of acetone and N,N-dimethylformamide (DMF) [[60]. Therefore, the inorganic filler concentration must be carefully chosen in order to obtain smooth nanofibers. Due to its biocompatibility, bioactivity, and osteoconductive properties, hydroxyapatite distinguished itself among materials used for bone tissue regeneration. However, the direct use of hydroxyapatite in such applications was associated with poor mechanical and chemical stability especially in the case of synthetic particles [67]. Therefore hybrid polymer/hydroxyapatite composites were developed, natural polymers such as collagen [68][69], polylactic acid [24][70], chitosan [71][72], and cellulose [73] being preferred, instead of synthetic ones [74], for the preparation of novel composite materials with superior bioactivity compared to pure components.
References
- Mathias Ulbricht; Advanced functional polymer membranes. Polymer 2006, 47, 2217-2262, 10.1016/j.polymer.2006.01.084.
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; Wiley: Hoboken, NJ, USA, 1995.
- Loeb, S. The Loeb-Sourirajan Membrane: How It Came About; American Chemical Society (ACS): Washington, DC, USA, 1981; Volume 153, pp. 1–9.
- Tiwari, R.R.; Jin, J.; Freeman, B.; Paul, D.R. Physical aging, CO2 sorption and plasticization in thin films of polymer with intrinsic microporosity (PIM-1). J. Membr. Sci. 2017, 537, 362–371.
- Zhou, H.; Tao, F.; Liu, Q.; Zong, C.; Yang, W.; Cao, X.; Jin, W.; Xu, N. Microporous Polyamide Membranes for Molecular Sieving of Nitrogen from Volatile Organic Compounds. Angew. Chem. Int. Ed. 2017, 56, 5755–5759.
- Zhou, H.; Jin, W. Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications. Membranes 2018, 9, 3.
- Voicu, Ş.I.; Dobrica, A.; Sava, S.; Ivan, A.; Naftanaila, L. Cationic surfactants-controlled geometry and dimensions of polymeric membrane pores. J. Optoelectron. Adv. Mater. 2012, 14, 923–928.
- Ionită, M.; Crica, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61.
- Voicu, Ş.I.; Pandele, A.; Tanasă, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone-graphene oxide composite films properties. Dig. J. Nanomater. Biostruct. 2013, 8, 1389–1394.
- Oana Serbanescu; Andreea Pandele; Florin Miculescu; Stefan Ioan Voicu; Synthesis and Characterization of Cellulose Acetate Membranes with Self-Indicating Properties by Changing the Membrane Surface Color for Separation of Gd(III). Coatings 2020, 10, 468, 10.3390/coatings10050468.
- Raicopol, M.D.; Andronescu, C.; Voicu, S.I.; Vasile, E.; Pandele, A.M. Cellulose acetate/layered double hydroxide adsorptive membranes for efficient removal of pharmaceutical environmental contaminants. Carbohydr. Polym. 2019, 214, 204–212.
- Thakur, V.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165.
- Satulu, V.; Mitu, B.; Pandele, A.; Voicu, S.; Kravets, L.; Dinescu, G. Composite polyethylene terephthalate track membranes with thin teflon-like layers: Preparation and surface properties. Appl. Surf. Sci. 2019, 476, 452–459.
- Roberto Castro-Muñoz; Kumar Varoon Agrawal; Joaquín Coronas; Ultrathin permselective membranes: the latent way for efficient gas separation. RSC Advances 2020, 10, 12653-12670, 10.1039/d0ra02254c.
- D. Stamatialis; Bernke J. Papenburg; Miriam Gironès; Saiful Saiful; Srivatsa N.M. Bettahalli; Stephanie Schmitmeier; Matthias Wessling; Medical applications of membranes: Drug delivery, artificial organs and tissue engineering. Journal of Membrane Science 2008, 308, 1-34, 10.1016/j.memsci.2007.09.059.
- Mihai Corobea; Oana Muhulet; Florin Miculescu; Iulian Vaile Antoniac; Zina Vuluga; Dorel Florea; Dumitru Mircea Vuluga; Maria Butnaru; Daniela Ivanov; Stefan Ioan Voicu; et al.V.K. Thakur Novel nanocomposite membranes from cellulose acetate and clay-silica nanowires. Polymers for Advanced Technologies 2016, 27, 1586-1595, 10.1002/pat.3835.
- D. Falkenhagen; W. Strobl; Jens Hartmann; A. Schrefl; I. Linsberger; K.-H. Kellner; F. Aussenegg; A. Leitner; Patient safety technology for microadsorbent systems in extracorporeal blood purification.. Artificial Organs 2002, 26, 84-90, 10.1046/j.1525-1594.2002.06884.x.
- Flendrig, L.M.; La Soe, J.W.; Jörning, G.G.; Steenbeek, A.; Karlsen, O.T.; Bovée, W.M.; Ladiges, N.C.; Velde, A.A.T.; Chamuleau, R.A. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J. Hepatol. 1997, 26, 1379–1392.
- Shih, C.; Lee, K.-R.; Lai, J. 60Co γ-ray irradiation modified poly(4-methyli-pentene) membrane for oxygenator. Eur. Polym. J. 1994, 30, 629–634.
- Igor Sauer; P. Neuhaus; J. C. Gerlach; Concept for modular extracorporeal liver support for the treatment of acute hepatic failure.. Metabolic Brain Disease 2002, 17, 477-484, 10.1023/a:1021938708670.
- Lara Leoni; Anthony Boiarski; Tejal A. Desai; Characterization of Nanoporous Membranes for Immunoisolation: Diffusion Properties and Tissue Effects. Biomedical Microdevices 2002, 4, 131-139, 10.1023/a:1014639332543.
- Corobea, M.S.; Albu-Kaya, M.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.I.; et al. Modification of titanium surface with collagen and doxycycline as a new approach in dental implants. J. Adhes. Sci. Technol. 2015, 29, 1–14.
- Voicu, S.I.; Condruz, R.M.; Mitran, V.; Cimpean, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Thakur, V. Sericin Covalent Immobilization onto Cellulose Acetate Membrane for Biomedical Applications. ACS Sustain. Chem. Eng. 2016, 4, 1765–1774.
- Pandele, A.M.; Constantinescu, A.E.; Radu, I.; Miculescu, F.; Voicu, S.I.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274.
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.; Stan, G.; Ciocan, L.T. Progress in Hydroxyapatite–Starch Based Sustainable Biomaterials for Biomedical Bone Substitution Applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512.
- Miculescu, F.; Maidaniuc, A.; Miculescu, M.; Batalu, N.D.; Ciocoiu, R.C.; Voicu, S.I.; Stan, G.; Thakur, V. Synthesis and Characterization of Jellified Composites from Bovine Bone-Derived Hydroxyapatite and Starch as Precursors for Robocasting. ACS Omega 2018, 3, 1338–1349.
- Andreea Maidaniuc; M. Miculescu; S. I. Voicu; L. T. Ciocan; M. Niculescu; Mihai Corobea; M. E. Rada; F. Miculescu; Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. Journal of Adhesion Science and Technology 2016, 30, 1829-1841, 10.1080/01694243.2016.1163808.
- Miculescu, F.; Mocanu, A.C.; Stan, G.; Miculescu, M.; Maidaniuc, A.; Cimpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157.
- Maidaniuc, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Matei, E.; Pencea, I.; Csaki, I.; Machedon-Pisu, T.; Ciocan, L.T.; Voicu, S.I.; et al. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166.
- Florin Miculescu; Aura-Cătălina Mocanu; Cătălina Andreea Dascălu; Andreea Maidaniuc; Dan Batalu; Andrei Constantin Berbecaru; Stefan Ioan Voicu; Marian Miculescu; V.K. Thakur; Lucian Toma Ciocan; et al. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614-622, 10.1016/j.vacuum.2017.06.008.
- Juho Antti Sirviö; Juha P. Heiskanen; Room-temperature dissolution and chemical modification of cellulose in aqueous tetraethylammonium hydroxide–carbamide solutions. Cellulose 2019, 27, 1933-1950, 10.1007/s10570-019-02907-x.
- Khiari, R.; Belgacem, M.N. Potential for using multiscale Posidonia oceanica waste. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 447–471.
- Gilad Alfassi; Rein; Avi Shpigelman; Yachin Cohen; Dmitry M. Rein; Partially Acetylated Cellulose Dissolved in Aqueous Solution: Physical Properties and Enzymatic Hydrolysis. Polymers 2019, 11, 1734, 10.3390/polym11111734.
- Global Industry Analysis. Cellulose Acetate (MCP-2035). Available online: https://www.strategyr.com/market-report-cellulose-acetate-forecasts-global-industry-analysts-inc.asp (accessed on 5 May 2020).
- Jun Xu; Zhenhua Wu; QiQi Wu; Yishan Kuang; Acetylated cellulose nanocrystals with high-crystallinity obtained by one-step reaction from the traditional acetylation of cellulose. Carbohydrate Polymers 2020, 229, 115553, 10.1016/j.carbpol.2019.115553.
- Araújo, D.; Castro, M.C.R.; Figueiredo, A.; Vilarinho, M.; Machado, A.; Green synthesis of cellulose acetate from corncob: Physicochemical properties and assessment of environmental impacts. J. Clean. Prod. 2020, 260, 120865.
- Juergen Puls; Steven A. Wilson; Dirk Hölter; Degradation of Cellulose Acetate-Based Materials: A Review. Journal of Polymers and the Environment 2010, 19, 152-165, 10.1007/s10924-010-0258-0.
- Mohammed Ahmad Wsoo; Shafinaz Shahir; Siti Pauliena Mohd Bohari; Nadirul Hasraf Mat Nayan; Saiful Abd Razak; A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: A new perspective. Carbohydrate Research 2020, 491, 107978, 10.1016/j.carres.2020.107978.
- Dobos, A.M.; Filimon, A.; Bargan, A.; Zaltariov, M.-F.; New approaches for the development of cellulose acetate/tetraethyl orthosilicate composite membranes: Rheological and microstructural analysis. J. Mol. Liq. 2020, 309, 113129.
- Violeta Martin-Gil; M.Z. Ahmad; Roberto Castro-Muñoz; V. Fila; Economic Framework of Membrane Technologies for Natural Gas Applications. Separation & Purification Reviews 2018, 48, 298-324, 10.1080/15422119.2018.1532911.
- L.K. Altunina; L.D. Tikhonova; E.G. Yarmukhametova; Method for Deriving Carboxymethyl Cellulose. Eurasian Chemico-Technological Journal 2016, 3, 49, 10.18321/ectj416.
- Loghman Golbaghi; Mehrdad Khamforoush; Tahmasb Hatami; Carboxymethyl cellulose production from sugarcane bagasse with steam explosion pulping: Experimental, modeling, and optimization. Carbohydrate Polymers 2017, 174, 780-788, 10.1016/j.carbpol.2017.06.123.
- Tao Shui; Shanghuan Feng; Gang Chen; An Li; Zhongshun Yuan; Hengfu Shui; Takashi Kuboki; Chunbao (Charles) Xu; Synthesis of sodium carboxymethyl cellulose using bleached crude cellulose fractionated from cornstalk. Biomass and Bioenergy 2017, 105, 51-58, 10.1016/j.biombioe.2017.06.016.
- K. Azzaoui; E. Mejdoubi; Abdellatif Lamhamdi; Shehdeh Jodeh; O. Hamed; M. Berrabah; S. Jerdioui; R. Salghi; N. Akartasse; A. Errich; et al.Á. RíosMohammed Zougagh Preparation and characterization of biodegradable nanocomposites derived from carboxymethyl cellulose and hydroxyapatite. Carbohydrate Polymers 2017, 167, 59-69, 10.1016/j.carbpol.2017.02.092.
- Karataş, M.; Arslan, N. Flow behaviours of cellulose and carboxymethyl cellulose from grapefruit peel. Food Hydrocoll. 2016, 58, 235–245.
- Chen, H. 5-Lignocellulose biorefinery product engineering. In Lignocellulose Biorefinery Engineering; Chen, H., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 125–165.
- Yining Chen; Guolian Cui; Nianhua Dan; Yanping Huang; Zhongxiang Bai; Changkai Yang; Weihua Dan; Preparation and characterization of dopamine–sodium carboxymethyl cellulose hydrogel. SN Applied Sciences 2019, 1, 609, 10.1007/s42452-019-0605-2.
- Vandana Singh; Sneha Joshi; Tulika Malviya; Carboxymethyl cellulose-rosin gum hybrid nanoparticles: An efficient drug carrier. International Journal of Biological Macromolecules 2018, 112, 390-398, 10.1016/j.ijbiomac.2018.01.184.
- Seiichi Ohta; Toru Nishiyama; Megumu Sakoda; Kyoko Machioka; Masaya Fuke; Shigetoshi Ichimura; Fuyuki Inagaki; Atsushi Shimizu; Kiyoshi Hasegawa; Norihiro Kokudo; et al.Makoto KanekoYutaka YatomiTaichi Ito Development of carboxymethyl cellulose nonwoven sheet as a novel hemostatic agent. Journal of Bioscience and Bioengineering 2015, 119, 718-723, 10.1016/j.jbiosc.2014.10.026.
- Hadi Samadian; Majid Salehi; Saeed Farzamfar; Ahmad Vaez; Arian Ehterami; Hamed Sahrapeyma; Arash Goodarzi; Sadegh Ghorbani; In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artificial Cells, Nanomedicine, and Biotechnology 2018, 46, 964-974, 10.1080/21691401.2018.1439842.
- Aneela Hayder; Arshad Hussain; Ahmad Nawaz Khan; Hizba Waheed; Fabrication and characterization of cellulose acetate/hydroxyapatite composite membranes for the solute separations in Hemodialysis. Polymer Bulletin 2017, 75, 1197-1210, 10.1007/s00289-017-2084-1.
- Andreea Pandele; F.E. Comanici; C.A. Carp; Marian Miculescu; S.I. Voicu; V.K. Thakur; B.C. Serban; Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 2017, 146, 599-605, 10.1016/j.vacuum.2017.05.008.
- Azzaoui, K.; Lamhamdi, A.; Mejdoubi, E.M.; Berrabah, M.; Hammouti, B.; Elidrissi, A.; Fouda, M.M.; Al-Deyab, S.S.; Lamhamdi, A. Synthesis and characterization of composite based on cellulose acetate and hydroxyapatite application to the absorption of harmful substances. Carbohydr. Polym. 2014, 111, 41–46.
- Ciobanu, G.; Ciobanu, O. High-performance ultrafiltration mixed-matrix membranes based on cellulose acetate and nanohydroxyapatite. Desalin. Water Treat. 2015, 57, 1–9.
- Ciobanu, G.; Ana-Maria, B.; Luca, C. Nystatin-loaded Cellulose Acetate/Hydroxyapatite Biocomposites. Revista de Chimie 2013, 64, 1426–1429.
- Anderson Luis Ohland; Vera Maria Martins Salim; Cristiano Piacsek Borges; Plasma functionalized hydroxyapatite incorporated in membranes for improved performance of osmotic processes. Desalination 2019, 452, 87-93, 10.1016/j.desal.2018.11.008.
- Zare, S.; Kargari, A. Membrane properties in membrane distillation. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 107–156.
- Hong Jiang; Yi Zuo; Lin Cheng; Hongli Wang; Aiqun Gu; Yubao Li; A homogenous CS/NaCMC/n-HA polyelectrolyte complex membrane prepared by gradual electrostatic assembling. Journal of Materials Science: Materials in Electronics 2010, 22, 289-297, 10.1007/s10856-010-4195-1.
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patent US1975504A, 2 October 1934.
- Azhar A. Hamad; Mohamed S. Hassouna; Thanaa I. Shalaby; Marwa F. Elkady; Mervat A. Abd Elkawi; Hesham Hamad; Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. International Journal of Biological Macromolecules 2020, 151, 1299-1313, 10.1016/j.ijbiomac.2019.10.176.
- Sasikumar Kandasamy; Valarmathi Narayanan; Shanmugam Sumathi; Zinc and manganese substituted hydroxyapatite/CMC/PVP electrospun composite for bone repair applications.. International Journal of Biological Macromolecules 2019, 145, 1018-1030, 10.1016/j.ijbiomac.2019.09.193.
- Mohamed El-Newehy; Mehrez. E. El-Naggar; Saleh Alotaiby; Hany El-Hamshary; Meera Moydeen; Salem Al-Deyab; Preparation of biocompatible system based on electrospun CMC/PVA nanofibers as controlled release carrier of diclofenac sodium. Journal of Macromolecular Science, Part A 2016, 53, 566-573, 10.1080/10601325.2016.1201752.
- Shi, D.; Wang, F.; Lan, T.; Zhang, Y.; Shao, Z. Convenient fabrication of carboxymethyl cellulose electrospun nanofibers functionalized with silver nanoparticles. Cellulose 2016, 23, 1899–1909.
- Gašparič, P.; Kurecic, M.; Kargl, R.J.; Maver, U.; Gradišnik, L.; Hribernik, S.; Kleinschek, K.S.; Smole, M.S. Nanofibrous polysaccharide hydroxyapatite composites with biocompatibility against human osteoblasts. Carbohydr. Polym. 2017, 177, 388–396.
- Raghavan, P.; Nageswaran, S.; Thakur, V.; Ahn, J.-H. Electrospinning of Cellulose: Process and Applications. In Nanocellulose Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2014; pp. 311–340.
- Travis J. Sill; Horst A. Von Recum; Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989-2006, 10.1016/j.biomaterials.2008.01.011.
- Elizângela H. Fragal; Thelma S.P. Cellet; Vanessa H. Fragal; Mychelle V.P. Companhoni; Tania Ueda-Nakamura; Edvani C. Muniz; Rafael Silva; Adley F. Rubira; Hybrid materials for bone tissue engineering from biomimetic growth of hydroxiapatite on cellulose nanowhiskers. Carbohydrate Polymers 2016, 152, 734-746, 10.1016/j.carbpol.2016.07.063.
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally crosslinked collagen/hydroxyapatite composite for enhanced in vivo bone repair. Colloids Surf. B Biointerfaces 2018, 163, 394–401.
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020.
- Baojin Ma; Jing Han; Shan Zhang; Feng Liu; Shicai Wang; Jiazhi Duan; Yuanhua Sang; Huaidong Jiang; Dong Li; Shaohua Ge; et al.Jinghua YuHong Liu Hydroxyapatite nanobelt/polylactic acid Janus membrane with osteoinduction/barrier dual functions for precise bone defect repair. Acta Biomaterialia 2018, 71, 108-117, 10.1016/j.actbio.2018.02.033.
- Domínguez, J.H.L.; Jiménez, H.T.; Cocoletzi, H.H.; Hernández, M.G.; Banda, J.A.M.; Nygren, H. Development and in vivo response of hydroxyapatite/whitlockite from chicken bones as bone substitute using a chitosan membrane for guided bone regeneration. Ceram. Int. 2018, 44, 22583–22591.
- Nazeer, M.A.; Yilgor, E.; Yilgor, E. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46.
- Selorm Torgbo; Prakit Sukyai; Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Materials Chemistry and Physics 2019, 237, 121868, 10.1016/j.matchemphys.2019.121868.
- Maddela Swetha; Kolli Sahithi; Ambigapathi Moorthi; Narasimhan Srinivasan; Kumarasamy Ramasamy; Nagarajan Selvamurugan; Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. International Journal of Biological Macromolecules 2010, 47, 1-4, 10.1016/j.ijbiomac.2010.03.015.




