
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Henriques Pons | + 1866 word(s) | 1866 | 2021-10-11 09:32:31 | | | |
| 2 | Catherine Yang | Meta information modification | 1866 | 2021-10-14 03:58:01 | | |
Video Upload Options
Mesenchymal stem cells (MSCs) are multipotent adult stem cells present in virtually all tissues; they have a potent self-renewal capacity and can differentiate into multiple cell types. They also affect the ambient tissue by the paracrine secretion of numerous factors in vivo, including the induction of other stem cells’ differentiation. In vitro, the culture media supernatant is named secretome and contains soluble molecules and extracellular vesicles that retain potent biological function in tissue regeneration.
1. Mesenchymal Stem Cells Can Be Isolated from Different Adult Tissues
Mesenchymal stem cells (MSCs) were described by Friedenstein in 1970 [1] and were first isolated from the bone marrow as non-hematopoietic stem cells. In 1991, Caplan introduced the term “mesenchymal stem cells” because it was believed that these cells would differentiate into mesodermal (middle germ layer) cells, such as bone, cartilage, tendon, fat, skin, muscle, and marrow stromal cells [2][3][4]. However, today we know that MSCs can also differentiate into ectodermal [5] and endodermal [6] cell lines, characterizing them as pluripotent stem cells [7][8]. They are adherent spindle-shaped cells found in virtually all adult tissues as a population of undifferentiated tissue-resident cells that facilitate tissue remodeling and repair during life [9]. MSCs generate progeny by self-renewal and can differentiate into various cell types depending on the particular tissue. However, in many organs, their pluripotency decreases with age, leading to reduced regenerative potential.
Although several trials are being conducted using MSCs to regenerate multiple tissues and organs, much remains to be elucidated about their biology and regenerative capacity. In general, MSCs are perivascular cells [10][11] that can be expanded for long periods in culture and, as observed in vivo, extended maintenance in vitro may reduce their pluripotency, limiting their applicability. Moreover, empirical evidence demonstrating that MSCs have the capacity for asymmetric cell division is still lacking [12], a characteristic of conventional stem cells [13]. Additionally, by definition, stem cells are rare cell populations that undergo self-renewal and yield progenitors to differentiate hierarchically into other cell types. However, MSCs appear to affect different cell types and alter the tissue microenvironment via paracrine signaling, inducing differentiation of other resident stem cells (cooperative activity), angiogenesis, chemotaxis, alteration of the local inflammatory response against pathogens and damage [14], tissue repair, proliferation, apoptosis control, and others [15][16].
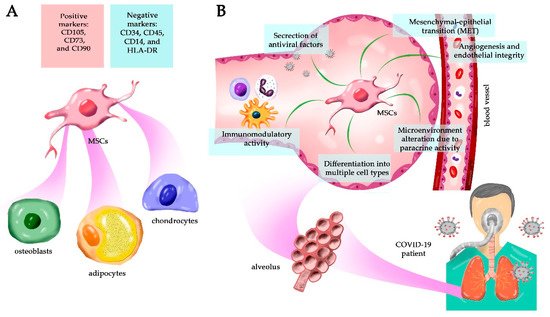
Finally, MSCs seem to be a much more heterogeneous population than initially envisioned. It is unclear, for example, whether these cells regenerate only in the tissues from which they originate, or if their heterogeneity allows the differentiation into other tissue parenchymal cell types in vivo [3][9]. Moreover, there are no ethical issues involved, in contrast to the use of embryonic stem cells in regenerative medicine, and they can be harvested in relatively large numbers, mainly from bone marrow and adipose tissue [19]. All these characteristics contribute significantly to the therapeutic use of MSCs [14].
The nomenclature “MSC” causes great confusion in the scientific literature and can also mean “mesenchymal stromal cells”. Thus, the International Society for Cellular Therapy (ISCT) suggests that the fibroblast-like plastic-adherent cells, regardless of the tissue from which they were isolated, be termed “multipotent mesenchymal stromal cells”. Then, the term mesenchymal stem cells should be used only for cells that meet specific stem cell criteria. The widely recognized acronym, MSC, may be used for both cell populations since researchers define the correct designation in their research [20]. In fact, mesenchymal stromal cells are present in the stroma of several organs and tissue and are a heterogeneous population comprising several cell types such as stem cells, progenitor cells, fibroblasts, and many others [21][22]. Thus, it is the stem cells present in this population that can differentiate into cells of the mesodermal, ectodermal, and endodermal lineage [23]. Therefore, the acronym MSC refers more appropriately to the stem cells defined by the term multipotent mesenchymal stromal cells.
2. MSC As an Alternative Treatment for COVID-19
References
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guin-ea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403.
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650.
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319.
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147.
- Kopen, G.C.; Prockop, D.J.; Phinney, D.G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they dif-ferentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl. Acad. Sci. USA 1999, 96, 10711–10716.
- Sato, Y.; Araki, H.; Kato, J.; Nakamura, K.; Kawano, Y.; Kobune, M.; Sato, T.; Miyanishi, K.; Takayama, T.; Takahashi, M.; et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 2005, 106, 756–763.
- Meirelles, L.D.S.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213.
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197.
- Klimczak, A.; Kozlowska, U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeo-stasis. Stem Cells Int. 2016, 2016, 4285215.
- Chen, W.C.W.; Park, T.S.; Murray, I.R.; Zimmerlin, L.; Lazzari, L.; Huard, J.; Péault, B. Cellular Kinetics of Perivascular MSC Precursors. Stem Cells Int. 2013, 2013, 1–18.
- Corselli, M.; Chen, W.C.; Crisan, M.; Lazzari, L.; Péault, B. Perivascular Ancestors of Adult Multipotent Stem Cells. Arter. Thromb. Vasc. Biol. 2010, 30, 1104–1109.
- Phinney, D.G. A SAGE View of Mesenchymal Stem Cells. Int. J. Stem Cells 2009, 2, 1–10.
- Bhartiya, D. The need to revisit the definition of mesenchymal and adult stem cells based on their functional attributes. Stem Cell Res. Ther. 2018, 9, 1–3.
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402.
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084.
- da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Rasini, V.; Dominici, M.; Kluba, T.; Siegel, G.; Lusenti, G.; Northoff, H.; Horwitz, E.M.; Schäfer, R. Mesenchymal stromal/stem cells markers in the human bone marrow. Cytotherapy 2013, 15, 292–306.
- Sheriff, L.; Alanazi, A.; Ward, L.S.; Ward, C.; Munir, H.; Rayes, J.; Alassiri, M.; Watson, S.P.; Newsome, P.N.; Rainger, G.E.; et al. Origin-Specific Adhesive Interactions of Mesenchymal Stem Cells with Platelets Influence Their Behavior After Infu-sion. Stem Cells 2018, 36, 1062–1074.
- Horwitz, E.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Deans, R.; Krause, D.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395.
- Alessio, N.; Squillaro, T.; Özcan, S.; Di Bernardo, G.; Venditti, M.; AB Melone, M.; Peluso, G.; Galderisi, U. Stress and stem cells: Adult Muse cells tolerate extensive genotoxic stimuli better than mesenchymal stromal cells. Oncotarget 2018, 9, 19328–19341.
- Galderisi, U.; Giordano, A. The Gap Between the Physiological and Therapeutic Roles of Mesenchymal Stem Cells. Med. Res. Rev. 2014, 34, 1100–1126.
- Wakao, S.; Kuroda, Y.; Ogura, F.; Shigemoto, T.; Dezawa, M. Regenerative Effects of Mesenchymal Stem Cells: Contribution of Muse Cells, a Novel Pluripotent Stem Cell Type that Resides in Mesenchymal Cells. Cells 2012, 1, 1045–1060.
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154.
- Metcalfe, S.M. Mesenchymal stem cells and management of COVID-19 pneumonia. Med. Drug Discov. 2020, 5, 100019.
- Golchin, A.; Seyedjafari, E.; Ardeshirylajimi, A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev. Rep. 2020, 16, 427–433.
- Golchin, A. Cell-Based Therapy for Severe COVID-19 Patients: Clinical Trials and Cost-Utility. Stem Cell Rev. Rep. 2020, 17, 56–62.
- Lalu, M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Canadian Critical Care Trials Group Safety of Cell Therapy with Mesenchymal Stromal Cells (SafeCell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559.
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 1–11.
- Sánchez-Guijo, F.; García-Arranz, M.; López-Parra, M.; Monedero, P.; Mata-Martínez, C.; Santos, A.; Sagredo, V.; Álvarez-Avello, J.M.; Guerrero, J.E.; Pérez-Calvo, C.; et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumo-nia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine 2020, 25, 100454.
- Tang, L.; Jiang, Y.; Zhu, M.; Chen, L.; Zhou, X.; Zhou, C.; Ye, P.; Chen, X.; Wang, B.; Xu, Z.; et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front. Med. 2020, 14, 664–673.
- Liang, B.; Chen, J.; Li, T.; Wu, H.; Yang, W.; Li, Y.; Li, J.; Yu, C.; Nie, F.; Ma, Z.; et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine 2020, 99, e21429.
- Chen, X.; Shan, Y.; Wen, Y.; Sun, J.; Du, H. Mesenchymal stem cell therapy in severe COVID-19: A retrospective study of short-term treatment efficacy and side effects. J. Infect. 2020, 81, 647–679.
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858.
- Fierabracci, A.; Del Fattore, A.; Luciano, R.; Muraca, M.; Teti, A.M.; Muraca, M. Recent Advances in Mesenchymal Stem Cell Immunomodulation: The Role of Microvesicles. Cell Transplant 2015, 24, 133–149.
- Muraca, M.; Pessina, A.; Pozzobon, M.; Dominici, M.; Galderisi, U.; Lazzari, L.; Parolini, O.; Lucarelli, E.; Perilongo, G.; Baraldi, E. Mesenchymal stromal cells and their secreted extracellular vesicles as therapeutic tools for COVID-19 pneumonia? J. Control Release 2020, 325, 135–140.
- Mansouri, N.; Willis, G.R.; Fernandez-Gonzalez, A.; Reis, M.; Nassiri, S.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight 2019, 4.
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754.




